Abstract
This article reviews the burden of cervical cancer in South Africa and shows that it remains the most common cancer among South African women, particularly women with least access to cervical cancer screening. It explains the rationale behind the South African cervical cancer screening policy, which is to offer all asymptomatic women three free cervical smears in a lifetime, beginning at age 30, 10 years apart. Further, it illustrates that cervical cancer screening offers unique opportunities for prevention at both the primary and secondary levels. The causal association of human papillomavirus infection of the cervix and the possibility for vaccination against the virus is discussed. The history of screening in South Africa and why it has failed to make a major impact to date on the morbidity and mortality of cervical cancer is also discussed. Finally, possible alternative approaches to cervical cytology for the prevention of cervical cancer are briefly reviewed.
Résumé
Cet article examine la charge du cancer du col de l’utérus en Afrique du Sud et montre qu’il demeure le cancer le plus fréquent chez les Sud-Africaines, en particulier celles qui ont le moins accès au dépistage. Il explique les justifications de la politique de dépistage de cette maladie en Afrique du Sud, qui est d’assurer à toutes les femmes asymptomatiques trois frottis gratuits pendant leur vie, dès l’âge de 30 ans, à dix ans d’intervalle. De plus, il montre que le dépistage du cancer du col de l’utérus offre des possibilités uniques de prévention aux niveaux primaire et secondaire. Il aborde l’association causale avec l’infection à papillomavirus humain et l’éventualité d’une vaccination contre le virus. Il décrit aussi l’histoire du dépistage en Afrique du Sud et explique pourquoi il n’a pu jusqu’à présent réduire sensiblement la morbidité et la mortalité par cancer du col de l’utérus. Enfin, il évoque brièvement d’autres approches possibles de la cytologie cervicale pour la prévention de ce cancer.
Resumen
En este artículo se examina el impacto del cáncer cervical en Sudáfrica, que continúa siendo el cáncer más común entre las mujeres sudafricanas, particularmente las que tienen el menor acceso a la detección sistemática del cáncer cervical. Se explica la lógica de la política sudafricana respecto a la detección sistemática del cáncer cervical: ofrecer a cada mujer asintomática tres pruebas gratuitas de Papanicolaou a lo largo de su vida, empezando a los 30 años de edad, a intervalos de 10 años. Además, se muestra que la detección sistemática del cáncer cervical ofrece oportunidades únicas para la prevención tanto en el primer nivel como en el segundo nivel de atención. Se trata la asociación causal de la infección por virus del papiloma humano del cérvix y la posibilidad de vacunación contra el virus, así como la historia de la detección sistemática en Sudáfrica y por qué hasta la fecha no ha logrado disminuir considerablemente las tasas de morbilidad y mortalidad por cáncer cervical. Por último, se examinan posibles alternativas a la citología cervical para la prevención del cáncer cervical.
Central to the development of cervical cancer is infection of the cervix with certain types of human papillomavirus (HPV),Footnote* the most common two being HPV 16 and 18. To date the most powerful tool for the prevention of cervical cancer has been the implementation of national, organised mass cytology-based screening programmes. Where this has been successful, the incidence of cervical cancer has been dramatically reduced. Today cervical cancer is most prevalent in areas where there is either no or very little effective screening activity. Establishing and sustaining cytology-based screening programmes requires a level of infrastructure that few developing countries can afford, prompting the evaluation of alternative methods of cervical cancer prevention. The newest development in this respect is the development of type specific vaccines against HPV types 16 and 18, and types 6, 11, 16 and 18. South Africa (SA) has a policy of offering asymptomatic women three free cervical smears in a lifetime, beginning at age 30 at 10 yearly intervals. There has been limited implementation of this policy, partly due to the demands on the health system of competing health needs, such as HIV infection, tuberculosis and maternal mortality. Other constraints include lack of financial infrastructure and human resources required to sustain preventative screening programmes. Implementation, however, is slowly improving and screening women has been prioritised by both national and most provincial departments of health.
Burden of cervical cancer in South Africa
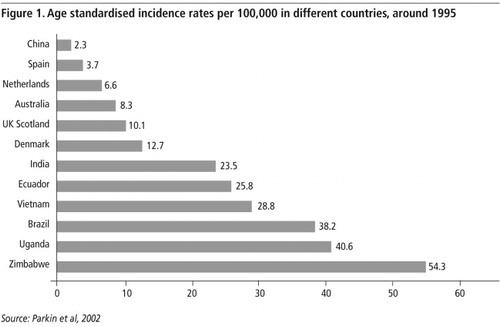
The high incidence of cervical cancer in the poor countries of the world is a reflection of the impact of the inequity of access to health care resources. In 2000, it was estimated that 471,000 new cases of cervical cancer were diagnosed worldwide and that 233,000 women died from cervical cancer worldwide.Citation1 Eighty per cent of the cases occurred in developing countries where cervical cancer comprises approximately 15% of all cancers in women (compared to less than 4% in women living in developed countries) and which has access to less than 5% of the world’s cancer care resources. shows the age standardised incidence rates (ASIR) recorded in a number of cancer registries around the world in 1995.Citation2 It shows very marked differences in incidences of cervical cancer, up to 20 fold, which is largely a reflection of different levels of cervical cancer screening. The areas of greatest incidence of cervical cancer include sub-Saharan Africa, Latin America, Caribbean, South and South East Asia, where there are few, if any, non-research related screening programmes. It should also be acknowledged that data collection in many poor countries is limited and the available information probably considerably underestimates the true incidence of the disease.
SA launched a pathology-based cancer registry in 1986, relying on information reported by 80 private and public laboratories. In 1986,Citation3 the total number of cancers reported in women was 16,559, of which 2,897 (17.4%) were new cases of histologically confirmed cervical cancer. In 1992, the total number of reported cancers in women had increased to 25,143, but the percentage of new cases of cervical cancer remained at the same proportion 17.8% (4,467) as was reported in 1986. There were 1,105 deaths from cervical cancer recorded in 1992.
In the 1993–1995 South African Cancer registry,Citation4 an average of 3,387 new cases of cancer of the cervix were reported per year, with 1,497 deaths reported for the year 1994. The ASIR of cervical cancer was 22/100,000; however, this figure was 27/100,000 for African women, the most disadvantaged women in SA in terms of access to health care. The lifetime risk of developing cervical cancer was 1 in 34 for African women compared to 1 in 93 for White women.
A total of 6,061 and 5,203 new cases of cervical cancer were reported to the South African Cancer registry in 1998 and 1999 respectively,Citation5 representing 20% and 17% of all cancers in women during this two year period. The ASIRs of cervical cancer in South African women were 34.4/100,000 and 28.7/100,000 in 1998 and 1999 respectively. About 84% of all women diagnosed with cervical cancer in 1998–1999 were African women with ASIRs of 42/100,000 in 1998 and 35/100,000 in 1999. One in 21 African women was at risk for developing cervical cancer in 1998. Proportionately, cervical cancer has remained the most common cancer in women since the onset of the cancer registry in 1986, and the available data most likely underestimate the true incidence of the disease as SA does not have a population-based registry and many cases may be not reported to pathology laboratories.
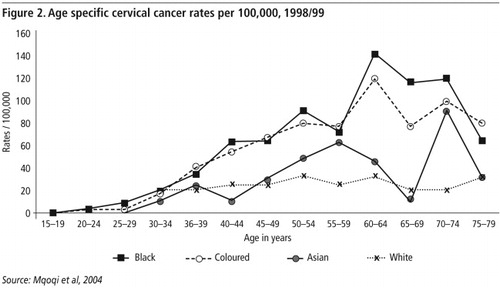
shows the age specific cervical cancer rates per 100,000 women in the different race groups in SA in 1998/99. In all groups the rate remained very low until age 30 years after which the rates rose steadily to peak at between 50 and 79 years. The highest age specific rate for all race and age groups occurred among African women aged 66–69 years with a rate of 152.5/100,000.
Not only is cervical cancer a common cancer in poor women, but cure rates are low because women present with advanced disease. In Groote Schuur Hospital, Cape Town, where approximately 200 new cases of cervical cancer are diagnosed and treated per year, 75–80% of women present with advanced disease (stage IIB and higher) (Personal communication, Dr Leon Van Wijk, Radiation Oncology, March 2006). In an evaluation of cervical cancer in African women in Durban, SA, Walker et al.Citation6 reported on hospital admissions for cervical cancer during 1994 and 1999. They found that the ASIR of cervical cancer was 45/100,000 for urban women and that the lesions of 66% of women were stage III and IV, with a mean age of presentation of 52 years.
Natural history of cervical cancer and rationale for prevention
There is now a considerable body of epidemiological, clinical and molecular evidence that infection of the cervix with high-risk types of HPV is necessary for the development of the vast majority of cases of cervical cancer and its immediate precursor, cervical intraepithelial neoplasia (CIN) 3 (also known as high grade squamous intra-epithelial lesion (HSIL). HPV is a very common, largely sexually transmitted infection of the genital tract which in the majority of cases is transient, asymptomatic and clinically insignificant. In a minority of women, however, the infection becomes persistent and may lead to the development of high-grade cervical cancer precursors and ultimately invasive cancer.
The strength of the association between infection of the cervix with high-risk types of HPV and invasive cervical cancer is almost unprecedented in cancer epidemiology, with odds ratios over 50 in most studies.Citation7Citation8 It is clear that high-risk HPV infection is a necessary although insufficient cause of invasive cancer. Other factors that appear to influence progression to invasive cancer are not well defined but multiparity and cigarette smoking have been consistently found to be increased among cervical cancer cases.Citation9,10 In addition, long-term use of oral contraceptives (>5 years)Citation11 and co-infection with other sexually transmitted infections (e.g. C. trachomatis and Herpes Simplex Virus)Citation12Citation13 have been found to increase the risk of HPV associated cervical cancer.
Cervical cancer provides unique opportunities for prevention because:
| • | Central to its aetiology is infection of the cervix with a well defined virus and preventing infection with the virus should have a major impact on the incidence of cervical cancer; and | ||||
| • | The process of developing cervical cancer takes at least 10 to 20 years, allowing opportunities to intervene long before cancer develops. | ||||
Cervical cancer and infection with HIV
While cervical cancer has been labelled by the Centers for Disease Control, USA as an AIDS-defining illness, the expected increase in cervical cancer attributable to HIV infection has not been strikingly demonstrated in many African countries. This is most likely due to women dying from other HIV-related disease prior to developing cervical cancer. In a case-control study in Johannesburg, Sitas et al.Citation14 showed an odds ratio of 1.6 (95% CI: 1.1–2.3) of cervical cancer in HIV infected women. Lomalisa et al.Citation15 presented data on 776 HIVseronegative women and 60 HIV-seropositive women treated in Johannesburg, SA in 2000 for cervical cancer. The HIV-seroprevalence was 7.2% and the HIV-positive women presented with invasive cancer almost 10 years earlier than HIV-negative women (mean age 44 versus 53 years respectively). Overall, there was no difference in the distribution of advanced lesions in the two groups (65% versus 55% respectively); however, when stratified by CD4 count, HIV-positive women with a CD4 count of less than 200/mm had significantly more advanced disease than HIV-negative women (77% versus 56% respectively).
Moodley et alCitation16 reported on a retrospective analysis of 206 women with cervical cancer in Durban, SA who underwent HIV testing and 45 (21.8%) were found to be HIV-seropositive. HIV-seropositive women were on average 13 years younger than HIV-seronegative women, but did not differ significantly in terms of stage of disease at presentation. There are very few natural history studies of cervical cancer in HIV-positive women, but available evidence suggests significantly greater expression of HPV infection and more rapid progression from initial HPV infection to the development of cervical cancer.Citation17 Similar results have been reported in SA in a case control study where HIV-positive women were nearly 5 times more likely to be infected with high risk HPV compared to HIV-negative women. Further, women infected with HIV and high-risk types of HPV were 40 times more likely to have a cervical cancer precursor than women not infected with either of the two viruses.Citation18 This has important implications for screening in HIV-positive women and the current recommendation is for annual screening of HIV-positive women who are immune compromised (CD4 <350) but this may be revised as more reliable natural history data emerge.
Primary prevention of cervical cancer
Primary prevention of cervical cancer would require the prevention of HPV infection of the genital tract de novo, or, at least, preventing persistent infection of the cervix with HPV. As the primary method for the transmission of genital HPV is via sexual intercourse, possible methods of primary prevention include abstinence, mutual monogamy in virgins and use of condoms.Citation19 Footnote* Another potentially very effective method of primary prevention is vaccination against cancerassociated types of HPV.
The recent publication of a number of randomised placebo-controlled trials of three different HPV vaccines has brought new hope and energy to the fight against cervical cancer. The results of the first trial on a double blind placebo controlled study of women aged 16–23 years who received three doses of vaccine or placebo given at day 0, month 2 and month 6, were published by Koutsky et al.Citation20 After a follow up period of a mean of 17.4 months, the incidence of persistent HPV 16 infection was 0 per 100 woman-years at risk in the vaccinated group (i.e. 100% efficacy) compared to 3.8 per 100 woman years at risk in the placebo group. In addition, the vaccine was shown to be safe and well-tolerated. In a publication on the same cohort of women followed through to 48 months,Citation21 the vaccine demonstrated 100% efficacy against CIN 2/3 lesions and 94% efficacy against persistent HPV infection. Harper et alCitation22 showed similar data using a bi-valent vaccine against HPV types 16 and 18. Vaccine efficacy was 91.6% against incident infection and 100% against persistent infection with HPV 16/18. These same authors have just published follow up data on this cohort at 4.5 years since vaccination.Citation23 The company which developed the bivalent vaccine used in the study has indicated that it will seek approval of the vaccine in Europe and other countries in 2006.
Two other companies have developed a quadrivalent vaccine that has efficacy against types 6 and 11 (which cause genital warts and are not associated with cancer) and types 16 and 18. Villa et al.Citation24 showed that the combined incidence of persistent infection or disease (CIN 2/3, genital warts, CIN 1) with HPV 6, 11, 16 or 18 fell by 90% in the vaccinated group. A USA based company has received the Food and Drug Administration approval for the vaccination of preadolescent and adolescent boys and girls in June 2006.
These rigorous and scientifically sound trials provide real hope that cervical cancer can be substantially reduced. It is estimated that vaccinating against types 16 and 18 only could prevent approximately 70% of cases of cervical cancer. Vaccinating against the 8 commonest types of HPV found in cervical cancer would prevent 95% of the cases.Citation25
While the data show clearly that these vaccines can prevent significant morbidity and mortality associated with HPV associated diseases, in addition to economic barriers, there will be considerable challenges to overcome prior to their introduction into the public health sector in a country like SA. To begin with, the intention is to vaccinate girls (and possibly boys) either pre-puberty or early adolescence, and very few developing countries have existing vaccine programmes designed to target this age group. In countries that provide hepatitis B vaccination to adolescents, a strong school-based public health system has been shown to be essential in order to achieve acceptable coverage of young people, and this is an infrastructure that is not yet well established in SA. It is also important to realise that in some developing countries, many girls do not attend school or attend only for short periods of time, and girls are the primary target group for vaccination.
Further, vaccination will require creating links and communication between the cancer prevention community (which deals with older women) and the immunisation community (which deals with children) and the reproductive health community (which in general is not linked to the immunisation community). Immunisation decision makers know little about HPV and cervical cancer and will most likely focus their attention on vaccines that can prevent diseases they normally treat e.g. rotavirus, pneumococcus vaccines. Another important issue will be the existence of competing vaccines and decisions as to the ‘stable’ of vaccines any government will provide will have to be made based on local epidemiology, the burden of disease and economic modelling of cost-effectiveness.
Finally, while vaccination offers a new approach to cervical cancer prevention, screening and efforts aimed at secondary prevention of cervical cancer remain critically important and should be greatly expanded. Even with 100% coverage, the HPV vaccines as currently formulated are only expected to prevent 70% of cervical cancer according to current data.
Secondary prevention of cervical cancer
While there have been no randomised trials of cytology-based screening programmes, it has been well documented that where these programmes have been successfully implemented, the incidence of cervical cancer has been dramatically reduced.
The International Agency for Research on Cancer (IARC) conducted a comprehensive analysis of data from several of the largest screening programmes in the world in 1986 and showed that well-organised screening programmes were effective in reducing the incidence of and mortality from cervical cancer.Citation26 In the Nordic countries, following the introduction of nationwide screening in the 1960s, cumulative mortality rates of cervical cancer showed a falling trend. The greatest fall was in Iceland (84% from 1965 to 1982) where the screening interval was the shortest and the target age range the widest. The smallest reduction in cumulative mortality (11%) was in Norway where only 5% of the population were part of organised screening programmes.Citation27 The falls in Finland, Sweden and Denmark were 50%, 34% and 27% respectively. The highest reduction in cervical cancer incidence was in the 30–49 age groups where the focus of screening was the most intense.
The association between mortality trends and the extent of coverage of the population by organised screening was most pronounced when the proportional reductions in the age-specific rates were related to the target ages of the screening programmes. The age-specific trends indicated that the target age range of a screening programme was a more important determinant of risk-reduction than the frequency of screening within the defined age range. This finding was in agreement with the estimates of the IARC working group, that for inter-screen intervals of up to five years, the protective effect of organised screening was high throughout the targeted age group (over 80%).Citation28 It is apparent therefore that the extent to which screening programmes have succeeded or failed to decrease incidence of and mortality from cervical cancer is largely reflected in:
| • | The extent of coverage of the population at risk by screening; | ||||
| • | The target age of women screened; and | ||||
| • | The reliability of cytology services in that programme.Citation29 | ||||
The rationale behind the South African cervical cancer screening policy is based on data from successful screening programmes in developed countries which is also supported by information from South African studies.
Fonn et alCitation30 performed Pap smears on 20,603 women from nine provinces in SA in 1998. Of interest is that 80% of the women had not had a Pap smear ever and 91% had not had a Pap smear in the preceding five years. Of the 468 women found to have low grade cervical cancer precursors, the average age was 33 years. Among the 366 women with high grade cervical cancer precursors, the average age was 38 years. The average age of women with a cancer diagnosis on their Pap smears was significantly older at 51 years. These data support the current understanding of the natural history of cervical cancer of an initial infection followed by a long latent phase in which intraepithelial precancers develop in women in their 30s and 40s followed by the slow evolution to cancer in women around the age of 50 years.
The study by Fonn et alCitation30 strongly supports the SA policy of targeting older women for screening as the majority of precursor lesions were found in women in their 30s and 40s. The ‘yield’ in terms of abnormalities detected per woman screened is much higher in women over 30 years of age compared to younger women. Similar data have been presented from screening programmes in developed countries, where cytology-based screening programmes have substantially reduced the morbidity and mortality from cervical cancer.Citation27
History of cervical cancer screening in South Africa
Until recently, SA did not have a national, organised cervical cancer screening policy and cervical screening was performed opportunistically, largely in family planning and antenatal clinics.Citation31 An example of the typical screening activity encountered in SA is illustrated in a study performed by Baillie in the Western Cape in 1994.Citation32 Baillie collected data on cervical smears analysed by the University of Cape Town Cytology laboratory over a five year period and showed that the highest proportion of smears were performed in maternity obstetric units on pregnant women under the age of 30 years. However, the highest proportion of smears showing CIN 3 was found among women aged 30–39 years who were screened in local clinics, and the highest proportion of smears showing malignancy were found among women over the age of 50 years, who were screened ‘opportunistically’ in day hospitals. The peak incidence of screening overall occurred in the 20–29 year age groups, and the screening incidence for women over 40 years or more was one third to one tenth of the peak screening incidence.
Cronje et alCitation33 reported a similar pattern of screening in the Free State Province in SA. Just over 36% of all smears performed in the province were in women aged 25–34 years and 29% of smears were performed in women aged 15–24 years. By contrast, 19% of smears were in women aged 35–44 years and only 16% were in women 45 years and older. Although White women constituted 21% of the population of the Free State (a group considered a relatively low-risk for cervical cancer due to their higher socio-economic status and greater access to screening), 53% of the women screened were White. In comparison, 76% of the population of the Free State were African women and only 43% of smears were performed in this group of women. Further, the vast majority of the smears (60%) were performed in the Bloemfontein, Free State, with less than 10% of smears performed in rural areas where the majority of African women lived.
In another study conducted outside the Pretoria area, Heystek et alCitation34 offered cervical smears to and assessed knowledge of screening among 1,095 women attending a local clinic for non-gynaecological complaints. Only 2% of the women interviewed had any knowledge about Pap smears but the incidence of preinvasive disease of the cervix was 54/1,000 women, which is relatively high.
Another important study of screening in SA was ‘Project Screen Soweto’ (PSS)Citation35 which illustrates the difficulties of establishing cytology-based cervical cancer screening. In 1980, Baragwanath Hospital, a large tertiary institution in Soweto documented an approximately 50% increase in the admissions for cervical cancer, from 150 cases in 1970 to 236 cases in 1980. In addition, more than 70% of women with abnormal cervical smears had not been followed up. In recognition of the poor quality of the existing ‘opportunistic’ screening programme and the apparently rising incidence of cervical cancer, PSS was initiated in 1986.Citation34
Prior to the establishment of PSS, a few basic goals were accomplished. Firstly, laboratory capacity for screening had been increased from 30,000 to 90,000 smears per annum. Pilot studies had identified the prime screening target as an existing and available pool of patients through the network of primary health care clinics. Laboratory-based follow-up services had been established and streamlined, and channels for communication between patients, clinicians and the laboratory had been created. The only unresolved problem was the development of an accessible public health education programme. PSS did not have enough resources and there were fears that a public health education programme would lead to a large number of women seeking services from an untested screening network. A decision was thus made to launch PSS prior to instituting any public health educational programme.
During the planning phase of PSS in 1982, 32,365 smears were taken. The number of smears taken decreased to 24,251 in 1983 and to 26,216 in 1984. In addition, there was a rapid decline in the diagnosis of both invasive and preinvasive cervical cancer, contrary to what was observed in a previously unscreened population. PSS was terminated after five years due to poor performance. However, had available laboratory facilities been utilised to full capacity during that five-year period, more than 300,000 women would have received at least one Pap smear test, which should have detected 6,000 preinvasive or invasive cancers.
The reasons for the failure of PSS are complex. One of the key reasons for the PSS’s failure was the low priority given by primary health care services to the collection of Pap smears, most likely due to other competing health needs. In addition, the failure to establish an effective public health educational programme resulted in women remaining ignorant about cervical cancer prevention and no consumer demand for screening was created.
Allocation of financial resources may also have played an important role and the long-term savings created by preventing cervical cancer may not have been appreciated. For instance, with the prevailing cervical cancer incidence rates in SA in 1984, it was estimated that a saving of US$34,000 would be obtained for every 1,000 women screened, with a very favourable cost-benefit ratio of 0.72.Citation36
The Women’s Health Research Unit formulated the Cervical Health Implementation Programme (CHIP) in order to develop and evaluate health systems interventions for implementing cervical screening within primary care services in SA. Three study sites were chosen and Smith et al.Citation37 reported on the situational analysis undertaken in the Mitchells Plain health district of the Western Cape. An audit of screening activity in the clinic showed that only 16% of all Pap smears performed were in the target age range of 30–59 years. Further only 56% of the smears taken were considered adequate for evaluation by the laboratory and endocervical cells were absent in 46% of smears. The rate of adherence to follow-up recommendations by the laboratory (i.e. follow-up smear in 3–6 months etc.) was 71%. Although 91% of the nurses were aware that some kind of policy existed, only 57% understood the policy and its rationale correctly. Twenty-two per cent of the nurses did not understand the purpose of Pap smears.
In 2003, Fonn published on the estimated human resource requirements for introducing a cervical cancer screening programme in SA.Citation38 Based on data published earlierCitation30 Fonn showed that to achieve 100% coverage of women eligible for screening over a ten-year period, each nurse would have to perform on average less than one Pap smear per day. She also indicated that if SA were to follow the guidelines that each cytotechnician only reads 48 smears per day, SA was under-resourced to provide this service. Further, providing services for women with abnormal cervical smears were considered markedly limited in SA and Fonn suggested that every regional hospital in the country would be required to create and run a weekly clinic to provide services to women with cervical abnormalities. In addition, extensive training of providers (doctors or nurses) would be required, particularly in colposcopy and treatment of cervical cancer precursors.
Michelow and DubbCitation39 investigated the status of public sector cytology laboratories in South Africa and the changes that would need to occur before a successful national cervical screening programme could be implemented. They identified 14 public sector laboratories which employed 97 cytotechnologists and cytotechnicians, almost all of whom performed functions other than screening Pap smears. These 14 laboratories examined half a million specimens per year and the proportion of their workload that involved screening Pap smears ranged from 70–80%. They estimated that a total of 100,000 additional slides could be screened annually on a national basis without altering the current staffing and structure of public sector laboratories. However, in the major urban centres e.g. Johannesburg and Cape Town additional screening could not be accommodated without increase in cytoscreeners and support personnel.
Michelow and Dubb point out the absolute necessity of computerising laboratories to maintain a careful check on the functioning of the screening programme. Despite extensive research the author was unable to find any laboratory register that is connected to a colposcopy and histology register. This leaves important gaps in knowledge about the percentage or proportion of women with abnormal smears actually undergoing colposcopy and further investigation and/or treatment. This deficiency requires urgent attention as it may be that the data from Leiman et al.Citation35 which showed that in the 1980s only 30% of women with abnormal smears attended colposcopy, is still correct. Fonn et al.Citation30 however, showed that 90% of women in their study of screening in 9 provinces did return for their results. The difference between the two scenarios is most likely due to having a system of tracking women with abnormal smears to ensure appropriate followup. Denny et al.Citation40 showed an over 90% return rate post screening in a research setting where intense attention was paid to recording women’s contact details, home addresses etc. and women who defaulted were tracked within a week of their missed appointment, suggesting that mechanisms for recalling women with abnormal smears for further investigation and treatment is as essential a part of the structure of a screening programme as is high quality laboratory services.
From these studies it is clear that the major challenges in developing a South African cervical cancer screening programme include:
| • | Creating consumer demand for Pap smears by educating the public to understand the rationale and purpose of cervical cancer screening; | ||||
| • | Stimulating political will and commitment to ensure appropriate allocation of resources, both human and financial to cervical cancer prevention; | ||||
| • | Training, educating and motivating nurses at primary care level to perform good quality Pap smears in the context of a woman centred and patient centred health care service; | ||||
| • | Performing Pap smears on the correct targeted age group (specifically women over 30 years); | ||||
| • | Improving the quality of the smears taken and ensuring the training of sufficient numbers of laboratory personnel to process slides according to acceptable international norms; | ||||
| • | Ensuring an efficient mechanism for giving women the results of their smears; and | ||||
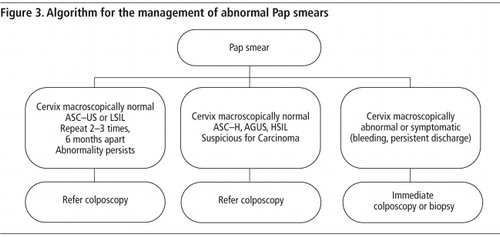
| • | Creating an infrastructure for the referral, treatment and follow-up of women with abnormal smears with adequate equipment and training of providers. (See – procedure for management of abnormal Pap smear.) | ||||
Screening in South Africa – the current situation
The current policy in SA is to offer all asymptomatic women aged 30 years and older, three free cervical smears in a lifetime, ten years apart. Based on mathematical models developed in the 1980s and assuming 100% coverage of the population, this policy is expected to reduce the cumulative incidence of cervical cancer by approximately two-thirds.Citation31
The latest data from the Western CapeFootnote* indicate that proportionately more women in the targeted age group (over 30 years old) are being screened. For instance, in 2005, 62,331 women were screened in the Western Cape of whom, 45,997 were over the age of 30 years (73.7%). Further, it is reported that 67.6% of smears contained endocervical cells and the smears were considered adequate for diagnosis. Abnormalities were found in 7.3% of the smears.
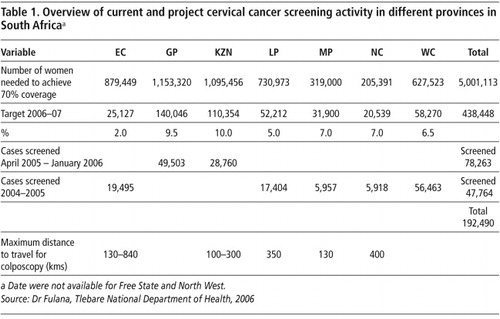
Similar trends were noted in other provinces of SA (Table 1). In the Eastern Cape in 2005, 18,485 smears were performed on women over the age of 25 years. It is estimated that to achieve 70% of the targeted age group 879,449 smears will need to be performed in Eastern Cape over the next 10 years. In Gauteng, between April 2005 and January 2006, 49,503 smears were performed, of which 10% (5,141) were found to be abnormal. In Limpopo province, 17,404 smears were performed in 2004–2005 and the target for 2006/07 is 52,212 i.e. to date one third of the target has been reached. In KwaZulu-Natal 28,760 smears were performed in 2005, which represents 26% of the targeted number of smears for 2006 and 2007.
These data, provided by national DoH and the National Health Laboratory Service (NHLS), Western Cape,Footnote† indicate that relatively large numbers of women remain unscreened. For instance in Gauteng province, where 79,568 women had smears done in 2005, the estimated number of women with a coverage of 70% in 10 years requiring smears is 1,153,320. Over the next 10 years the DoH estimates that nationally over 5 million women over the age of 30 years need to be screened to achieve 70% coverage of the population. Experience from countries that have successfully implemented screening programmes indicate that with high levels of commitment and good coordination of the different aspects of screening programmes, a significant impact on cervical cancer morbidity and mortality may be achieved. The creation of a robust, reliable, sustainable and patient centred programme has been shown in many countries to have a highly favourable cost-effectiveness and cost-benefit ratio.
Alternatives to cytology-based screening programmes
While it is the intention of the South African cervical cancer prevention programme to use cytology as its primary screening test, and despite the many challenges in implementing such a programme, there is every possibility that a successful programme may be created in SA. However, within the context of the HIV epidemic and the many other diseases of poverty, including tuberculosis, and malaria and a high maternal mortality, achieving adequate coverage of South African women may be very difficult. Certainly in poorer countries in Africa, where in general the health infrastructures are much less well resourced than in SA, implementing effective cytology-based screening programmes has proved prohibitively difficult.
These challenges have prompted a number of researchers to look at alternative screening tests to cytology, to both reduce the costs of screening by finding simpler tests and to simplify the process to an outpatient one-stop ‘screen and treat’ protocol. Two tests that have been extensively investigated in both cross-sectional and randomised trials are VIA (Visual Inspection with Acetic Acid) and HPV DNA testing.
VIA involves training a health worker (usually a nurse) to wash the cervix with 3–5% acetic acid and to examine the cervix thereafter for the presence of a ‘white’ lesion (acetic acid turns precancerous lesions white) using a bright light. (There is only one commercially available kit for detecting HPV DNA known as Hybrid Capture II [Digene, Gaitherburg, MD], which is able to identify DNA from 13 high-risk types of HPV.) The test is robust, reproducible and large numbers of tests can be performed per day. Although it is also a laboratory based test and is currently too expensive for developing countries, new HPV DNA tests are being developed that may become affordable in developing countries in the future.
The test characteristics of VIA have been evaluated in several cross-sectional studies in less developed countries and have shown a similar or often higher sensitivity that cytology, although a consistently lower specificity.Citation41 These studies together have involved more than 150,000 women and have reported promising results supporting its use as an alternative to cervical cytology. The sensitivity of VIA to detect high-grade precursor lesions and invasive cervical cancer has varied from 49% to 96% and the specificity from 49% to 98%.Citation35 Pooled estimates of sensitivity vary from 62% to 80% and specificity from 77% to 84% for VIA to detect high-grade cervical intraepithelial neoplasia.
There are also many studies showing the significantly higher sensitivity than cytology or VIA of HPV DNA testing for the detection of cervical cancer and its precursors and currently there is a large body of opinion that believes HPV DNA testing should be used for primary screening of women (over 30 years of age) and cytology reserved for those women who are high risk HPV DNA positive.Citation42 In addition to the improved sensitivity and easier infrastructure, both HPV DNA testing and VIA offer the potential of ‘screening and treating’ women without intervening steps of colposcopy and histological sampling. Denny et al. performed a randomised controlled trial of 6,555 non-pregnant, unscreened women, aged 35–65 years.Citation43 All women were screened using HPV DNA testing (Hybrid Capture II) and with VIA. Women were randomised thereafter to one of three groups: cryotherapy (performed by trained nurses) if the HPV test was positive, cryotherapy if VIA was positive or delayed treatment regardless of the result of the screening test. The prevalence of high grade CIN (defined histologically) was significantly lower in the two screen and treat groups at six and 12 months post randomisation compared to the delayed evaluation group. HPV DNA testing followed by cryotherapy however was twice as effective as VIA followed by cryotherapy in reducing histologically proven CIN 2 and 3 at both 6 and 12 months of follow up (). This was the first study of alternative screening approaches to the secondary prevention of cervical cancer that evaluated effectiveness (as opposed to safety and feasibility), both of which were addressed in a study conducted in Thailand.Citation44
Conclusions
Cervical cancer remains a significant and pervasive cause of death among women in SA and other areas with restricted resources. Yet, this is a largely preventable cancer. The failure to implement screening programmes for the secondary prevention of cervical cancer in poor countries is unlikely to be easily resolved in the future due to the considerable costs and infrastructure associated with cytologically based secondary prevention approaches.
SA is unique in Africa in that resources for the diagnosis and treatment of cervical cancer are relatively sophisticated in most urban centres, with availability of advanced surgery, radiotherapy and chemotherapy. These resources are however very expensive and cure rates remain low due to the late presentation of most patients. Further, rural women are considerably discriminated against due to lack of access to these facilities. Many studies have confirmed that cervical cancer prevention has a very favourable cost-effectiveness and cost-benefit ratio and efforts to prevent the disease are likely to be highly rewarding.
StudiesCitation31,32,34 on the situation with regard to screening in SA suggest that the country has the capacity to develop an effective cytology based screening programme, but clearly this will require not only significant political will, but the allocation of considerable resources both for development of skills and infrastructure. While it is critical to pursue secondary prevention of cervical cancer, it may well be prudent for countries such as SA to implement in demonstration projects some of the alternative approaches to cervix cancer prevention mentioned above, particularly in areas that are significantly under-resourced. This should happen in parallel to the development and expansion of cytology and colposcopy services.
In addition, attention needs to be paid to the huge potential benefits of vaccinating women and girls who have not been exposed to HPV to prevent infection with HPV. The results of numerous mathematical models suggest that used as a vaccine to prevent a prevalent and lethal cancer, the HPV vaccine will be a highly cost-effective intervention.
Note
This paper is reprinted from the South African Health Review 2006 on cervical cancer with kind permission of the editor and the author. The Review retains the copyright.
Notes
* Human papillomavirus is a name of a group of viruses that include more than 100 different strains or types. More than 30 of these viruses are sexually transmitted.
* While condoms do seem to have some protective effect against HPV infection, it is nowhere near as effective as preventing HIV transmission and therefore is not a reliable method of primary prevention.
* Data provided by Ms Irene Le Roux, Cytology Coordinator, Coastal Branch, NHLS.
† Personal communication, Dr Pulani Tlebere and Ms Irene Le Roux, NHLS, June 2006.
References
- BF Ferlay, P Pisani, DM Parkin. Globocan 2002. Cancer Incidence, Mortality and Prevalence Worldwide. IARC Cancer Base No. 5 Version 2.0. 2004; IARC Press: Lyon.
- DM Parkin, SL Whelan, J Ferlay, L Teo, DB Thomas. Cancer Incidence in Five Continents, Vol VIII. IARC Scientific Publications No. 155. 2002; IARC: Lyon.
- Cancer Registry of South Africa, Annual Report 1987.
- F Sitas, J Madhoo, J Wessie. Incidence of Histologically Diagnosed Cancer in South Africa, 1993–1995, National Cancer Registry of South Africa. 1998; South African Institute of Medical Research: Johannesburg.
- N Mqoqi, P Kellet, F Sitas, M Jula. Incidence of Histologically Diagnosed cancer in South Africa, 1998–1999. National Cancer Registry of South Africa. December. 2004; National Health Laboratory Service: Johannesburg.
- ARP Walker, PM Michelow, BF Walker. Cervix cancer in African women in Durban, South Africa. International Journal of Gynecology and Obstetrics. 2002 Apr 30; 45–46.
- N Munoz, FX Bosch, S de Sanjose, R Herrero, X Castellsague, KV Shah. Epidemiologic Classification of Human Papillomavirus Types Associated with Cervical Cancer. N Engl J Med. 348: 2003; 518–527.
- GM Clifford, JS Smith, M Plummer, N Munoz, S Franceschi. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. British Journal of Cancer. 88: 2003; 63–73. URL: http://www.nature.com/bjc/journal/v88/n1/abs/6600688a.html
- N Munoz, S Franceschi, C Bosetti, V Moreno, R Herrero, JS Smith. Role of parity and human papillomavirus in cervical cancer: the IARC multicentric case-control study. Lancet. 359(9312): 2002 Mar 30; 1093–1101.
- International Collaboration of Epidemiological Studies of Cervical Cancer. Carcinoma of the cervix and tobacco smoking: Collaborative reanalysis of individual data on 13, 541 women with carcinoma of the cervix and 23, 017 women without carcinoma of the cervix from 23 epidemiological studies. Int J Cancer. 118(6): 2006; 1481–1495. URL: http://www3.interscience.wiley.com/cgi-bin/abstract/112099381/ABSTRACT
- V Moreno, FX Bosch, N Munoz, CJ Meijer, KV Shah, JMM Walboomers. Effect of oral contraceptives on risk of cervical cancer in women with human papillomavirus infection: the IARC multicentric casecontrol study. Lancet. 359(9312): 2002 Mar; 1085–1092. URL: http://image.thelancet.com/extras/01art3030web.pdf
- JS Smith, R Herrero, C Bosetti. Herpes Simplex Virus-2 as a human pappilomavirus cofactor in the etiology of invasive cervical cancer. J Natl Cancer Inst. 94(21): 2002; 1604–1613.
- KL Wallin, F Wiklun, T Luostarinen, T Angstrom, T Anttila, F Bergman. A population-based prospective study of Chlamydia trachomatis infection and cervical carcinoma. Int J Cancer. 101(4): 2002 Oct 1; 371–374. URL: http://www3.interscience.wiley.com/cgi-bin/abstract/97518948/ABSTRACT?CRETRY=1&SRETRY=0
- F Sitas, R Pacella-Norman, H Carrara, M Patel, P Ruff, R Sur. The spectrum of HIV-1 related cancers in South Africa. Int J Cancer. 88(3): 2000; 489–492. URL: http://www3.interscience.wiley.com/cgi-bin/abstract/73504895/ABSTRACT
- P Lomalisa, T Smith, F Guidozzi. Human immunodeficiency virus infection and invasive cervical cancer in South Africa. Gynecol Oncol. 77(3): 2000 Jun; 460–463.
- M Moodley, S Mould. Invasive cervical cancer and human immunodeficiency virus (HIV) infection in KwaZulu-Natal, South Africa. J Obstet Gynaecol. 25: 2005; 706–710.
- T Harris, RD Burke, JM Palefsky, LS Massad, J Yon Bang, K Anastos. Incidence of Cervical Squamous Intraepithelial Lesions Associated with HIV Serostatus, CD4 Cell counts, and Human Papillomavirus Test Results. JAMA. 293: 2005; 1471–1476.
- J Moodley, M Hoffman, H Carrara, B Allan, D Cooper, L Rosenberg. HIV and pre-neoplastic and neoplastic lesions of the cervix in South Africa: A casecontrol study. BMC Cancer. 6: 2006; 135–141.
- LE Manhart, LA Koutsky. Do Condoms Prevent Genital HPV Infection, External Genital Warts, or Cervical Neoplasia? A Meta-Analysis. Sex Trans Dis. 29: 2002; 725–735.
- LA Koutsky, KA Ault, CM Wheeler, DR Brown, E Barr, F Alvarez. A controlled trial of a human papillomavirus type-16 vaccine. N Engl J Med. 347: 2002; 1645–1651.
- C Mao, LA Koutsky, KA Ault, CM Wheeler, DR Brown, DJ Wiley. Efficacy of human papillomavirus-16 vaccine to prevent cervical intraepithelial neoplasia: a randomized controlled trial. Obstet Gynecol. 107(1): 2006; 18–27. URL: http://www.ob-gyn.ca/pdf/Suggested%20Readings/ForMay09_BadawiHPV_Vaccine.pdf
- DM Harper, EL Franco, C Wheeler, DG Ferris, D Jenkins, A Schuind. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomized controlled trial. Lancet. 364(9447): 2004 Nov; 1757–1765.
- DM Harper, EL Franco, CM Wheeler, A Moscicki, CM Roteli-Martins, D Jenkins. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomized control trial. Lancet. 367(9518): 2006 April; 1247–1255.
- LL Villa, RL Costa, CA Petta, RP Andrade, KA Ault, AR Giuliana. Prophylactic quadrivalent human papillomavirus types 6, 11, 16, and 18 L1 virus-like particle vaccine in young women: a randomized doubleblind placebo-controlled multicentre phase 11 efficacy trial. Lancet Oncol. 6(5): 2005 May; 271–278.
- N Munoz, FX Bosch, X Castellsague, M Diaz, S de Sanjose, D Hammounda. Against which Human Papillomaviurs types shall we vaccinate and screen? The International Perspective. Int J Cancer. 111: 2004; 278–285.
- Summary Chapter: IARC Working Group on Cervical Cancer Screening. M Hakama, AB Miller, NE Day. Screening for Cancer of the Uterine Cervix. 1986; International Agency for Research on Cancer: Lyon, 133–142.
- E Laara, NE Day, M Hakama. Trends in Mortality from Cervical Cancer in the Nordic Countries: Association with organized screening programmes. Lancet. 1987 May 30; 247–249.
- IARC Working Group on Evaluation of Cervical Cancer Screening Programmes. Screening for squamous cervical cancer: the duration of low risk after negative result of cervical cytology and its implication for screening policies. Br Med J. 293: 1986; 659–664.
- M Hakama, K Louhivuori. A screening programme for cervical cancer that worked. Cancer surveys. 17(3): 1988; 403–416.
- S Fonn, B Bloch, M Mabina, S Carpenter, H Cronje, C Maise. Prevalence of pre-cancerous lesions and cervical cancer in South Africa – a multicentre study. S Afr Med J. 92(2): 2002 Feb; 148–156.
- S Fonn, B Klugman, K Dehaeck. Towards a National Screening Policy for Cancer of the Cervix in South Africa. Paper no. 31. 1993; The Centre for Health Policy, Department of Community Health, Medical School, University of Witswatersrand: Johannesburg.
- Baillie R. Cervical Cytology Screening: Towards the Development of a Rational Policy for the Western Cape, South Africa. Doctoral Thesis. Department of Community Health. University of Cape Town; December 1994.
- HS Cronje, MD Trumpelmann, PD Divall, LL Scott, BD Middlecote, JI De Wet. Cervical cytological services in the Orange Free State. Demographic Characteristics. S Afr Med J. 76: 1989; 116.
- MJ Heysteck, ET de Jonge, HP Meyer, BG Lindeque. Screening for cervical neoplasia in Mamelodi – lessons from an unscreened population. S Afr Med J. 85(11): 1995 Nov; 1180–1182.
- Leiman Gladwyn. ‘Project Screen Soweto’ – A planned cervical cancer screening programme in a high-risk population. The S Afr Journal of Epidemiology and Infection. 2: 1987; 61–68.
- S Emdon, U Gerard, R Jones. Knowledge about and utilisation of facilities for cervical smears among black women in Johannesburg. S Afr Med J. 65: 1984; 289–290.
- N Smith, J Moodley, M Hoffman. Challenges to cervical cancer screening in the Western Cape province. S Afr Med J. 93(1): 2003 Jan; 32–35.
- S Fonn. Human resource requirements for introducing cervical screening – who do we need where?. S Afr Med J. 93(10): 2003; 901–903.
- P Michelow, M Dubb. The implementation of a national cervical screening programme: are our cytology laboratories up to the challenge?. The S Afr Journal of Epidemiology and Infection. 18: 2003; 38–41.
- L Denny, L Kuhn, A Pollack, TC Wright Jr. Direct visual inspection for cervical cancer screening. Cancer. 94(16): 2002; 1699–1707. URL: http://www3.interscience.wiley.com/cgi-bin/abstract/91016204/ABSTRACT
- R Sankaranarayanan, L Gaffikin, M Jacob, J Sellors, S Robles. A Critical Assessment of Screening Methods for Cervical Neoplasia. Int J Gynaecol Obstet. 89 Suppl 2: 2005 May; S4–S12.
- J Cuzick, C Clavel, KU Petry, CJ Meijer, H Hoyer, S Ratnam. Overview of the the European and North American studies on HPV testing in primary cervical cancer screening. Int J Cancer. 119(5): 2006 Sep; 1095–1101.
- L Denny, L Kuhn, M De Souza, AE Pollack, W Dupree, TC Wright. Screen-and-Treat Approaches for Cervical Cancer Prevention in Low-Resource Settings. A Randomized Controlled Trial. JAMA. 294: 2005; 2173–2181. URL: http://jama.ama-assn.org/cgi/content/abstract/294/17/2173
- L Gaffikin, PD Blumenthal, M Emerson, K Limpaphayom, Royal Thai College of Obstetricians and Gynaecologist (RTCOG)/JHPIEGO Corporation Cervical Cancer Prevention Group. Safety, acceptability, and feasibility of a single-visit approach to cervical-cancer prevention in rural Thailand; a demonstration project. Lancet. 361(9360): 2003 Mar; 814–820.