Figures & data
Figure 1. Generation of stable MDCK tet-off cell clones with inducible expression of PLEKHA7 constructs. (A) Schematic diagrams showing the putative minimal regions of PLEKHA7 which can interact with afadin (residues 120–374 in human PLEKHA7), p120ctn (538–696), CGNL1 (620–769), or nezha (680–821),Citation21,Citation23,Citation29 and schematic structure of the PLEKHA7 constructs (Full-length FL, N-terminal = N-term., C-terminal = C-term., Central) used to generate stable clones of MDCK tet-off cells. Constructs were tagged N-terminally with YFP, and C-terminally with myc. Putative structural domains are indicated: WW (purple), PH (brown), Pro-rich (P, red), and coiled-coil (CC, blue, CC1 = residues 686–835, and CC2 = residues 1055–1095, based on Phyre2 analysis). Numbers indicate position of PLEKHA7 amino acid residues within constructs. (B) Immunoblotting analysis of lysates of MDCK cell lines (two clones for each construct shown) cultured either in the presence (+) or in the absence (-) of doxycycline, using antibodies against the myc tag (to detect exogenous PLEKHA7), E-cadherin (E-cad.), cingulin (CGN) and tubulin (loading control). Numbers on the right indicate the migration of pre-stained markers (kDa). (C) Confocal immunofluorescent analysis of induced cultures of stable clones showing the localization of exogenous PLEKHA7 constructs (green, YFP) and α-tubulin (red), on the apical plane of focus (ZO-1 labeling is shown for control cells, panel E). Arrows = junctional labeling; arrowheads = cytoplasmic labeling; circle = cytoplasmic dots; n = nuclear labeling. (D) Table summarizing the subcellular localization of each YFP-tagged construct, based on immunofluorescence on paraformaldehyde-fixed cells. (E) Immunofluorescent labeling of control Tet-off cells showing tubulin and ZO-1 in the apical plane of focus. Bar = 10 μm.
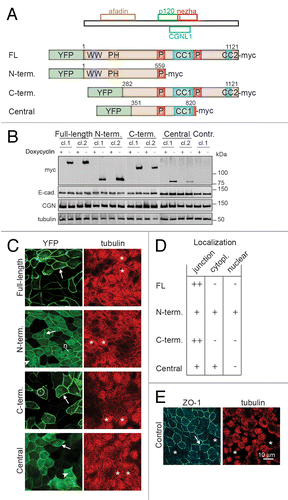
Figure 2. Exogenous expression of full-length, N-terminal, C-terminal, but not Central PLEKHA7 constructs enhances ZA recruitment and PA clustering of E-cadherin. Confocal immunofluorescence analysis of clonal lines of MDCK cells showing labeling for exogenous constructs (green) and E-cadherin, either in the apical plane of focus (ZO-1, gray, left panels), which contains ZA (zonula adhaerens), or in the basal plane (nuclei, blue DAPI, right panels), which contains PA (puncta adhaerentia). (A) Full-length, uninduced (FL+DOX); (B) Full-length, induced (FL); (C) N-terminal, induced (N-TERM); (D) C-terminal, induced (C-TERM; (E) Central, induced. Matched arrows indicate matched normal junctional labeling (using ZO-1 as a reference fore the apical plane), and matched arrowheads indicate matched decreased junctional labeling (B, C, D, apical plane) or diffuse lateral labeling (A and E, basal). Within each panel, arrows indicate stronger labeling than arrowheads. The basal plane was typically 10–15 μm below the apical plane. Bar = 10 μm.
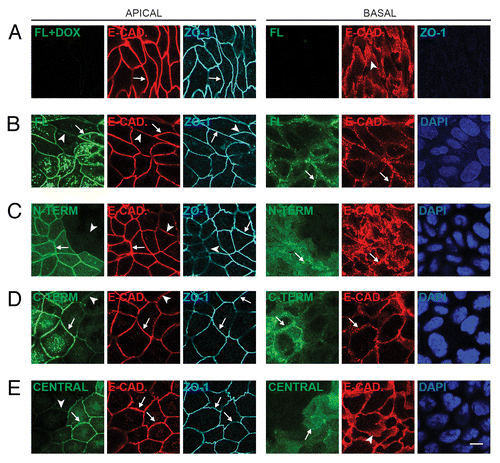
Figure 3. Exogenous expression of full-length PLEKHA7 enhances the ZA recruitment of paracingulin, but not cingulin, ZO-1, occludin and afadin. Confocal immunofluorescence analysis of MDCK cells expressing the Full-length construct cells with labeling for exogenous protein (green) and either paracingulin (CGNL1, (A)), cingulin (CGN, (B)) occludin (OCCL, (D)), or afadin (AFAD), (E)) in red. ZO-1 labeling in triple-stained cells is shown in gray in apical, left panels, and in basal plane (B). See legend to for arrow/arrowheads description. Bar = 10 μm.
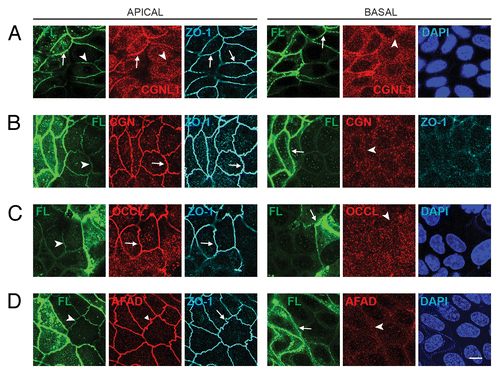
Figure 4. Expression of exogenous PLEKHA7 constructs does not affect TER development during the calcium switch in MDCK cells. Transepithelial electrical resistance (TER, ohm.cm2) profiles of representative clonal lines of MDCK cells expressing YFP (Control) or YFP-tagged constructs of PLEKHA7 (see legends), either in the absence (red) or presence (blue) of doxycycline, during the calcium switch.
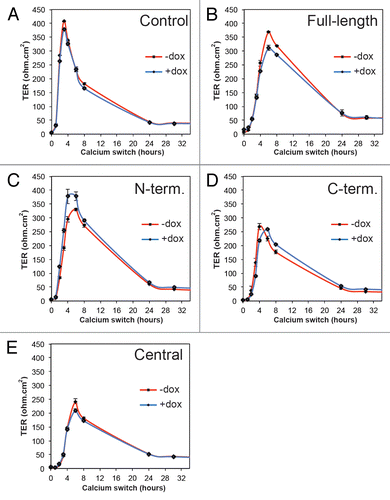
Figure 5. Exogenous expression of PLEKHA7 constructs does not affect paracellular permeability to large solutes. Histogram showing paracellular permeability to 3 kDa dextran of clonal MDCK lines expressing PLEKHA7 constructs, and control MDCK Tet-off cells, either at normal extracellular calcium concentration (NC, value at 2 h), or after extracellular calcium depletion (LC, value at 30 min after calcium depletion), either in the presence (+DOX, uninduced) or in the absence (-DOX, induced) of doxycycline.
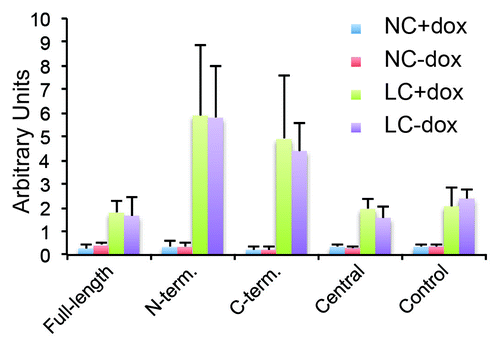
Figure 6. Exogenous expression of Full-length, N-terminal and C-terminal but not Central PLEKHA7 constructs attenuates barrier disruption by extracellular calcium removal, in a microtubule-dependent manner. (A) TER values (expressed as % of value of uninduced, +dox cultures) of clonal lines of MDCK cells after trypsinization and incubation of confluent monolayers at normal extracellular calcium for either 18 h (NC + dox 18hr, NC-dox 18hr) or 64 h (NC + dox 64hr, NC-dox 64hr). The values at 64 h were the average of two experiments. (B) TER values (expressed as % of value after overnight incubation in NC) of clonal lines of MDCK cells under different conditions: normal calcium (NC) or 30 min low calcium treatment (LC), uninduced or induced (+dox, -dox), treated or untreated with nocodazole (DMSO, noco). For cultures maintained at normal calcium, the TER values were expressed as % of the value before treatment with either DMSO or nocodazole. For cultures undergoing calcium depletion (only the 30 min time point is shown), the TER values were expressed as % of value at time 0, after treatment with either DMSO or nocodazole, and just prior to calcium removal. * = P < 0.05.
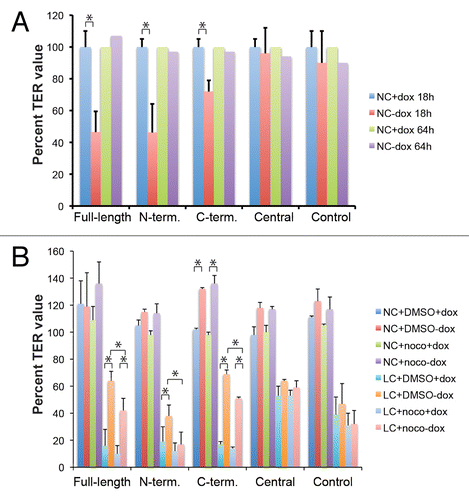
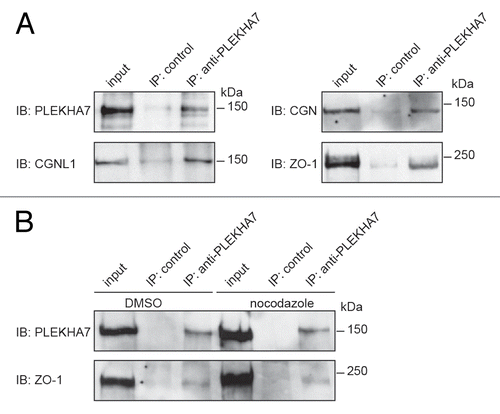
Figure 7. PLEKHA7 forms a complex with TJ proteins. (A) Immunoblot analysis of PLEKHA7 immunoprecipitates from lysates of confluent human intestinal colon carcinoma cells (Caco2) with antibodies against PLEKHA7, ZO-1, cingulin and paracingulin. (B) Immunoblot analysis of PLEKHA7 immunoprecipitates, with antibodies against either PLEKHA7 or ZO-1, from lysates of Caco2 cells treated with either DMSO or nocodazole for 1 h prior to lysis. Numbers on the right indicate migration of molecular size markers (kDa).