Figures & data
Figure 1. Transcriptional stimulatory and repressive functions of Bre1 at the same set of genes. (A) Schematic diagram showing different regions of Bre1 (1–700 amino acids). (B) Schematic diagram showing the stimulatory and repressive functions of Bre1 in transcription. Bre1 interacts with chromatin via its RING domain at the C-terminal.Citation21 Rad6 interacts with a domain within the first 210 amino acids at the N-terminal of Bre1,Citation21 and leads to targeted histone H2B ubiquitylation.Citation21 Paf1C interacts with Bre1Citation11,Citation21 via its non-RING domain to promote histone H2B ubiquitylation activity of Rad6-Bre1. Thus, bre1∆RING, ∆paf1, ∆rad6, and H2B-K123R mutant strains impair histone H2B ubiquitylation, RNA polymerase II association with active gene, and transcription.Citation5-Citation11,Citation21,Citation22 The non-RING part of Bre1 has a repression domain (RD) that lowers the association of RNA polymerase II with the active gene and hence transcription,Citation11 possibly via interaction with repressor(s) or by impairing the recruitment/activity of transcriptional stimulatory/elongation factor(s). The transcriptional stimulation is lost in the absence of histone H2B ubiquitylation in the bre1∆RING, ∆rad6, and H2B-K123R mutant strains. When the whole BRE1 is deleted, transcriptional repression is lost in the absence of the repression domain of Bre1, in addition to the impairment of transcriptional stimulation. These two opposing activities counteract, and hence the defect in transcription (or RNA polymerase II association with active gene) is not apparently observed in the ∆bre1 strain. PID, Paf1C interaction domain; RID, Rad6p interaction domain; Ub, ubiquitin.
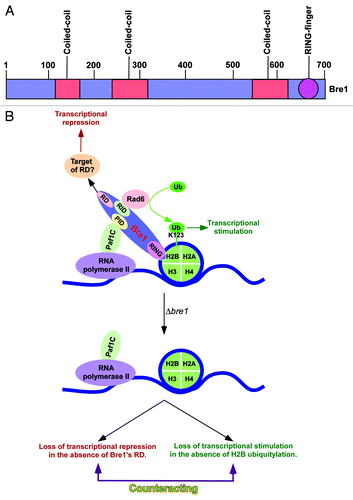
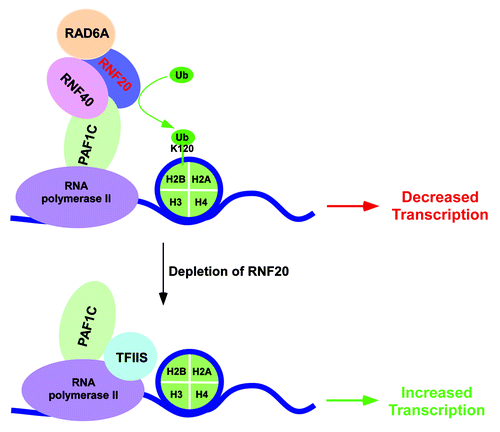
Figure 2. Schematic diagram showing the transcriptional repressive function of human BRE1 or RNF20/RNF40 at a set of genes. The enzymatic activity of RNF20/RNF40/RAD6A complex impairs the association of TFIIS with PAF1C (possibly via histone H2B ubiquitylation), hence lowering recruitment of TFIIS to the RNF20-repressed genes. Following depletion of RNF20, histone H2B ubiquitylation declines, and interaction between TFIIS and PAF1C is enhanced, which subsequently promotes recruitment of TFIIS to the chromatin to facilitate transcription.