Figures & data
Figure 1. class switch recombination at the Immunoglobulin Heavy Chain locus. (A) The configuration of the unrearranged immunoglobulin heavy chain locus in immature B cells according to NG_005838.1. Here, VH, DH and JH represent the various unrearranged gene segments that will generate the VDJ exon following V(D)J recombination and are followed by the various constant regions genes (Cμ-α, yellow boxes). Each constant region gene is preceded by a switch sequence (Sμ-α, black oval); switch sequences are non-coding regions transcribed by their own transcriptional regulatory promoter elements. Two important regions that have enhancer functions and influence various recombination events in the IgH locus are shown in a blue box and are labeled as Eμ and Eα (also known as 3′ regulatory region, 3′RR). (B) Following V(D)J recombination, various B cell signaling pathways induce transcription at switch sequence promoter regions. Transcription at the upstream switch sequence Sμ is constitutive whereas transcription at the downstream switch sequence (in this case, Sγ1) is induced due to activation of its promoter elements by various signaling pathways. (C) A schematic of a simplified IgH locus that is poised to undergo CSR to IgG1 following transcription activation at Sμ and Sγ1. A region of the switch sequences, known as the core switch region (G-rich on the non-template strand), is capable of forming stable RNA/DNA hybrids that lead to ssDNA structure “R-loop” formation. (D) Transcription at switch sequences induces formation of R-loops which become targets for AID activity. AID converts cytidine residues to uracils, that are then recognized by the base excision pathway uracil DNA deglycosylase (UNG). (E) UNG activity induces generation of abasic residues that are then cleaved by the apurinic endonuclease family of proteins (APE1/2) to generate DNA double strand breaks (DSBs) at both upstream (Sμ) and downstream (Sx, in this case, Sγ1) switch sequences. (F) Recognition of these two DSBs by two cellular DNA damage repair pathways known as non homologous end-joining (NHEJ) and alternative end joining (AEJ) leads to joining of the two distant switch sequences that have DSBs leading to the completion of CSR. (G) The final configuration of the antibody heavy chain molecule coding mRNA is shown.
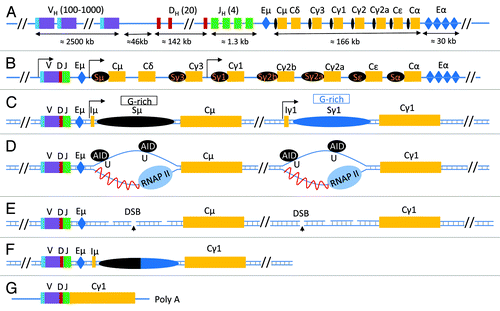
Figure 2. Fate of cytidine residues following AID mediated DNA deamination. (A) AID deaminates cytidine residues to uracils that are identified by the cellular base excision repair pathway (UNG) or the mismatch repair pathway (MSH2/MSH6) for repair. Neighboring residues (A/T based pairs) may be mutated in this process of DNA lesion repair that depends upon the DNA polymerase η. (B) Multiple possibilities exist explaining how a lesion could be repaired. Based on the activity of error prone DNA polymerases (DNA pol η), change of the neighboring A/T based pair could be to T/A or G/C or C/G base pairs. The nascently formed uracil residues are substrates of the apurinic endonucleases (APE1/2), and this reaction eventually leads to creation of single-strand DNA (ssDNA) nicks. ssDNA nicks on both strands of switch sequences can generate DNA double strand breaks that are intermediates during CSR.
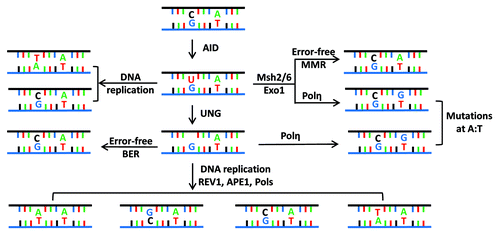
Figure 3. Subcellular control of AID activity by various post-transcriptional regulatory pathways. AID mRNA is recognized by two miRNAs (miR-155, miR-181) that bind to its 3′UTR to regulate AID mRNA translational efficiency and prevent AID hyperactivity caused by its overexpression.Citation55-Citation58 One proposed mode of cytoplasmic AID protein regulation is the chaperone complex HSP90Citation59 and EF1a,Citation60 controlling AID’s ability to translocate into the nucleus of B cells. AID uses its nuclear localization signal (NLS) to translocate into the nucleus where its steady-state nuclear protein levels are further controlled by another chaperone, REGγ Citation61. AID translocated in the nucleus can have multiple fates that include its ubiquitinationCitation62,Citation63 and its phosphorylation at various serine, threonine, or tyrosine residues (see (B) for details of known phosphorylation sitesCitation64-Citation68). Phosphorylated AID forms a complex with its cofactors 14–3-3Citation69 and RPACitation17,Citation70 and PTPBP2Citation71 and binds to the stalled RNA polymerase II complex (marked by RNAP II stalling marker Spt5)Citation40 at AID target sequences, where it interacts with the DNA/RNA hybrid and the 3′-5′ RNA exonuclease, RNA exosome.Citation48 It is postulated that the macromolecular complex RNA exosome provides AID the ability to deaminate both strands of its DNA substrate by processing the RNA present in the RNA/DNA hybrid associated with the transcription complex. The DNA DSBs in the immunoglobulin switch regions are intermediates that ultimately are utilized by the cellular DNA DSB response factors to complete CSR. (B) Schematic representation of AID phosphorylation sites along with AID’s cytidine deaminase domain, nuclear localization signal (NLS), APOBEC-like region, and nuclear export signal (NES) motif. (C) A detailed chart of various regulatory elements of AID that are known to directly control its activity. The protein factors and AID modifications are schematized in (A). The mRNA stability of AID is regulated by various miRNAs, as indicated in (C).Citation55-Citation58
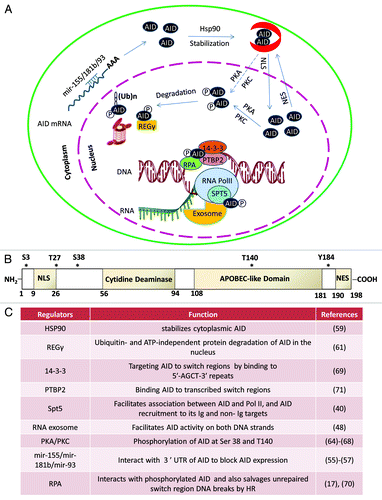
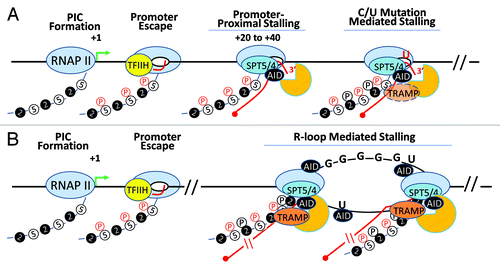
Figure 4. AID in the transcription complex. AID, RNA exosome and Spt5 can associate with transcribing RNA polymerase II in two distinct transcription complexes. (A) Following transcription initiation, RNA polymerase II at many transcribed genes enters a phase called “transcription stalling.” The association of RNAP II co-factors NELF and Spt5 and TFIIH leads to the resolution of RNAP II stalling and promotes RNAP II to transition into its transcription elongation phase. AID, by virtue of its association with Spt5, accesses this complex and its associated DNA. Moreover, RNA exosome is recruited to this complex and can associate with AID and Spt5-associated RNAP II to promote DNA deamination during somatic hypermutation in the first 100–200 base pairs downstream of the transcription start site. However, during SHM as well as in CSR, it is possible that the RNAP II is able to overcome the promoter proximal stalling and enter the elongation phase. Here, if it encounters dU residues (incorporated by preceding RNAP II-associated AID complexes), it may stall and recruit RNA exosome and catalyze robust cytidine deamination at DNA regions kilobases downstream from the transcription start sites. (B) If the transcription complex does continue into the elongation phase, it may generate stable DNA secondary structures like R-loops depending upon the physical properties of the transcribed DNA. For example, transcription of switch sequences is proposed to generate stable large R-loop structures due to the presence of G-richness on the template strand. If R-loops or other transcription complex impedance factors are recruited (or preexist), elongating RNAP II molecules that are loaded on the DNA secondary structure containing templates may undergo a second “stalling” event that is analogous to RNAP II pre-termination. In this pre-termination complex RNA exosome actively degrades the nascent transcript to prevent continuity of abortive transcription and aberrant DNA/RNA hybrids that can initiate genomic instability. This RNA exosome-associated RNAP II pretermination complex can potentially associate with AID and provide another hub for AID and its associated co-factors to catalyze DNA deamination. This scenario parallels the transcription stalling associated AID DNA deamination activity observed during class switch recombination.