Figures & data
Figure 1. FAST encodes an eIF4E-binding motif. Amino acid sequence alignment comparing the eIF4E-binding motifs of FAST with those found in human eIF4GI (AF012088), human eIF4GII (AF012072), human 4E-BP1 (L36055), human 4E-BP2 (L36056), human 4E-BP3 (AF038869), human 4E-T (AF240775), Drosophila eIF4G (AF030155), S. cerevisiae eIF4GI (p39935), S. cerevisiae eIF4GII (p39936), S.cerevisiae p20 (X15731) and wheat iso-eIF4G (M95747). Residues which are critical for eIF4E binding are boxed and shaded. The additional identical or reserved residues are boxed.
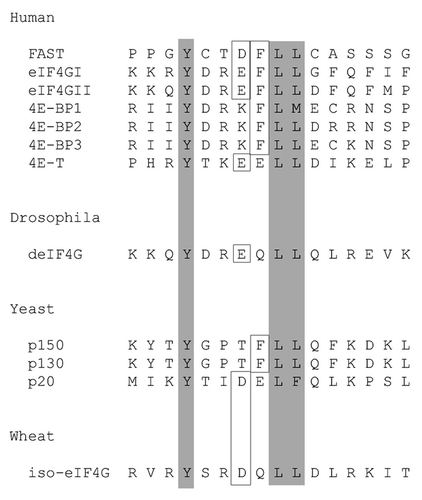
Figure 2.FAST interacts with eIF4E through its eIF4E-binding motif.A, FLAG-tagged eIF4E was overexpressed together with the indicated constructs in COS-7 cells. Cell lysates were immunoprecipitated with anti-HA Ab, then subjected to SDS-PAGE immunoblot analysis. Left upper panel: immunoblotting with anti-FLAG Ab after IP; Left lower panel: immunoblotting with anti-HA Ab after IP; Right upper panel: immunoblotting with anti-FLAG Ab before IP; Right lower panel: immunoblotting with anti-HA Ab before IP. B, HA-tagged WT-FAST and Y428G-FAST were overexpressed in HeLa cells. Cell lysates were immunoprecipitated with a mouse anti-eIF4E Ab followed by SDS-PAGE immunoblot analysis. Left panel: immunoblotting with anti-HA Ab after immunopreciptitation; Right panel: immunoblotting with anti-HA Ab before immunoprecipitation.
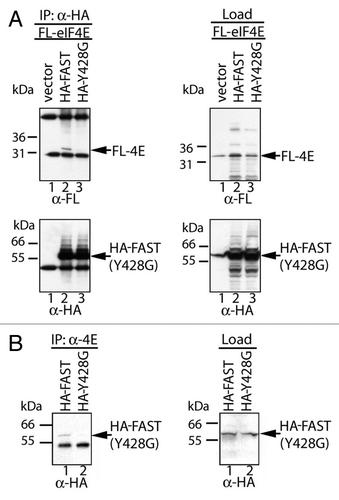
Figure 3. FAST:eIF4E interactions are resistant to RNase. U2OS cells were transfected with pMT2-HA vector (-) or pMT2-HA-FAST encoding wild type (WT) or Y428G (MUT) variants. Transfected cells were lysed in the presence or absence of RNase A as indicated, and subjected to immunoprecipitation using mouse IgG antibodies (IP:IgG) or eIF4E (IP:eIF4E) antibodies. Co-immunoprecipitation efficiency was quantified by immunoblotting using anti-HA and anti-eIF4E antibodies. Load: loading control (1/20th of total protein lysate).
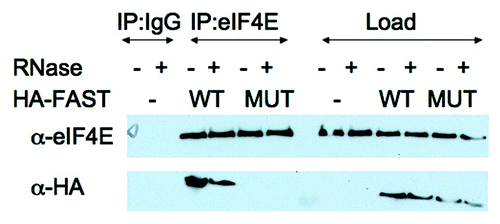
Figure 4. m7GTP cap pull-down analysis. U2OS cells were transfected with pMT2-HA vector (-) or pMT2-HA-FAST encoding wild type (WT) or Y428G (MUT) variants. Transfected cells were lysed in the presence of RNase A and subjected to pull-down using m7GTP Sepharose (m7GTP). Pulled-down proteins were quantified by immunoblotting using anti-HA and anti-eIF4E antibodies. Load: loading control (1/20th of total protein lysate)
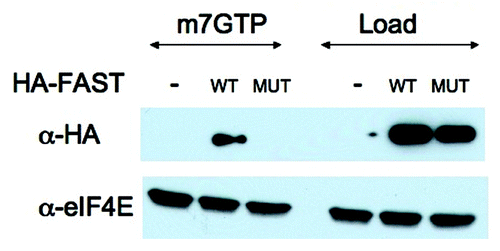
Figure 5.FAST displaces eIF4G from eIF4E. U2OS cells were transfected with pMT2-HA vector (lane 1) or with an increasing amount of pMT2-HA-FAST encoding wild type FAST protein. Transfected cells were lysed in the presence of RNase A, and subjected to immunoprecipitation using eIF4E antibodies (IP:eIF4E). Co-immunoprecipitation efficiency was quantified by immunoblotting using anti-HA, anti-eIF4G and anti-eIF4E antibodies. Load: loading control (1/20th of total protein lysate).
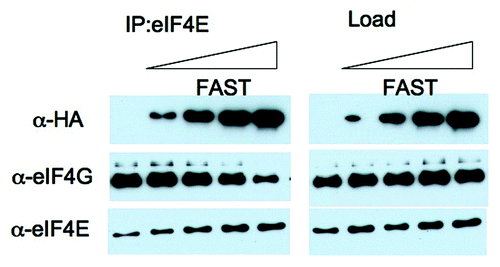
Figure 6.FAST and FAST (Y428G) have different effects on the expression of co-transfected β-gal and cIAP-1. β-gal (A and B) or Myc-cIAP-1 (D) was expressed together with the indicated constructs in COS-7 cells. Total cellular RNA (A) or total proteins (B and D) were extracted and subjected to RNA gel blot and SDS-PAGE immunoblot analysis, respectively. (C) The mean relative amount of β-gal mRNA in each transfectant was quantified using densitometry and presented as a bar graph (± standard errors, n = 3). The amount of β-gal mRNA expressed in cells transfected with the vector control was arbitrarily designated as 1. The relative amount of β-gal mRNA expressed in cells transfected with HA-FAST or HA-FAST (Y428G) was calculated by dividing their absolute expression levels by the absolute expression level in cells transfected with the vector control. Calculated P values are shown for selected samples.
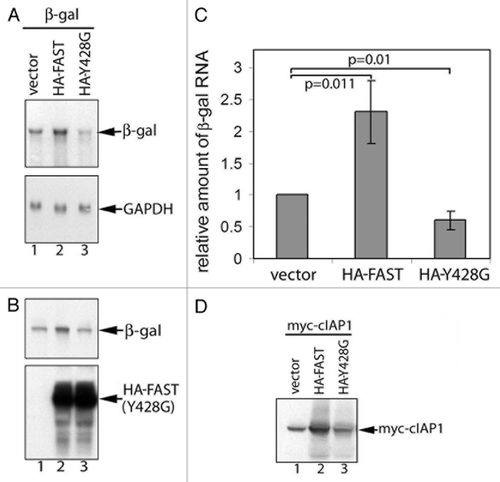
Figure 7.FAST and FAST (Y428G) have different effects on the stability of co-transfected β-gal transcripts.(A) COS-7 cells were co-transfected with β-gal together with either WT FAST or Y428G FAST. After 48 h, cells were treated with actinomycin D (5 mg/ml) for 0, 1.5, or 3 h. Total RNA was extracted and subjected to blot analysis.(B)The mean relative amounts of β-gal mRNA is plotted as a function of time in the presence of actinomycin D (± standard errors, n = 3). The relative amount of β-gal mRNA from samples treated with actinomycin D for 0 h (untreated) was arbitrarily designated as 1. The relative amount of the β-gal mRNA from samples treated with actinomycin D for 1.5 or 3 h was calculated by dividing their absolute values by the absolute amount of the β-gal mRNA of their corresponding untreated samples. In both cases, the t1/2 of β-gal mRNA in vector transfected cells (right panel) was 2.9. In contrast, the t1/2 of β-gal mRNA in HA-FAST transfected cells (left panel) was 9.4 and the t1/2 of β-gal mRNA in HA-Y428G transfected cells was 1.6. Calculated P values are shown for the samples treated with actinomycin D for 3 h.
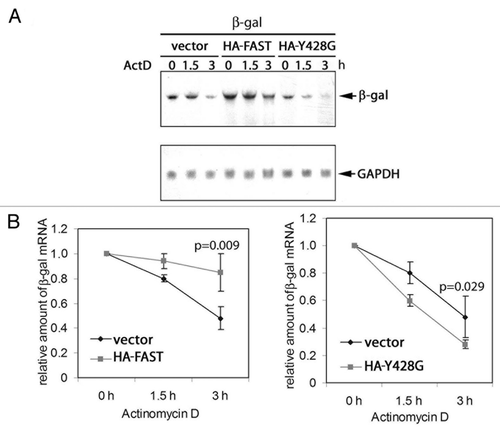
Figure 8.The Y428G mutant of FAST binds TIA-1. FLAG-tagged eIF4E and/or TIA-1 was overexpressed together with the indicated constructs in COS-7 cells. Cell lysates were immunoprecipitated with anti-HA Ab, then subjected to SDS-PAGE immunoblot analysis. Upper left panel: immunoblotting with anti-FLAG Ab after IP; Upper right panel: immunoblotting with anti-FLAG Ab before immunoprecioutation; Lower left panel: immunoblotting with anti-HA Ab after i.p.; Lower right panel: immunoblotting with anti-HA Ab before immunoprecipitation
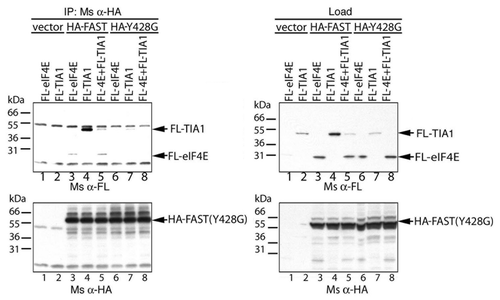
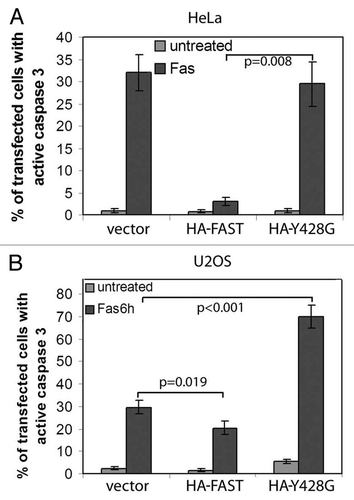
Figure 9.WT FAST, but not its Y428G mutant, inhibits Fas-induced activation of caspase-3. HeLa cells (A) and U2OS cells (B) were co-transfected with β-gal together with vector control, WT-FAST, or Y428G-FAST. After 48 h, cells were processed for immunofluorescence microscopy to quantify the expression of active caspase-3 in β-gal transfectants.