Abstract
The methods used for abortion in the second trimester have changed considerably in recent years. The surgical procedure dilatation and evacuation (D&E) has replaced hysterotomy. Instead of injecting different compounds, such as hypertonic saline, prostaglandin analogues are administered by non-invasive routes. The most effective medical method is combining a prostaglandin analogue with mifepristone. The consequence of these developments is that abortion in the second trimester can be be performed significantly more effectively and that the currently recommended methods being used are associated with fewer side effects and complications.
Résumé
Les méthodes utilisées pour l'avortement du deuxième trimestre ont changé considérablement ces dernières années. La procédure chirurgicale par dilatation et évacuation a remplacé l'hystérectomie. Au lieu d'injecter différents composés, comme un soluté salin hypertonique, des analogues de la prostaglandine sont administrés par des voies non invasives. La méthode médicamenteuse la plus efficace associe un analogue de la prostaglandine avec la mifépristone. Par conséquent, l'avortement du deuxième trimestre peut être pratiqué avec nettement plus d'efficacité, et les méthodes actuellement recommandées et utilisées sont associées avec moins d'effets secondaires et de complications.
Resumen
Los métodos utilizados para el aborto en el segundo trimestre han cambiado considerablemente en los últimos años. El procedimiento quirúrgico de dilatación y evacuación (D&E) sustituyó a la histerotomía. En vez de inyectar diferentes compuestos, como solución salina hipertónica, se administran análogos de prostaglandina por vías no invasivas. El método médico más eficaz es la combinación de un análogo de prostaglandina con mifepristona. La consecuencia de estos sucesos es que el aborto en el segundo trimestre puede ser efectuado con una eficacia considerablemente mayor y que los métodos recomendados y utilizados actualmente están asociados con menos efectos secundarios y menos complicaciones.
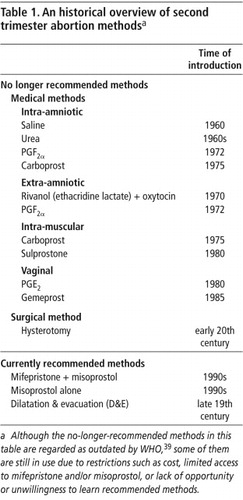
In the first half of the 20th century only surgical abortion methods such as dilatation and curettage (D&C) and hysterotomy were available. The situation changed in the middle of the century when compounds such as hypertonic saline, urea and ethacridine lactate (Rivanol) were introduced. These compounds, administered into the uterus, stimulate uterine contractility, resulting in the expulsion of the fetus and placenta. In the years since then, there have been continuing efforts to improve on medical abortion methods in terms of effectiveness, rates of complications, ease of provision and use, and acceptability. The introduction of prostaglandins in 1971 was the first step of this development. Initially prostaglandins were administered intra-uterine. Even more important was the development of prostaglandin analogues short thereafter. These could be given intramuscularly or vaginally. More recently misoprostol, an orally active prostaglandin E1 analogue, was developed. Today misoprostol is widely used for induced abortion. In the early 1990s the antiprogestogen mifepristone became available for clinical use and together with a prostaglandin was found to be a highly effective and safe method for termination of second trimester pregnancy (Table 1).
Today the methods recommended by the World Health Organization are medical abortion with mifepristone and misoprostol and dilatation and evacuation (D&E), which has replaced hysterotomy as the preferred surgical procedure. These methods are described in other articles in this publication.
The aim of this article is to describe some of the older methods and the reasons for the change to the newer methods. It is based on a literature search of the Medline database. Mainly randomised, controlled trials and large case series have been included.
Invasive routes
Intra-amniotic
This method was mainly used from gestational week 15 and onwards, when the amniotic cavity is easier to puncture. Several compounds were evaluated historically, such as urea, hypertonic saline, prostaglandin F2α (PGF2α), and 15 methyl PGF2α (carboprost).
Hypertonic saline
Hypertonic saline instillation started to be more widely used in the late 1960s.Citation1
After local anaesthesia a spinal needle was inserted through the abdomen into the amniotic sac. To be sure that the needle was in the right place, a free flow of amniotic fluid was established before instillation of the saline solution. Some physicians preferred to withdraw different amounts of amniotic fluid prior to the amnio-infusion to obtain a higher concentration of saline. The amount of 20% saline in sterile water was usually 200 ml (150–250 ml).
Reports of the instillation-to-abortion time varied considerably, but most fell within a 24–36 hour range. About 80% of patients expelled the fetus within 48 hours and almost all patients within 72 hours.Citation2
Complications reported with hypertonic saline included hypernatremia, blood coagulation disorders, haemorrhage, infection and cervical damage.Citation2
Prostaglandin F2α and PGE2
Naturally occurring prostaglandins, mainly PGE1, PGE2 and PGF2α, are potent stimulants of human uterine contractility at any stage of pregnancy and also cause cervical ripening and dilatation. The first studies on the use of prostaglandins for termination of pregnancy via instillation into the amniotic sac were reported by Bygdeman et alCitation3 and Karim and Sharma.Citation4 in 1971.
Following systemic administration, primary PGs are very rapidly metabolised and the metabolites are inactive. The estimated half-life of, for example, PGE2 in the circulation is less than 15 seconds. To obtain a longer duration of action in order to be practical for clinical use, primary PGs were therefore administered either into the amniotic sac (intra-amniotic) or between the fetal membranes and the uterine wall (extra-amniotic). The half-life for PGF2α following intra-amniotic administration was 13.5–20 hours. Another way to overcome the rapid metabolism was the development of prostaglandin analogues such as carboprost, gemeprost and misoprostol.Citation5
The technique used to instill PGF2α was similar to that used with intra-amniotic instillation of saline. First a small amount of amniotic fluid was withdrawn to verify the location of the needle in the amniotic sac, and then the compound was injected. A catheter was sometimes left in to avoid multiple punctures if additional prostaglandin doses were required. Both single- and multiple-dose schedules were found to be effective in inducing abortion. Doses ranged from 25 mg repeated after 6, 24 and 30 hours, if necessary, or 40 mg followed by 10–40 mg after 24 hours, or a single dose of 50 mg.Citation2
The use of PGF2α generally resulted in a shorter induction-to-abortion interval than with saline. In a randomised, multicentre study, the mean induction-to-abortion interval for PGF2α was 19.7 hours compared with 30.4 hours for saline.Citation6 The success rate after 48 hours was around 85% and within 72 hours almost all patients had aborted, at least if repeated doses were given.Citation7 The reason for the more rapid abortion with PGF2α is that this compound has a direct stimulatory effect on the myometrium while the effect of saline is probably secondary to an increased release of endogenous prostaglandins.Citation8
Gastrointestinal side effects such as vomiting and diarrhoea were common after PGF2α. Other complications reported include haemorrhage, infection, bronchospasm, hypotension, bradycardia, and cervical damage.Citation2
PGE2 was used as an alternative to PGF2α especially in Great Britain. The doses used were 5–20 mg as a single or 3–10 mg for repeated instillations after 12 and/or 24 hours. The outcome of the therapy was essentially the same as for PGF2α.Citation9
Urea
After puncture of the amniotic sac through the abdominal wall, 200 ml of amniotic fluid was removed and a solution of 100–250 ml of 30-60% urea in 5% dextrose and water was injected. With urea alone the efficacy was low and the induction-to-abortion interval long, and the treatment was therefore combined with a simultaneous injection of 5–20 mg PGF2α and/or an oxytocin infusion.
With combination of urea plus 5–10 mg PGF2α and addition of an oxytocin infusion if needed, almost all patients aborted within 48 hours with a mean induction-to-abortion interval of between 16 to 19 hours.Citation10
With this treatment, nausea and vomiting, but not diarrhoea, were common side effects. The potential risk of haemorrhage, infection and cervical damage applied to urea as with all mid-trimester abortion methods. In contrast to hypertonic saline, urea was relatively harmless if the compound gained rapid access to the systemic circulation.
Carboprost (15-metyl PGF2α)
As mentioned previously, primary prostaglandins are rapidly metabolised. In order to be more practical for clinical use, a number of analogues have been developed. The first was carboprost in which the hydrogen at carbon 15 in the PGF2α molecule is replaced by a methyl group. Following intra-amniotic administration of carboprost the half-life of the compound was 31–37 hours, which is approximately twice that of PGF2α.Citation5
The dose of carboprost used was 2.5 mg injected intra-amniotically in the same way as for PGF2α. The outcome of the treatment was evaluated in several studies, the largest of which was a multicentre, multinational study involving 1,521 patients, where carboprost was compared with single injections of 40 mg and 50 mg PGF2α. With carboprost the success rate was 95.6% within 48 hours, which was significantly higher than for both 40 and 50 mg PGF2α. The mean induction-to-abortion interval was 18–20 hours for all three treatments.Citation11
Nausea, vomiting and diarrhoea were common side effects with carboprost. The risk for haemorrhage, fever and cervical damage existed also. Other side-effects such as dyspnoea, flushing and chest pain were found only occasionally.
Since vaginal administration of gemeprost, and especially misoprostol, was introduced for termination of pregnancy and the efficacy and simplicity of these methods became widely known in the 1990s, intra-amniotic methods are no longer being used.
Extra-amniotic (administration between the fetal membranes and the uterine wall)
Rivanol (ethacridine lactate)
Rivanol is a yellow dye with antiseptic properties and a weak oxytocic effect in experimental animals. It was initially used as an abortion method in Japan.Citation12 Studies by Gustavi et alCitation13 indicated that at least partly the abortifacient effect of Rivanol was due to an increased endogenous production of prostaglandins.
A sterile Nelaton or Foley catheter was inserted 4-5 cm past the internal os into the extra-amniotic space and a 0.1% solution of Rivanol was instilled, 10 ml per week of pregnancy up to a maximum of 150 ml. After the instillation the catheter was tied off and left in place until abortion occurred.
The mean induction-to-abortion interval was about 24 hours and 90% of the women aborted within 72 hours. Addition of intravenous oxytocin was commonly used. A common side effect was temperature elevation, while vomiting and diarrhoea were uncommon. Data on serious complications were collected from 2,058 consecutive second trimester abortions. There were no deaths, no septicaemia and no other life-threatening complications. One cervical fistula and one case of cervical laceration were found, which is a lower frequency than normally found following prostaglandin treatment.Citation14
The use of Rivanol is slowly stopping. It should be mentioned that despite reassuring human data on safety issues in clinical practice, the WHO toxicology review panel denied permission for WHO-supported clinical trials on Rivanol in view of significant acute toxicity data in animals.
Prostaglandins PGF2α, PGE2 and carboprost
Extra-amniotic administration of prostaglandins for termination of second trimester pregnancy was first described by Wiquist and Bygdeman.Citation15
The prostaglandins were administrated through a catheter into the extra-amniotic space in the same way as for Rivanol. The catheter was left in place until the abortion took place. PGF2α or PGE2 was given by repeated instillations with an initial test dose of 250 μg PGF2α or 50 μg PGE2 followed by 750 μg or 200 μg respectively every second hour for up to 36 hours. A schedule like this would be expected to have a success rate of 80–90% within 24 hours and over 90% within 36 hours. Side effects included occasional episodes of vomiting and diarrhoea.Citation16
To overcome the risk of infection with a remaining catheter and repeated injections the use of carboprost in a viscous gel was evaluated in an international multicentre study.Citation17 A dose of 0.92 mg of carboprost was given to 660 women through a small polyethylene catheter, which was removed after the instillation in the 10th to 20th week of gestation. With this therapy 80.2% of the patients aborted within a 36-hour period, with a mean induction-to-abortion interval of 14.2 hours. The only common side effects were vomiting and diarrhoea. However, these symptoms were not severe. Cervical lacerations were found in one patient. No serious complications were reported.
Since the efficacy and easy treatment with the vaginal prostaglandin analogues gemeprost and especially misoprostol started to be widely known in the 1990s, these prostaglandins are no longer used.
Non-invasive routes
During these years, there was always the wish to develop abortion methods based mainly on oral or vaginal administration of the active drug, in order to avoid serious complications due to inadvertent administration and because the simplicity of the treatment would allow e.g. trained midwives or nurses to be responsible for the management of the patient.
Intramuscular administration
Carboprost
Carboprost is the only prostaglandin analogue that is currently approved for intramuscular administration. An effective regimen is an initial dose of 0.2 mg followed by 0.3 mg every 3 hours. The treatment results in abortion in 80–90% of the women by 30–36 hours but is associated with a high rate of vomiting and diarrhoea.Citation18Citation19 For this reason carboprost is of limited value when given by intramuscular injection, but can be useful as a supplement when other methods have failed to complete the abortion process.
Sulprostone (16-phenoxy-w-tetranor PGE2 methyl sulphonylamide)
Sulprostone is another prostaglandin E2 analogue which was given by intramuscular injections for termination of second trimester pregnancy. In an international, multicentre study 0.5 mg every 4 hours and 1.0 mg every 8 hours were compared in 295 women. Both doses were equally effective and 84% of the women aborted within 30 hours from the onset of treatment. The induction-to-abortion time was approximately 15 hours in both groups. The frequency of vomiting and diarrhoea was lower than that normally reported for other prostaglandin analogues. Fifty per cent of the women had no vomiting and 90% no diarrhoea. The frequency of cervical laceration was between 1.3–2.1%.Citation20
In a randomised study, intramuscular injections of carboprost (0.25 mg every second hour) and sulprostone (0.5 mg every fourth hour) were compared. To increase the efficacy a laminaria tent was introduced into the cervical canal 12 hours before treatment to dilate it. Both treatments were equally effective. All but two women (98.3%) had aborted within 24 hours of prostaglandin treatment. The mean interval between the first injection and the abortion was the same in the two treatment groups, approximately ten hours. Gastrointestinal side effects, vomiting and diarrhoea were significantly more common following carboprost. With sulprostone almost half the women (48%) did not experience any gastro-intestinal side effect and the frequency of diarrhoea was only 6%. No cervical damage was found. However, sulprostone for intramuscular injection has been withdrawn from the market since myocardial infarctions attributed to coronary spasm induced by sulprostone occurred in three women in early pregnancy.Citation21
Vaginal administration
PGE2
PGE2 is still available in vaginal tablets of 20 mg. The treatment is a 20 mg vaginal tablet every 3 hours. In a non-randomised study, the treatment was as effective as 40 mg PGF2α given intra-amniotically followed by a further 20 mg every 8 hours. The induction-to-abortion time was significantly shorter following PGE2 treatment, but vomiting and diarrhoea as well as temperature elevation were more common.Citation22
Carboprost methyl ester
In a WHO international, multicentre study,Citation23 310 women who were 13-20 weeks pregnant were treated with vaginal suppositories containing 1.5 mg of carboprost methyl ester every 3 hours for up to 30 hours. Within this time period, the success rate was 91.9% with a mean induction-to-abortion interval of 14.2 hours. However, the frequency of vomiting and diarrhoea was high, comparable to that of carboprost administered intramuscularly. The compound is no longer available.
Gemeprost (16, 16-dimethyl-trans-Δ2–PGE1 methyl ester)
Gemeprost is the newest prostaglandin analogue available as vaginal suppositories and still on the market in many countries. Following administration of 1.0 mg vaginally the maximum plasma levels are seen after 2–3 hours and detectable levels are still found after 6 hours.Citation24
A retrospective study of 932 second trimester terminations with gemeprost was published in 1992.Citation25 A single course of 1 mg vaginal suppository was administrated every 3 hours five times. If abortion had not occurred after the first course of treatment, a further course of 1 mg vaginal suppositories every three hours five times was administered the next day. Oxytocin was given intravenously after 36 hours if abortion had not occurred. 80% and 95% of the women aborted within 24 and 48 hours, respectively, and the mean induction-to-abortion interval was 17 hours.
Twenty-six per cent of the women had diarrhoea and 23% vomiting following administration of gemeprost. The incidence of pelvic sepsis (0.1%), cervical tears (0.1%) bleeding more than 500 ml (1.6%) and blood transfusion (0.6%) was low.
Present medical abortion methods
The WHO-recommended abortion method today is 200 mg mifepristone followed 36–48 hours later by repeated administration of either gemeprost or misoprostol. This procedure is highly effective, the duration of labour is much shorter than with older methods (6–10 hours) and side effects are less frequent than if prostaglandin is given alone. If mifepristone is not available vaginal or sublingual administration of misoprostol alone is also an effective and well-accepted procedure. (These methods are described in more detail in another article in this issue.Citation26)
Surgical procedures
Hysterotomy
The traditionally used surgical method for terminating a second trimester pregnancy was hysterotomy, a surgical procedure akin to caesarean section and sometimes called mini-caesarean. Since a number of studies, mainly in the USA in the 1970s and 1980s, showed that of all abortion methods, hysterotomy was associated with the highest mortality rate,Citation27 it is no longer recommended and should no longer be used. The main reasons are that it involves abdominal surgery and that the lower uterine segment where the incision at caesarean section is performed has not yet developed.
Present surgical procedure
The WHO-recommended surgical procedure today is dilatation and evacuation (D&E). Normally this operation is performed with local anaesthesia and sedation. It involves pre-operative dilatation of the cervical canal by e.g. laminaria tent or misoprostol, evacuation of the amniotic fluid with vacuum aspiration and extraction of the fetus and placenta using forceps. (This method is also described in more detail in another article in this issue.Citation28)
Discussion
The development of modern abortifacients started in the 1970s when prostaglandins became available for clinical use. Primary prostaglandins are naturally occurring and involved in a number of different functions in the human body. Primary prostaglandins such as PGE1, PGE2 and PGF2α are regarded as the last step in the complicated process leading to cervical ripening and labour at term in women. They represented a new principle for abortifacient drugs, having a direct stimulatory effect on the myometrium in contrast to methods such as hypertonic saline, which has no effect on the myometrium but acts, at least partly, by stimulating endogenous prostaglandin production.
Prostaglandins are local hormones produced and acting locally in a number of organ systems and rapidly metabolised systemically. Systemic administration is therefore not practical and is also associated with side effects, mainly from the gastrointestinal tract. To overcome this drawback, PGF2α and PGE2 were administered intra- or extra-amniotically. The next stage was the development of prostaglandin analogues, the first of which was carboprost. Intra-amniotic administration of PGF2α and carboprost were compared with hypertonic saline in several studies.
Both efficacy and complication rates are, however, difficult to compare, since the definition of success and complications vary from one study to another. The WHO Prostaglandin Task Force carried out a number of multicentre, partly randomised studies comparing saline with PGF2α and carboprost. An overview of these studies was published in 1978.Citation29 These studies showed that the most effective treatment following a single intra-amniotic injection was carboprost if the outcome of treatment was evaluated after 48 hours. PGF2α in appropriate doses was more effective than unaugmented saline. For both prostaglandin compounds, the induction-to-abortion time was also significantly shorter.
It is equally clear that the prostaglandin treatment was associated with a higher frequency of nausea, vomiting and diarrhoea. The frequency of haemorrhage (estimated loss of more than 500 ml) and need for blood transfusion was higher following PGF2α but not following carboprost. Some studies have also indicated that the risk of cervical damage is higher following prostaglandin than following saline treatment.Citation30
Which method is safer has been a matter of discussion. From a theoretical point of view, prostaglandin would be expected to be safer, since cardiovascular effects appear to be fewer, clotting factors do not change significantly, tissue damage following inappropriate administration appears to be less and the compounds are rapidly metabolised if accidentally injected into the bloodstream instead of the muscle. However, Grimes et al, based on studies in the USA, found a higher rate of major complications following PGF2α.Citation31 Yet a similar difference could not be found in later data (1977–1981). The mortality following PGF2α was found to be lower than for saline (2.8 and 4.3 per 100,000 abortions, respectively).Citation27
Intra-amniotic carboprost has been compared with vaginal administration of misoprostol. Successful abortion rates at 24 and 48 hours were the same. However, vaginal misoprostol resulted in a significant shorter mean induction-to-abortion interval. Among side effects, fever and shivering were more common with vaginal misoprostol.Citation32
The extra-amniotic route had the advantage that the method was applicable in the early second trimester, which is not suitable for intra-amniotic administration. The doses of prostaglandin used were also low and serious complications due to the prostaglandin compounds were not expected. The disadvantage was the necessity of repeated instillations, which was inconvenient for hospital staff. To allow repeated instillations it was necessary to keep the catheter in the uterus during the abortion period, which was associated with a potential risk of infection.
An alternative was extra-amniotic administration of Rivanol. This method also involved a remaining catheter but only one instillation. To shorten the induction-to-abortion interval, treatment with Rivanol was combined with an intravenous infusion with oxytocin. The combination of the anti-diuretic effect of oxytocin and an intravenous infusion represented, however, an additional risk. Randomised comparisons are lacking but comparative studies indicated that the Rivanol method was more effective and associated with fewer side effects than extra-amniotic saline and might well compete with extra-amniotic PGF2α with regard to efficacy and side effects.Citation14
In a study by Boza et al (also in this issue),Citation33 vaginal administration of misoprostol was compared with an historical cohort treated with Rivanol. The conclusion of the study was that both methods were highly effective but that misoprostol treatment resulted in a shorter induction-to-abortion interval. At 24 hours, the frequency of abortion was 92.6% with misoprostol compared with 76.2% with the Rivanol/oxytocin method (OR 4.2. 95%CI 2.3–8.0).
Administration of prostaglandins and other compounds into the uterus was replaced by vaginal administration of prostaglandin analogues, mainly gemeprost and misoprostol. The most important reasons were the simplicity of the treatment which can be managed by e.g. trained midwives, the elimination of the risk of inadvertent administration and a shorter induction-to-abortion interval and shorter hospital stay.
Carboprost was found effective following both intramuscular and vaginal administration but a high frequency of gastro-intestinal side effects limited its use. Today, intramuscular injections of carboprost are mainly used to finish an abortion when other methods have failed. Sulprostone is a better alternative for intramuscular injections because of a lower frequency of side effects. It is also a highly effective abortion method, especially if the woman is pre-treated with an intracervical laminaria tent. Sulprostone is, however, not available for intramuscular injection.
PGE2 and the E-analogues gemeprost and misoprostol are all used following vaginal administration. Vaginal administration of PGE2 has been compared with misoprostol and found to be less effective. Side effects, mainly fever, vomiting and diarrhoea, were also significantly more common.Citation34 Even if vaginal administration of PGE2 was combined with a concentrated oxytocin infusion, it tended to be less effective (p=0.06) than misoprostol at 24 hours. Diarrhoea, nausea/emesis and placental retention were significantly less common while fever was more common following misoprostol.Citation35
Both vaginal administration of gemeprost and misoprostol are highly effective abortifacient methods. In a prospective randomised study 1 mg gemeprost every 3h for a maximum of 5 doses was compared with 400 μg misoprostol also given every 3h for a maximum of 5 doses. The percentage of women who achieved abortion within 24 hours was significantly higher for misoprostol and the mean induction-to-abortion interval shorter. If the treatment was repeated all women aborted. There were no significant differences in side effects except for diarrhoea, which was more common following gemeprost, and fever, which was more common following misoprostol.Citation36 In a recent systemic review of the six randomised trials published comparing vaginal gemeprost and misoprostol, the conclusion was that misoprostol was as effective as gemeprost. However, in three of these studies the women were pre-treated with mifepristone which might have influenced the outcome.Citation37 Other reasons why misoprostol is preferred are that, in contrast to gemeprost, it is available in a large number of countries, it is cheaper, and does not need to be kept in a refrigerator.
Today the preferred medical method for second trimester abortion is mifepristone in combination with misoprostol. With this combined method, the induction-to-abortion interval is shortened from 16–20 hours if misoprostol is used alone, to an average of 6–7 hours, and the total prostaglandin dose and side effects are reduced.
D&E is the safest and most effective surgical technique for abortion from the 13th week of pregnancy and has replaced hysterotomy.
There are different opinions as to which method is to be preferred, D&E or non-invasive administration of prostaglandin analogues, especially if combined with mifepristone. Both methods are highly effective and safe. The abortion process following a medical method in the second trimester is very similar to delivery, and therefore both midwives and doctors are familiar with the process and its complications. D&E, at least after the 15th week of pregnancy, needs a skilled doctor and a sufficient workload to remain experienced. Local conditions might be the most important factor in the choice of method.Citation38
References
- TD Kerenyi, N Mandelman, D Scherman. Five thousand consecutive saline induction. American Journal of Obstetrics and Gynecology. 116: 1973; 593–600.
- TD Kerenyi. Intraamniotic techniques. JE Hodgson. Abortion and Sterilization. 1981; Academic Press: New York, 359–377.
- M Bygdeman, N Wiquist. Induction of mid-trimester abortion by intra-amniotic administration of PGF2α. Acta Physiologica Scandinavica. 82: 1971; 415–416.
- SMM Karim, SD Sharma. Second trimester abortion with single intra-amniotic injection of prostaglandin E2 or F2. Lancet. ii: 1971; 47–48.
- M Bygdeman. Pharmacokinetics of prostaglandin. Best Practice & Research Clinical Obstetrics & Gynecology. 17: 2003; 707–716.
- WHO Prostaglandin Task Force. Comparison of intra-amniotic prostaglandin F2α and hypertonic saline for induction of second-trimester abortion. British Medical Journal. 2: 1976; 1373–1376.
- D Edelman, W Brenner, A Metha. A comparative study of intraamniotic saline and two prostaglandin F2α dose schedules for midtrimester abortion. American Journal of Obstetrics and Gynecology. 125: 1976; 188–195.
- B Gustavii, K Green. Release of prostaglandin F2α following injection of hypertonic saline for therapeutic abortion: a preliminary study. American Journal of Obstetrics and Gynecology. 114: 1972; 1099–1100.
- M Toppozada, AA Ismail. Intra-uterine administration of drugs for termination of pregnancy in the second trimester. Baillière's Clinical Obstetrics and Gynecology. 4: 1990; 327–349.
- RT Burkman, NH Dubin, TM King. The use of hyperosmolar urea for the elective abortion of mid-trimester pregnancy. GI Zatuchni, JJ Sciarra, JJ Speidel. Pregnancy termination. 1979; Harper and Row: Hagerstown, 261–267.
- Prostaglandin Task Force. Comparison of single intra-amniotic injection of 15-methyl prostaglandin F2α for termination of second-trimester pregnancy: an international, multicenter study. American Journal of Obstetrics and Gynecology. 129: 1977; 601–606.
- Y Manabe. Artificial abortion at midpregnancy for mechanical stimulation of the uterus. American Journal of Obstetrics and Gynecology. 105: 1969; 132–146.
- B Gustavii, G Hauff, U Brunk. Rivanol induced alterations of cultured cells. Contraception. 16: 1977; 89–96.
- CA Ingemanson. The ethacridine-catheter method in second trimester abortion. GJ Zatuchni, JJ Sciarra, JJ Speidel. Pregnancy termination. 1979; Harper and Row: Hagerstown, 282–289.
- N Wiquist, M Bygdeman. Therapeutic abortion by local administration of prostaglandin. Lancet. ii: 1970; 716–717.
- N Wiquist, F Beguin, M Bygdeman. Induction of abortion by extra-amniotic prostaglandin administration. Prostaglandins. 1: 1972; 37–43.
- WHO Prostaglandin Task Force. Single extra-amniotic administration of 15-methyl prostaglandin F2α in Hyscon for termination of pregnancies in weeks 10 to 20 of gestation: An international multicenter study. American Journal of Obstetrics and Gynecology. 129: 1977; 597–600.
- NH Lauerson, KH Wilson. Termination of mid-trimester pregnancy by serial injections of 15(S)-15-methyl PGF2α. American Journal of Obstetrics and Gynecology. 123: 1976; 169–176.
- WHO Prostaglandin Task Force. Intramucular administration of 15-methyl PGF2α for induction of abortion in weeks 10 to 20 of pregnancy. American Journal of Obstetrics and Gynecology. 129: 1977; 593–596.
- WHO Prostaglandin Task Force. Termination of second-trimester pregnancy by intramuscular injection of 16.phenoxy-w-17,18,19,20-tetranor-PGE2 methyl sulphonylamide. International Journal of Gynecology and Obstetrics. 20: 1982; 383–386.
- M Bygdeman, NJ Christensen. Termination of second-trimester pregnancy by laminaria and intramuscular injection of 15-metyl PGF2α or 16-phenoxy-w-17,18,19,20-tetranor PGE2 methylsolfonylamide. Acta Obstetricia Gynecologica Scandinavica. 62: 1983; 535–536.
- JP Lebed, A Rubin, AE Millman. Comparison between intra-amniotic PGF2α and vaginal PGE2 for second trimester abortion. Obstetrics and Gynecology. 56: 1980; 90–96.
- WHO Prostaglandin Task Force. Repeated vaginal administration of 15-methyl PGF2α methylester for termination of pregnancy in the 13th to 20th week of gestation. Contraception. 16: 1977; 175–181.
- V Dimov, K Green, M Bygdeman. Metabolism of 16,16-dimethyl-trans-Δ2–PGE1 methylester following intravenous and vaginal administration to pregnant women. Drug Metabolism and Disposition. 14: 1986; 494–502.
- KJ Thong, AJ Robertson, DT Baird. A retrospective study of 932 second trimester terminations using gemeprost. Prostaglandins. 44: 1992; 65–74.
- K Gemzell-Danielsson, S Lalitkumar. Second trimester medical abortion with mifepristone-misoprostol and misoprostol alone: a review of methods and management. Reproductive Health Matters. 16(31 Suppl): 2008; 162–172.
- NJ Binkin. Trends in induced legal abortion morbidity and mortality. Clinical Obstetrics and Gynaecology. 13: 1986; 83–94.
- PA Lohr. Surgical abortion in the second trimester. Reproductive Health Matters. 16(31 Suppl): 2008; 151–161.
- M Bygdeman. Comparison of prostaglandin and hypertonic saline for termination of pregnancy. Obstetrics and Gynecology. 52: 1978; 424–429.
- R Lowensohn, CA Ballard. Cervico vaginal fistula. An apparent increased incidence with prostaglandin F2α. American Journal of Obstetrics and Gynecology. 119: 1974; 1057–1061.
- DA Grimes, KF Schulz, W Cates. Midtrimester abortion by intraamniotic prostaglandin F2α: Safer than saline?. Obstetrics and Gynecology. 49: 1977; 612–616.
- LL Su, A Biswas, M Choolani. A prospective, randomized comparison of vaginal misoprostol versus intra-amniotic prostaglandins for midtrimester termination of pregnancy. Obstetrics and Gynecology. 193: 2005; 1410–1414.
- A Velazco Boza, R Gómez Ponce de León, L Salas Castillo. Misoprostol preferable to ethacridine lactate for abortions at 13–20 weeks of pregnancy: Cuban experience. Reproductive Health Matters. 16(31 Suppl): 2008; 189–195.
- JK Jain, DR Mishell. A comparison of intravaginal misopostol with prostaglandin E2 for termination of second trimester pregnncy. New England Journal of Medicine. 33: 1994; 290–293.
- PS Ramsey, K Savage, T Lincoln. Vaginal misoprostol versus concentrated oxytocin and vaginal PGE2 for second trimester labour induction. Obstetrics and Gynecology. 104: 2004; 138–145.
- KS Wong, CSW Ngai, AYK Wong. Vaginal misoprostol compared with vaginal gemeprost in termination of second trimester pregnancy. A randomized trial. Contraception. 58: 1998; 207–210.
- IM Dodd, CA Crowther. Misoprostol versus cervagem for the induction of labour to terminate pregnancy in the second and third trimester. A systemic review. European Journal of Obstetrics & Gynecology and Reproductive Biology. 125: 2006; 3–8.
- PA Lohr, JL Hayes, K Gemzell-Danielsson. Surgical versus medical methods for second trimester induced abortion. Cochrane Database Syst Rev. 1: 2008 Jan 23; CD006714.
- Safe Abortion: Technical and Policy Guidance for Health Systems. World Health Organization, Geneva 2003.