Abstract
The intrauterine contraceptive device (IUD) is highly effective and cost-effective. IUD use is limited in some regions, however, due to concerns about increased risk of pelvic inflammatory disease (PID) and subsequent complications such as infertility and ectopic pregnancy. Recent reviews suggest that the overall risk of PID with modern IUDs is lower than previously thought, at least in regions with a low prevalence of sexually transmitted infections (STIs). Risk of PID may be higher, however, in places where gonorrhoea and chlamydia are prevalent, where screening for STIs is limited and where aseptic conditions for insertion are difficult to ensure. A World Health Organization multi-centre study and other studies have confirmed regional differences in STI prevalence, and the WHO study established that PID risk is temporally related to IUD insertion procedures. Studies of the effectiveness of antibiotic prophylaxis to prevent infectious complications are inconclusive due at least in part to use of sub-therapeutic regimens for pathogens commonly implicated in PID. In summary, the IUD can be safe and effective if inserted under aseptic conditions in women free of cervical infection. Further study is needed to define appropriate standards of care for IUD insertion where STI prevalence is high and ability to rule out infection is limited. Even with safe insertion, IUD promotion in areas of high STI/HIV prevalence must address women's needs for dual protection from infection and unwanted pregnancy.
Résumé
Le stérilet est très efficace et rentable. Pourtant, il est peu utilisé dans certaines régions par crainte d'un risque accru de maladies inflammatoires pelviennes (MIP) et de séquelles comme la stérilité et la grossesse ectopique. De récentes études indiquent que le risque global de MIP avexc les stérilets modernes est inférieur aux prévisions, au moins dans les régions présentant une faible prévalence de MST. Le risque de MIP peut s'accroı̂tre dans des régions où la blennorragie et la chlamydiase sont prévalentes, où le dépistage des MST est limité et où il est difficile de garantir des conditions d'asepsie pour l'insertion. Plusieurs travaux, notamment une étude de l'OMS dans plusieurs centres, ont établi que le risque de MIP est liée aux procédures d'insertion du stérilet. Les études sur l'efficacité de la prophylaxie antibiotique pour prévenir les complications infectieuses sont peu concluantes, en partie du fait de l'utilisation de doses sous-therapeutiques pour des pathogènes fréquemment impliqués dans les MIP. Le stérilet peut être sûr et efficace s'il est insére dans des conditions aseptiques chez des femmes ne présentant pas d'infections gynécologiques. De nouvelles études devront définir des normes appropriées pour l'insertion d'un stérilet quand la prévalence des MST est élevée et la capacité à eliminer le risque infectieux est limitée. Même avec une insertion sûre, dans les régions à forte prévalence de MST/VIH, la promotion du stérilet doit tenir compte du besoin qu'ont les femmes d'une protection double, contre l'infection et les grossesses non désirées.
Resumen
El dispositivo intrauterino (DIU) es muy eficaz y rentable. No obstante, en algunas regiones, su uso es limitado debido a un riesgo más elevado de enfermedad inflamatoria pélvica (EIP) y subsiguientes complicacaciones como la infecundidad y el embarazo ectópico. Los últimos estudios sugieren que el riesgo general de EIP con el DIU moderno es más bajo que lo previsto, en regiones con un bajo indice de infecciones de transmisión sexual (ITS). Puede que el riesgo de EIP sea más alto en lugares con un alto ı́ndice de gonorrea y clamidia, donde es difı́cil garantizar condiciones asépticas para la inserción del DIU. En un estudio realizado por la Organización Mundial de la Salud en múltiples centros y en otros estudios se confirmaron las diferencias regionales en el indice de ITS, y el estudio de la OMS estableció que el riesgo de EIP está relacionado temporalmente con la inserción del DIU. Los estudios sobre la eficacia de la profilaxis antibiótica para la prevención de complicaciones infecciosas son inconclusos debido, en parte, al uso de regı́menes subterapéuticos para patógenos comúnmente implicados en la EIP. El DIU es seguro y eficaz si es insertado bajo condiciones asépticas en mujeres sin infección cervical. Es necesario realizar más estudios para definir las normas apropiadas de atención para la inserción del DIU cuando existe un alto ı́ndice de ITS y la capacidad de descartar la infección es limitada. Aun cuando la inserción es segura, la promocion del DIU en áreas con alto ı́ndice de ITS/VIH debe abordar las necesidades de las mujeres de doble protección contra infección y embarazo no deseado.
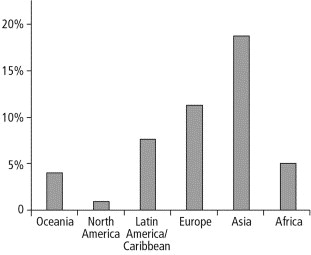
The intrauterine contraceptive device (IUD) is a highly effective method of contraception. It is also one of the most cost-effective methods available, providing long-term protection at a public sector cost as low as US$5 per woman/year of contraception in developing countries.Citation1 Use of the IUD is limited in some regions Footnote(Figure 1) , however, due to concerns about increased risk of pelvic inflammatory disease (PID)—estimated to range from two to nine timesCitation2—and subsequent complications such as infertility and ectopic pregnancy. A recent review concluded that the overall risk of PID with modern IUDs is lower than suggested by earlier studies, is concentrated in the weeks to months following insertion and is related to prevalence of sexually transmitted infections (STIs).Citation3 In other words, the IUD appears to be very safe under conditions where women can be determined to be free of infection at the time of insertion.
Figure 1 IUD use among women of reproductive age (15–49 years) in a marital or consensual unionCitation9
Others have argued that evidence of safety from regions with low prevalence of STI may not apply in settings where gonorrhoea and chlamydial infection are prevalent, and where it is not possible to rule out infection prior to insertion.Citation4 In fact, the technical difficulty of ruling out cervical infection due to these organisms in low-resource, high-prevalence areas is a broader problem of women's sexual health that has resisted easy solution.Citation5 This constraint, together with the challenges of ensuring aseptic conditions for IUD insertion, raises questions about appropriate standards of care for safe IUD use in low-resource settings.
These issues assume greater relevance in the light of a recent report from a USAID-supported inter-agency workshop that advocates wider use of the IUD even in countries with high STI prevalence.Citation6 Other materials designed to promote the IUD among health care providers in such countries are also beginning to appear.Citation7
WHO classifies the IUD as Category 3 (use of method not usually recommended unless other more appropriate methods are not available or not acceptable) when there is a very high individual likelihood of exposure to gonorrhoea or chlamydial infection. In the presence of current purulent cervicitis or chlamydial infection or gonorrhoea, it is classified as Category 4 (method not to be used) for initiation of use and Category 2 (generally use the method) for continuation of useCitation8 (Peterson H. Personal communication, 2004).
Against this background, we reviewed the literature addressing two issues: 1) the question of safety, specifically the risk of PID following IUD insertion in low-resource settings where gonorrhoea and chlamydia are prevalent, and 2) the value of antibiotic prophylaxis prior to IUD insertion in settings when it is not possible to rule out existing infection.
The risk of PID following IUD insertion
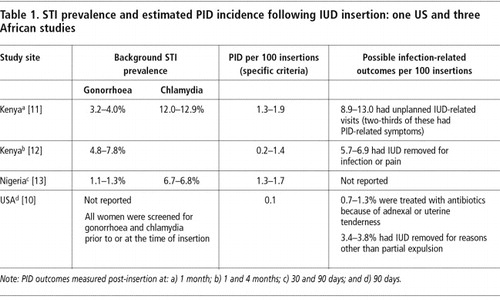
Reported PID incidence ranges from one per 1000 measured in a US-based studyCitation10 to two per 100 in the month following insertion in several African studies. Citation11 Citation12 Citation13 Within this approximately 20-fold range should lie some explanation for why the IUD is a significantly lower-risk method of contraception in some settings than in others. At least two factors—the background prevalence of STI and the conditions under which the procedure is performed—influence the risk of PID following IUD insertion.
PID can be caused either by sexually transmitted infections (STIs) or introduction into the uterus of bacteria from the vagina or outside contamination. Among common pathogens, gonorrhoea is more likely than chlamydial infection to cause acute symptomatic PID and to come to the attention of clinicians. Because of its more insidious progression and lack of symptoms, chlamydial PID may be a more important cause of complications such as tubal infertility.Citation14 In a Nairobi study, however, only one-third of clinically diagnosed PID episodes were in women with proven gonococcal or chlamydial infection.Citation11 The contribution of non-sexually transmitted contaminants to PID following IUD insertion under poor aseptic conditions has been given little attention, but is well documented for puerperal and post-abortion infections.Citation15 The role of bacterial vaginosis in IUD-related complications is also under investigation.Citation16
There is little debate about how IUD insertion increases risk of PID in the period immediately following the procedure.Citation3, Citation17 Disruption of the cervical mucus barrier by a foreign object that deposits bacteria directly into the uterine cavity is a well-established mechanism for infection following transcervical procedures. Intrauterine microbial contamination has been found to be highest in the month following IUD insertion and to decrease with time.Citation18
Since organisms cannot be pushed into the upper genital tract if they are not present in the first place, the risk of PID following IUD insertion is related to both local STI prevalence and to the capacity of health care services to identify pre-existing cervical infection and ensure aseptic conditions for insertion. In other words, both a source of infection and a transport mechanism are important. In epidemiological terms, there is a strong interaction (as opposed to confounding) between IUD insertion and the prevalence of pathogens capable of causing PID.
How often does this happen? The true risk of insertion in low-resource settings with moderate-to-high STI prevalence cannot be accurately estimated from studies conducted in low STI prevalence settings, or where screening reduces the likelihood of STI at the time of insertion and hygienic conditions are optimal. Unfortunately, data from higher-prevalence, low-resource settings are few, and estimates of PID risk following IUD insertion are often generalised from inappropriate settings.
Moreover, even where data are available, commonly used case definitions and outcome measures for PID underestimate the extent of the problem and introduce classification bias.Citation14, Citation19, Citation20 Because PID symptoms are often mild or absent, a clinical diagnosis only picks up about a third of all PID cases compared to laparoscopy.Citation14 A few studies have used broader case definitions in an attempt to increase sensitivity.Citation10, Citation12 While still imprecise, such definitions yield higher rates that may be closer estimates of true PID incidence. The importance of accurate measurements is highlighted by one meta-analysis which found stronger associations between IUD use and asymptomatic PID compared to symptomatic PID.Citation21
Taking these issues into account, we take another look at estimates of relative and absolute risk of PID following IUD insertion from high-prevalence, low-resource settings, and factor in a correction for low diagnostic sensitivity of methods used in those studies.
Relative risk
A strong association between the time of IUD insertion and the development of PID has been documented in several studies.Citation22, Citation23 An extensive WHO multi-centre reviewCitation22 estimated the overall risk of PID to be concentrated in the first 20 days after insertion when the risk was six-fold higher than at later times. Such evidence is a direct measure of risk related to insertion that avoids a number of biases in choice of comparison group in case–control and prospective studies. Notably, most of the IUD insertions in the study took place in low STI prevalence settings; one-fifth were from China in the 1980s when gonorrhoea and chlamydia were rare.Citation24 In fact, no cases of PID were reported from Chinese IUD users, a finding in line with evidence that IUD-related PID risk correlates highly with STI prevalence.Citation3, Citation23
Absolute risk
Studies from Africa report PID rates () that are significantly higher than those found in developed countries. Citation10 Citation11 Citation12 Citation13 In these series, specific but insensitive PID case definitions requiring three signs of tenderness (abdominal, cervical motion and adnexal) plus evidence of lower genital tract inflammation or fever were used. The studies were conducted under research conditions in university teaching hospitals with better-than-average hygienic conditions and careful selection of IUD candidates. These factors all work to underestimate the risk of PID in typical service delivery settings. One study reported considerably higher rates (>5%) using more sensitive criteria for “infection-related complications” (women with IUD removals for infection or pain and women with signs of pelvic, cervical motion or adnexal tenderness during physical examination).Citation12 Adjustment of the measured PID rates for asymptomatic or minimally symptomatic cases (assuming 30–50% sensitivity of study methods) would also yield incidence estimates in the range of 3–6% during the post-insertion period for most African samples. This is far higher than can be attributed to observed gonorrhoea/chlamydia prevalence alone, and greatly exceeds infection rates measured in a US study.Citation10
Preventing infection with IUD insertion
Data from ChinaCitation22 and high-resource countriesCitation3, Citation23 suggest that PID risk following IUD insertion can be minimised if 1) gonorrhoea and chlamydia prevalence is low or infection is ruled out prior to insertion, and 2) insertion is performed under aseptic conditions. Unfortunately, these conditions do not exist in many low-resource settings.Citation5, Citation15 A number of studies report on attempts to identify women with cervical infection by means of clinical examination or demographic and behavioural risk assessments, but these have proven disappointing, especially when used for screening in family planning populations.Citation5, Citation25
What are the options? First, IUD use can be restricted in areas of high STI prevalence or where safe insertion under aseptic conditions cannot be assured. This approach would certainly prevent iatrogenic infections but at the cost of limiting choice of effective and affordable contraception. An alternative strategy would be to assume that infection is present if it cannot be reliably ruled out, and to provide antibiotic coverage effective against organisms known to cause PID.
Antibiotic prophylaxis versus presumptive treatment
A number of studies have looked at the value of antibiotic coverage at the time of IUD insertion to reduce the risk of PID.Citation26 The available evidence is difficult to interpret, however, for several reasons. One trial showing no benefit of prophylaxis was conducted in a developed country setting with low STI prevalence among women who were screened for infection prior to IUD insertion.Citation10 In the absence of STI at insertion, the value of antibiotics to prevent development of PID is difficult to evaluate. In Kenya, with higher gonorrhoea and chlamydia prevalence and no prior laboratory screening, prophylaxis reduced both PID and unplanned IUD-related clinic visits by one-third, although the PID reduction was not significant (p=0.17).Citation11
A fundamental limitation of the controlled trials on antibiotic prophylaxis conducted to date is the choice of the antibiotic regimens (doxycycline 200 mg or azithromycin 500 mg) evaluated. There is no evidence to support the therapeutic effectiveness of these single-dose regimens against organisms commonly associated with PID. While a single dose of doxycycline 200 mg has been shown to be effective in reducing infectious complications of elective abortion in low prevalence settings,Citation26 it is insufficient for treatment of cervical infections due to gonorrhoea or chlamydia.Citation20, Citation27 A seven-day course of doxycycline (100 mg twice a day) is standard for chlamydial cervicitis. Gonorrhoea has been shown to be highly resistant to the tetracyclines (including doxycycline) in all regions. One study used azithromycin 500 mg as prophylaxis based on standard loading dose recommendations.Citation10 Current recommendations for treatment of gonorrhoea and chlamydia with azithromycin advise at least one gram; two grams are recommended for gonorrhoea in some guidelines.Citation20
The existing direct evidence thus considers the value of a sub-curative dose in reducing the progression of infection to the upper genital tract. There are no trials evaluating the protective effect of a full curative dose of antibiotics (presumptive treatment) effective against either N. gonorrhoeae or C. trachomatis. Such treatment would be expected to reduce the risk of PID associated with IUD insertion and other transcervical procedures for several reasons. First, treatment of potential pathogens in the endocervix removes an important source of upper genital tract infection. Second, cure rates above 95% for gonorrhoea and chlamydia can be achieved; several single-dose regimens are recommended as treatment for cervical infection and provide coverage for other pathogens implicated in PID. Finally, the fact that these same regimens are also recommended for treatment of these organisms in established PID argues that coverage at the time of the procedure would prevent iatrogenic spread of infection.Citation20, Citation27
Cost–benefit considerations
The risk of complications following PID is high, especially in low-resource settings. The risk of infertility following a single episode of PID has been estimated to be 50–60% in settings with limited access to treatment (two to three times higher than in developed countries). Ectopic pregnancy risk increases 7–10 times following PID and the mortality rate associated with this event is especially high in low-resource settings where emergency surgical services are uncommon.Citation28
There are precedents for treating cervical infections presumptively under certain situations where background prevalence is high.Citation27 Current WHO STI treatment guidelines recommend presumptive treatment to partners of patients with STI, and to women with genital complaints and a high likelihood of infection. Cost–benefit analyses of syndromic algorithms that include presumptive antibiotic treatment of cervical infection for women with symptomatic vaginal discharge have been conducted (Ndowa F. Personal communication, 2003). Above a breakpoint of 5–10% gonorrhoea/chlamydia prevalence, the cost of treating women for cervical infection is balanced by averted cost of care for PID and its complications, even in low-resource settings where the cost of such care is low. It should be noted that vaginal discharge is a poor predictor of cervical infection or PID.Citation5 Women undergoing IUD insertion, on the other hand, have a greater risk of developing PID in the month following insertion. Factoring in a relative risk of sixCitation22 would reduce the cost–benefit breakpoint to much lower levels (1–2%) of gonorrhoea/chlamydia prevalence.
Programmatic implications
Would presumptive treatment to reduce risk of post-insertion PID constitute a medical barrier to contraception? We think not. Even with an additional US$1–2 to cover the cost of effective single-dose treatment for each insertion, the IUD would remain one of the most cost-effective methods of contraception available. While supplier (UNFPA, USAID) prices for copper IUDs range from US$0.35 to $1.72 per unit, the programme cost of the IUD is in the order of US$5 per year. The incremental cost of providing full antibiotic coverage at the time of insertion to prevent PID would add approximately 2–10% to the programme costs of IUD provision, assuming an average of five years of use per woman. With longer IUD use, the incremental cost would be even less.
A related concern sees IUD access tied to the ability of poorly resourced health care systems to obtain and manage sufficient quantities of effective antibiotics. Ensuring adequate supplies of commodities is a sine qua non of family planning programmes, however, and essential commodities include supplies related to safe provision of services. The alternative—to promote IUD insertions where access to antibiotics is compromised—would spell disaster for women who do develop PID, and ultimately for the reputation of the family planning programme. In fact, availability of essential antibiotics should be seen as a basic requirement of health care services that cannot be ignored by any programme working in the health sector.
Research gaps, safer procedures and reproductive health
Over 90% of global expenditure on medical research focuses on diseases causing 10% of the global burden of disease.Citation29 This “90/10 research gap” means that health problems confined to poorer regions receive inadequate attention, and that evidence increasingly reflects conditions (of prevalence or access to technology) that do not exist in poorer settings. This research gap also applies to important areas of reproductive health.Citation30
The safety of IUD use under the right conditions is not in question. In the US, where litigious fallout from the Dalkon Shield has kept IUD use at low levels, “rehabilitation” efforts to reassure potential users and their health care providers are justified. Even where STI rates are relatively low, however, including in the US, authors stress a standard of care—careful client selection and prior screening for STI—that is simply not attainable in low-resource settings.
A programme of research to support safer IUD use in low-resource settings should address outstanding issues of service provision and outcome measurement. Priorities include evaluation of safety under typical service delivery conditions, development of affordable and accurate diagnostic and screening tools for cervical infections, and more sensitive methods for measuring PID outcomes. Operational research to improve safety of health care procedures is already a priority to reduce parenteral transmission of HIV and hepatitis B and C, and should be expanded to include transcervical procedures, including IUD insertion. This should include better methods for identifying STI as well as ways to reduce contamination with other organisms during procedures. For settings where cervical infection cannot be reliably ruled out, the effectiveness of treatment with a full curative course of antibiotics at insertion could be evaluated by randomised, placebo-controlled trial.
Attention to several ethical and standard of care issues should be more overt in the research and service provision agendas. To what extent does effective contraception, taking into account the considerable health benefit of preventing unwanted pregnancy, balance risks associated with its provision? Arguably, family planning programmes—especially those financed by developed countries—are ethically obligated to ensure a standard of care (comparable to donor country standards) that minimises those risks.
The alternative, promotion of dual standards of care for low-resource and high-resource countries, would be hard to justify. From an ethical standpoint the principle of justice calls for “treating like persons alike”. How is a young woman seeking family planning in Africa different from a young woman in Europe or North America in terms of her contraceptive needs? Although she has the same desire to control and preserve her fertility, her risk of complications related to IUD use is actually much higher. To deny these risks and promote a method under sub-standard conditions pits fertility control against health concerns, a position which goes squarely against the paradigm shift initiated at ICPD.
Furthermore, even with safe insertion, IUD promotion in areas of high STI/HIV prevalence must address women's needs for dual protection from infection and unwanted pregnancy. The commitment to women's reproductive health, reiterated at ICPD+5, states: “All primary health care and family planning facilities should offer the widest achievable range of safe and effective family planning methods, essential obstetric care, prevention and management of reproductive tract infections, including sexually transmitted diseases, and barrier methods to prevent infection.” As solutions are sought to achieve these goals, efforts to prevent iatrogenic infections should be seen as an integral part of the process.
References
- An extremely low-cost option: IUCD Method Brief No. 3. 2003; Kenya Ministry of Health: Nairobi.
- AE Washington, SO Aral, P Wolner-Hanssen. Assessing risk for pelvic inflammatory disease and its sequelae. JAMA. 266: 1991; 2581–2586.
- DA Grimes. Intrauterine device and upper-genital-tract infection. Lancet. 356(9234): 2000; 1013–1019.
- C Coggins, NL Sloan. Prophylactic antibiotics before insertion of intrauterine devices [Comment]. Lancet. 351(9120): 1998; 1962–1963.
- GA Dallabetta, AC Gerbase, KK Holmes. Problems, solutions, and challenges in syndromic management of sexually transmitted diseases. Sexually Transmitted Infections. 74(Suppl.1): 1998; S1–S11.
- Increasing Access to the IUD: An Inter-Agency Workshop organized by Family Health International's Research to Practice Initiative in collaboration with USAID's Service Delivery Improvement Division. 2003; FHI: Chapel Hill NC.
- Expanding Women's Contraceptive Choices: IUCD Method Brief No. 1. 2003; Kenya Ministry of Health: Nairobi.
- Improving Access to Quality Care in Family Planning. Medical Eligibility Criteria for Contraceptive Use. 2nd ed. WHO/RHR/00.02. 2000; World Health Organization: Geneva. At: 〈http://www.who.int/reproductive_health/publications/RHR_002_medical_eligibility_criteria_second_edition〉.
- World Contraceptive Use. 2001; UN Population Division, Department of Economic and Social Affairs: New York.
- TL Walsh, DA Grimes, R Frezieres. Randomized controlled trial of prophylactic antibiotics before insertion of intrauterine devices. IUD Study Group. Lancet. 351: 1998; 1005–1008.
- SK Sinei, KF Schulz, PR Lamptey. Preventing IUCD-related pelvic infection: the efficacy of prophylactic doxycycline at insertion. British Journal of Obstetrics & Gynaecology. 97(5): 1990; 412–419.
- SK Sinei, CS Morrison, C Sekadde-Kigondu. Complications of use of intrauterine devices among HIV-1-infected women. Lancet. 351: 1998; 1238–1241.
- OA Ladipo, G Farr, E Otolorin. Prevention of IUD-related pelvic infection: the efficacy of prophylactic doxycycline at IUD insertion. Advances in Contraception. 7(1): 1991; 43–54.
- L Weström, D Eschenbach. Pelvic inflammatory disease. KK Holmes, PF Sparling, P-A Mårdh. Sexually Transmitted Diseases. 1999; McGraw-Hill: New York, 783–809.
- C AbouZahr, E Åhman, R Guidotti. Puerperal sepsis and other puerperal infections. CJL Murray, AD Lopez. Health Dimensions of Sex and Reproduction. 1998; Harvard School of Public Health: Boston.
- R Ferraz do Lago, JA Simoes, L Bahamondes. Follow-up of users of intrauterine devices with and without bacterial vaginosis and other cervicovaginal infections. Contraception. 68: 2003; 105–109.
- JD Shelton. Risk of clinical pelvic inflammatory disease attributable to an intrauterine device. Lancet. 357(9254): 2001; 443.
- R Lu, N Wang, J Zhao. Investigation of intrauterine microbes after intrauterine operation [in Chinese]. Chung-Hua Fu Chan Ko Tsa Chih (Chinese Journal of Obstetrics & Gynecology). 33(3): 1998; 168–169.
- J Paavonen. Pelvic inflammatory disease. From diagnosis to prevention. Dermatologic Clinics. 16(4): 1998; 747–756.
- Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines. MMWR—Morbidity & Mortality Weekly Report 2002;51(No. RR-6).
- IF Gareen, S Greenland, H Morgenstern. Intrauterine devices and pelvic inflammatory disease: meta-analyses of published studies, 1974-1990. Epidemiology. 11(5): 2000; 589–597.
- TM Farley, MJ Rosenberg, PJ Rowe. Intrauterine devices and pelvic inflammatory disease: an international perspective [Comment]. Lancet. 339(8796): 1992; 785–788.
- EA Wright, AO Aisien. Pelvic inflammatory disease and the intrauterine contraceptive device. International Journal of Gynecology & Obstetrics. 28(2): 1989; 133–136.
- MS Cohen, GE Henderson, P Aiello. Successful eradication of sexually transmitted diseases in the People's Republic of China: implications for the 21st century. Journal of Infectious Diseases. 174(Suppl.2): 1996; S223–S229.
- CS Morrison, C Sekadde-Kigondu, WC Miller. Use of sexually transmitted disease risk assessment algorithms for selection of intrauterine device candidates. Contraception. 59: 1999; 97–106.
- Grimes DA, Schulz KF. Antibiotic prophylaxis for intrauterine contraceptive device insertion. Cochrane Database of Systematic Reviews 2001(1).
- World Health Organization. Guidelines for the Management of Sexually Transmitted Infections. WHO/HIV_AIDS/2001.01, WHO/RHR/01.10. 2001; WHO: Geneva.
- A Meheus. Women's health: importance of reproductive tract infections, pelvic inflammatory disease and cervical cancer. A Germain, KK Holmes, P Piot. Reproductive Tract Infections: Global Impact and Priorities for Women's Reproductive Health. 1992; Plenum Press: New York.
- World Health Organization. Investing in Health Research and Development: Report of the Ad Hoc Committee on Health Research Relating to Future Intervention Options. 1996; WHO: Geneva.
- J Villar, G Carroli, AM Gülmezoglu. The gap between evidence and practice in maternal healthcare. International Journal of Gynecology and Obstetrics. 75: 2001; S47–S54.