More than 300,000 women in the United States and many more around the world undergo surgery with breast implants every year, either to replace breasts lost to cancer or to increase the size of healthy breasts.Citation1 When a woman decides whether to get breast implants, she must consider how that decision will affect her health and her life. Despite dozens of studies published in peer-reviewed journals that indicate implant problems, and informational booklets on implants required to be given to women before surgery in the United States, our non-profit center, the National Research Center for Women & Families,Footnote* has received thousands of calls and e-mails in recent years from women all over the world who tell us that their plastic surgeons did not adequately warn them about the risks of breast implants or fully advise them about their options when problems arise. Many women with leaking implants and the pattern of autoimmune symptoms typical of women with such leaks tell us that their physicians assured them that their leaking implants did not pose a health risk and could not possibly be causing their health problems.
In this commentary, I will focus on what research tells us about the risks and benefits of breast implants, then discuss what we do not know and consider the informed consent process for women who choose breast implants.
Breast augmentation is performed under intravenous or general anaesthesia, and a pocket is created under the breast tissue or muscle. Invented in the 1960s for breast augmentation, and not widely used for either augmentation or reconstruction until the 1980s, breast implants consist of silicone envelopes filled with silicone gel or saline. The patient is told to wear a surgical bra for about two weeks and avoid strenuous exercise for 4–6 weeks.
Prior to 1990, most plastic surgeons told their patients that their implants would “last forever” but in the last 20 years implant manufacturers and plastic surgeons have instead stated that implants “do not last a lifetime”. That is not very informative; in fact, the average implant seems to stay intact for about ten years, but for reasons that are not known some implants break within a few months and others last more than 15 years. The thickness of implant gels and shells has changed over the years, which has influenced how long they last. Without long-term data, it is difficult to predict how long the newest implants will stay intact. Only two companies currently have FDA approval to sell breast implants in the US: Mentor and Allergan (previously Inamed). They also are the major manufacturers selling breast implants all over the world. Prior to thousands of lawsuits on behalf of breast implant patients in the 1990s, Dow Corning was the major manufacturer of breast implants.
Health and cosmetic risks
At the time breast implants first became available, medical devices were not required to be proven safe or effective in the United States or most other countries. Clinical trials were not required to prove the safety of any breast implants in the US until 1991, and are still not required in most countries today. Even when existing clinical trials were deemed inadequate in 1991, implants were allowed to stay on the market in most countries, with some restrictions. The standard for US Food and Drug Administration (FDA) approval of any implanted medical device is that they must be “reasonably safe and reasonably effective”. This is lower than the standard of “proven safe and effective” required of prescription drugs. Saline breast implants were not approved by the FDA until 2000 and silicone gel implants only in 2006.Citation2 In both cases, approval was based on short-term data (2–3 years). An unusual condition of approval was the requirement that plastic surgeons provide patients with an information booklet approved by the FDA, and that the implant manufacturers conduct large, ten-year longitudinal studies to determine longer-term risks.
Adverse effects
The most common complication of breast implants is capsular contracture, the tightening or hardening of the scar tissue surrounding the implant, which usually causes the breast to feel unnaturally firm, and may eventually result in breasts that are hard and very painful.Citation2 Capsular contracture can occur almost immediately after getting implants, or, more likely, years later. The resulting shape – very noticeable on movie stars – is unnaturally round breasts, often with flat chest space between them. In some cases, the breast(s) can also become elongated or asymmetrical in shape or appearance.Citation3 Subsequent surgery to remove and replace the implants results in more natural-looking cleavage for a few months or years, but capsular contracture may recur. Although widely acknowledged as a risk, there is no agreement about how often capsular contracture occurs. However, one long-term study of breast augmentation funded by Dow Corning found that 62% had “clinically significant” capsular contracture and only half had “satisfactory overall breast appearance”.Citation4
The risk of rupture and leaking
When the FDA required companies to conduct long-term safety studies, they did not require that the data be made public, and indeed, the companies have not made their data publicly available. But numerous other studies have indicated that all breast implants have short-term and long-term risks.
Rupture happens when an implant develops a break or tear in the shell, whether or not it deflates or changes size, and is widely acknowledged as a risk. For example, an editorial in Plastic and Reconstructive Surgery, the journal of the American Society of Plastic Surgeons, states that breast implants do not last forever and women will need additional operations to replace ruptured implants.Citation5 This article, co-authored by the former director of FDA’s Office of Women’s Health, Dr Susan Wood, and a former president of the American Society of Plastic Surgeons, Dr Scott Spear, is a crucial contribution to the literature on breast implant safety. It points out that ruptures of saline breast implants are obvious because of immediate deflation, while ruptures of silicone gel implants are often “silent”, meaning there are no obvious signs or symptoms. Until recently, almost all plastic surgeons recommended clinical exams and mammograms to check for the rupture of silicone gel implants, but Wood and Spear agree with the FDA that magnetic resonance imaging (MRI) “is the most accurate way to detect a rupture”Citation5 and that:
“Mammograms are often inaccurate in detecting rupture, and if an implant is already broken, the pressure from a mammogram could cause the silicone gel from the implant to leak outside the capsule.”Citation5
Our Center did a survey of major medical centers in all 50 US states and found that breast MRIs cost an average of $2,000. This is a substantial expense, and MRIs to detect implant rupture are almost never covered by health insurance, even for women with implants after a mastectomy. Although the need for MRIs is noted in the information for patients with breast implants on the FDA website, the thousands of women who contact our Center usually do not know this, and we have never been contacted by any women who have undergone regular MRIs to check for rupture or leakage.
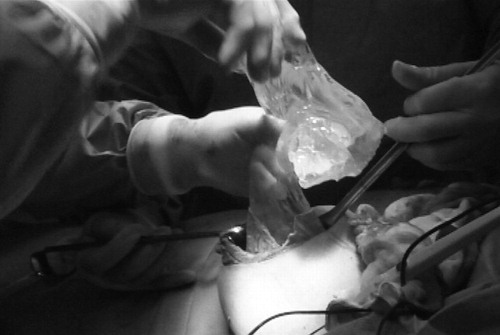
Silicone gel being removed from a ruptured implant, screen grab from the documentary film Absolutely Safe
Risks related to removal
In their editorial, Wood and Spear note that women who notice any changes that could be caused by a ruptured implant or who have had an MRI showing rupture are advised to make an appointment to discuss removal. They note that the removal of implants is more complicated than the initial surgery, especially if a silicone implant has ruptured and the silicone has leaked into the surrounding tissue. Even if the implant is not broken or leaking, after removal “the resulting stretching and sagging which may have occurred may be ‘cosmetically unacceptable’” and a breast lift or reconstructive surgery may be needed to make the breasts look like they did before implants.Citation5 However, replacing the implants also has risks:
“After a second surgery, the risk of more complications, especially capsular contracture and rupture is higher than before. Revisions or secondary corrections do not reduce the need or likelihood of future surgery.”Citation5
Negative effect on breastfeeding
In their review of the research literature on breast implants, the US Institute of Medicine concluded that all breast surgery, including breast augmentation surgery, increases the likelihood of insufficient lactation when a woman tries to breastfeed.Citation7 A recent study confirms this.Citation8
Risk of reduced accuracy of mammograms
Breast cancer detection is another potential problem raised by Wood and Spear. In addition to the risk of rupture during mammography, implants have been found to hide approximately half of cancerous tumours during a mammogram. An implant shows up as a solid white orb on a mammogram, which can hide tumours above or below the implant.Citation9 Specially trained technicians can partially compensate by taking additional views of the implanted breast, but additional views generally cost more, take more time, and expose the woman to more radiation. Since most women wanting breast implants plan to have them for the rest of their lives, it is important to stress this issue to women of all ages before they make a decision about getting breast implants. Most mastectomy patients also need to consider the impact on mammograms, since many choose to have two breast implants: one to replace the breast lost to cancer, and the other to make the remaining breast higher and fuller so that it looks similar to the breast that was replaced. The implant that replaces the removed breast is unlikely to interfere with cancer detection, since the breast tissue has been removed; it is in the other breast that the problem can occur.
Until recently, most women undergoing breast augmentation were young, in their late teens and 20s, or perhaps early 30s. The widely advertised “mommy makeovers” have changed that. “Mommy makeovers” are being promoted by plastic surgeons to women who are done with childbearing and breastfeeding and want to make their bodies look younger again. These women, in their 30s, 40s and 50s, are or will soon be at an age when regular mammograms are recommended.
The need for subsequent surgery
The risks of surgery, such as post-operative bleeding and haematoma, reactions to anaesthesia and infection, are relatively rare.Citation2 During implant surgery, however, nerves in the nipple area can be damaged, leading to a loss of sensation; this is a more common complication of surgery, and can be temporary (lasting weeks or months) or permanent.Citation2
Most complications are related to the “ageing” implant, not the surgery, and increase over the lifetime of the product. Thus, the risk of complications requiring surgery increases over time, so that a young woman may need at least 5–10 additional surgeries, with the associated risks, in her lifetime.Citation7 Breast augmentation for teens and young women raises additional concerns, both because their bodies are still developing and their financial resources may be limited.Citation10
Inadequate informational material for women
Wood and Spear’s candid discussion of several of the risks of breast implants was unprecedented in a plastic surgery journal, and reflect Wood’s perspective as a women’s health advocate. Unfortunately, the concerns raised in the editorial are not reflected in information provided to patients by the same medical society that published the article.
Moreover, the Breast Implant Safety website (<www.breastimplantsafety.org>), hosted by the two major US plastic and cosmetic surgery medical societies, provides a very different message. The section on “Patient safety” provides very little risk information other than vague statements such as: “All surgery has risk. Breast implant surgery is no different. Silicone breast implants in particular have been the focus of much scrutiny. A review of the current scientific literature supports the use of silicone gel-filled implants as a choice for women seeking breast implant surgery. However, like all surgery, breast implant surgery may pose some risk. And like all medical devices, breast implants are not meant to last a lifetime. Women need to be aware that they have a responsibility to maintain good breast health with an annual mammogram and follow-up visits with their plastic surgeon.” And this section concludes that: “Saline-filled breast implants have always been safe, a fact that has been supported by more than 30 years of clinical experience throughout the plastic surgery community. Research and reports from the last decade have shown that silicone gel-filled implants do not pose additional risk to women’s short-term or long-term health.”
In the section on “Breast augmentation procedure”, there is a brief warning about interference with mammography and the possible loss of nipple sensation. The fact that this risk information is in the section on the logistics of the procedure, but not in the sections on safety or risks, raises questions about whether it is intentionally hidden where women might not notice it. In fact, if one puts the keywords “nipple sensation”, “mammogram” or “mammography” in the search box for this website, the response is: “Webpage cannot be found”.
Following the section on breast lifts, if the reader hasn’t yet given up, she may find a section entitled “Risks related to breast implants”. This has a short page on capsular contracture, and a link at the end sends the reader to another page that has two or three sentences each on bleeding, implant ruptures, infection, visible skin wrinkling and rippling, which concludes: “The subject of risks and potential complications of surgery is best discussed on a personal basis between you and your plastic surgeon, or with a staff member in your surgeon's office.” No mention is made of interference with mammography or loss of nipple sensation in this section. In another section, on breastfeeding, the research and Institute of Medicine report referenced here, and more recent research indicating that breast surgery can undermine the ability to breastfeed, is not mentioned, and says only: “The presence of a breast implant will have no effect on your ability to become pregnant, deliver a baby, or even breastfeed.”
Benefits of breast implants: myth and reality
Research indicates that the public have an unrealistic view of cosmetic surgery as low risk and painless.Citation11 This may be even greater for adolescents and young women.Citation10
The Breast Implant Safety website is very positive about the benefits of breast implants, stating that: “Studies confirm that the vast majority of women who choose breast augmentation experience improvements in body image, self-esteem and quality of life.” Indeed, studies asking women how they feel shortly after getting breast implants contain favourable responses. However, scientifically valid research does not support the view that breast implants improve body image or self-esteem overall, over the long-term.Citation10 Self-esteem tends to be a long-standing personality trait that is not changed by surgery, and although women may feel better about how their breasts look, research indicates that they don’t feel better about themselves, their social lives or their quality of life.Citation10 In fact, numerous studies indicate that women who have breast augmentation are two to six times more likely to kill themselves than other women with a similar demographic background, or even other plastic surgery patients..Citation12–15 This, and the fact that women with implants for a longer period of time are more likely to take anti-depressants,Citation16 suggests that implant problems may contribute to serious psychological problems. However, studies conducted by researchers from the International Epidemiology Institute, a for-profit research centre whose implant studies were funded by Dow Corning, conclude that the women probably had mental problems before their augmentation surgery. Regardless of the cause, however, the increased risk of suicide and depression after breast augmentation compared to other plastic surgery patients and the general population of women with a similar demographic background, challenges the claim that breast implants are a solution for low self-esteem or depression.
The unresolved questions surrounding autoimmune diseases
The greatest controversy about breast implants is the question of whether breast implants, particularly leaking implants, can cause or exacerbate autoimmune diseases. Although silicone is considered biocompatible, that only means that most people won’t have an allergic or auto-immune reaction to a silicone implant; it does not mean that no one will. As can be seen on the FDA website, the implant manufacturers warn in their labels and implant information brochures that their implants were not studied in women with autoimmune diseases.Citation17 What they don’t say is that these women were not eligible for the studies because of concern that they would be more likely to have an autoimmune reaction to the silicone in the implants. Meanwhile, FDA scientists have reported more fibromyalgia and several other autoimmune diseases in women with leaking implants compared to those with intact implants,Citation18 and rheumatologists have reported that women with implants and arthritis or other rheumatology symptoms often improve when their implants are removed.Citation19
Financial risks
As part of the decision-making process, women of all ages need to consider the long-term financial costs of breast implants. Many women contact our Center seeking help because they are not able to afford surgery to have their implants removed, even those with leaking implants or serious rheumatological illnesses. Unfortunately, no financial assistance is available for most of them, and many of them have very limited resources because of their illnesses. Moreover, women who bought breast implants on the installment plan are surprised to learn that they are not able to get them removed on the installment plan; they must usually pay the total fee prior to surgery.
In addition to the initial surgery to insert the implants, subsequent operations to fix implant problems or remove broken implants can cost at least as much as the initial surgery, if not more.Citation20 The additional cost of every mammography appointment and $2,000 every other year for MRIs for silicone implants must also be considered in the US. In addition, when our Center examined US health insurance questionnaires, we found that many ask about “breast cancer or breast implants” in the same question, and may drop coverage or raise premiums for women who have undergone breast implant surgery.Citation5
The research literature
Why is the information on the plastic surgery societies’ website so inconsistent with the article by Wood and Spear in the American Society of Plastic Surgeons’ own publication, as well as the FDA’s website information on breast implants? How can plastic surgeons repeatedly claim that the research evidence clearly shows that breast implants are safe?
A review of all articles listed on PubMed on breast implants found dozens of published studies, most within the last ten years. Most of the major studies were conducted by researchers affiliated with the International Epidemiology Institute, which were funded by Dow Corning, a former breast implant manufacturer. In 1998 Dow Corning agreed to a $3.2 billion legal settlement with women harmed by implants.Citation21 Several months after the settlement, Dow Corning filed for bankruptcy, and the settlement was lowered to $3.25 billion and went into effect in 2004. Despite compensating tens of thousands of women with autoimmune illnesses and leaking implants, the company continues to claim that their implants did not cause those problems, and the studies they funded seem to support that claim. Although using large samples and registries in Scandinavia, most of the studies are poorly designed, including women with implants for just a few days or months, using inappropriate comparison groups, and inadequate outcome measures.Citation22Citation23 For example, in several studies, hospitalisation was used as the only health outcome measure. This reduces the likelihood of finding significant differences in illness between women with and without breast implants. Lastly, some studies do not mention statistically significant findings in their abstracts or conclusions (e.g. a study by Breiting et al which found significant increases in chronic breast painCitation16) and yet conclude that breast implants are not harmful.
Informed consent
Informed consent forms and the consent process are intended to support the right of self-determination and patient choice. Bioethicists point out that in cosmetic surgery both supply and demand can be stimulated by manufacturers’ and physicians’ financial interests, which can compromise informed consent.Citation24
The FDA requires that each implant manufacturer provide patient booklets that clearly describe the risks (see, for example, the one on the FDA website <www.accessdata.fda.gov/cdrh_docs/pdf3/P030053d.pdf>). However, this “booklet” is 40–50 pages long and quite technical, resembling a textbook description more than the simple explanations that health educators recommend for informed consent information. Moreover, although the FDA requires doctors to provide the FDA-approved booklet to all their patients, the requirement is not enforced. In Europe, in contrast, the European Parliament’s Public Health Committee has urged European Union member states to ban direct advertising to the public of breast implants, require risk information on the labelling, and promote alternatives to breast implants.Citation25 Whether they have all done so is another question.
In the US, rather than warn about risks, most of the information from manufacturers and plastic surgeons seems to be focused on reassuring patients that implants are safe. On the plastic surgery medical societies’ website described earlier, controversy is dismissed, complications are barely mentioned, and benefits are exaggerated. Surgery is offered on the installment plan,Citation26 and mastectomy patients are strongly encouraged to have reconstructive surgery on breasts lost to cancer and augmentation in their healthy breasts to make their two breasts symmetrical..Citation27–29 Breast cancer patients tell us that if they express doubts about having implants, they are told that they will feel much better about themselves if they have reconstructive surgery, although research shows no difference in the quality of life or self-esteem of women who chose reconstruction and those who did not.Citation30
When questions are raised about the legal settlements or implant horror stories in the media, implant manufacturers and plastic surgeons claim that the newest implant styles, made from a more cohesive silicone gel, are less likely to leak than older implants, and are therefore safer.Citation31Citation32 Will the gel that looks so solid when the implant is cut with a knife in marketing videos look just as cohesive after more than ten years in a woman’s body? Until longer-term data are available, these claims cannot be verified. Although not approved in the US, these newer styles have been sold in Europe since 1995, but there is a lack of published data thus far showing longer-term safety.
Reliable sources of information for women
Women need accurate, easy-to-understand information about the risks and costs of breast breast implants. This includes brief, easy-to-read descriptions of the risks and benefits written by experts who do not have a financial stake in whether the woman chooses implants or not. They can get a better understanding of the risks from a documentary entitled Absolutely Safe, by Carol Ciancutti-Leyva, a filmmaker whose mother became sick from her breast implants after a mastectomy (see <www.absolutelysafe.com/index.html>). The film includes personal stories of women with implants and interviews with experts about implant safety. It is available on DVDs and has been shown on college campuses by the non-profit organisation Our Bodies, Ourselves.
Women can also find information online, although most websites about breast implants are written by plastic surgeons whose income relies on the procedures. Some websites are paid an advertising fee for each plastic surgeon they list. Even Wikipedia’s breast implant article is written primarily by plastic surgeons. When I tried to edit it to make it more balanced, much of the information I provided was deleted by one plastic surgeon, despite support for my edits by other health advocates from around the world. After several months of an editing war between women’s health advocates and plastic surgeons, the article was closed to further revisions by Wikipedia administrators, and I gave up trying to improve it.
Since its founding in 1999, the National Research Center for Women & Families has provided research-based information on breast implants and their risks that is intended for a lay audience, at <www.center4research.org>. However, it soon became clear that we were not attracting women who were considering implants, since those women tended to go to a website with “implant” in the URL rather than “research.” We therefore developed a website entirely focused on implants, <www.breastimplantinfo.org>, with separate sections on breast augmentation and breast reconstruction. That website includes summaries and critiques of peer-reviewed studies, but focuses primarily on providing easy-to-understand information that a patient or potential patient would want, such as personal stories of women with implants and a “Frequently asked questions” section. In addition, the Center provides personal responses to women who e-mail questions to our online health hotlines on both websites, as well as our new website on cancer at <www.stopcancerfund.org>.
Since our websites don’t include blogs or make recommendations about plastic surgeons, we often recommend that women with implant problems go to <www.explantation.com>, a website by implant patients where they talk candidly about their experiences with implants and implant removal, and also talk about the plastic surgeons who they found helpful or not helpful. If a patient wants information to take to their doctor, we often recommend the FDA website (<www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/default.htm>), which has good information, though some of it may be too technical for a lay audience. We also sometimes recommend <www.intheknow.org>, a website that features stories of actresses with implant problems; we have spoken with several of the women and are impressed with their candour and understanding of their health problems.
Women need accurate and unbiased information from their plastic surgeons, but if that is not forthcoming, they need informed advice from other medical professionals. Physicians and other health professionals who treat women considering implants or who already have implants also need unbiased, research-based information, not just summaries funded by implant manufacturers or news stories based on promotional press releases. More than two million women in the US currently have breast implants and many more around the world. These women need help to decide whether implants present a particular risk for them, e.g. if they have an autoimmune disease or a history of breast cancer, and what to do if implants break and leak, or if symptoms or other problems arise. Only then will women who are considering breast implants or seeking help for breast implant problems have a true informed choice.
Notes
* The National Research Center for Women & Families is a non-profit research and educational organization that focuses on improving the health and safety of adults and children. We receive approximately 1,000 e-mails and calls every year from women wanting information about breast implants, either because they are considering getting implants or because they are having problems with their implants. Most are from the US but some are also from at least a dozen other countries. We respond to each one individually.
References
- American Society of Plastic Surgery Statistics. 2010 Report of the 2009 Statistics, National Clearinghouse of Plastic Surgery Statistics. At: <www.plasticsurgery.org/Documents/Media/statistics/2009-US-cosmeticreconstructiveplasticsurgeryminimally-invasive-statistics.pdf. >. Accessed 3 May 2010.
- Food and Drug Administration. Breast Implant Questions and Answers. At: <www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm063719.htm#2. >. Accessed 3 May 2010.
- See, for example, US Food & Drug Administration. Photographs and/or Illustrations of Breast Implant Complications. At: <www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/UCM064348>. Accessed 8 May 2010.
- LR Hölmich, VB Breiting, JP Fryzek. Long-term cosmetic outcome after breast implantation. Annals of Plastic Surgery. 59(6): 2007; 597–604.
- SF Wood, SL Spear. What do women need to know and when do they need to know it?. Plastic and Reconstructive Surgery. 120(7 Suppl.1): 2007; 135S–139S.
- SL Brown, JF Todd, HD Luu. Breast implant adverse events during mammography: reports to the Food and Drug Administration. Journal of Women's Health. 13(4): 2004; 371–378.
- Institute of Medicine. Safety of Silicone Breast Implants. 1999; Washington, DC: National Academy Press.
- NI Cruz, L Korchin. Breastfeeding after augmentation mammaplasty with saline implants. Annals of Plastic Surgery. 64(5): 2010; 530–533. At: <www.ncbi.nlm.nih.gov/pubmed/20354430. >. Accessed 7 May 2010.
- DL Miglioretti, CM Rutter, BM Geller. Effect of breast augmentation on the accuracy of mammography and cancer statistics. JAMA. 291: 2004; 442–450.
- D Zuckerman, A Abraham. Teenagers and cosmetic surgery: focus on breast augmentation and liposuction. Journal of Adolescent Health. 43(4): 2008; 318–324.
- GS Hamilton, JS Carrithers, LH Karnell. Public perception of the terms “cosmetic”, “plastic”, and “reconstructive” surgery. Archives of Facial Plastic Surgery. 6(5): 2004; 315–320.
- LA Brinton, JH Lubin, MC Burich. Cancer risk at sites other than the breast following augmentation mammoplasty. Annals of Epidemiology. 11(4): 2001; 248–256.
- E Pukkala, I Kulmala, H Sirpa-Liis. Causes of death among Finnish women with cosmetic breast implants, 1971–2001. Annals of Plastic Surgery. 51(4): 2003; 339–342.
- PH Jacobsen, LR Hölmich, JK McLaughlin. Mortality and suicide among Danish women with cosmetic breast implants. Archives of Internal Medicine. 164(22): 2004; 2450–2455.
- L Lipworth, O Nyren, W Ye. Excess mortality from suicide and other external causes of death among women with cosmetic breast implants. Annals of Plastic Surgery. 59(2): 2007; 119–123.
- VB Breiting, LR Hölmich, B Brandt. Long-term health status of Danish women with silicone breast implants. Plastic Reconstructive Surgery. 114(1): 2004; 217–226.
- For example, see the Food and Drug Administration. Important information for augmentation patients about Mentor MemoryGel silicone gel-filled breast implants, Updated 11/3/06. At: <www.accessdata.fda.gov/cdrh_docs/pdf3/P030053d.pdf>. Accessed 3 May 2010.
- SL Brown, G Pennello, WA Berg. Silicone gel breast implants rupture, extracasular silicone, and health status in a population of women. Journal of Rheumatology. 28(5): 2001; 996–1003.
- FB Vasey, SA Zarabadi, M Seleznick. Where there’s smoke there’s fire: the silicone breast implant controversy continues to flicker: a new disease that needs to be defined. Journal of Rheumatology. 30(10): 2003; 2092–2094.
- Einstein Industries. Breast implant removal and prices. At: <www.docshop.com/education/cosmetic/breast/revision/cost. >. Accessed 7 May 2010.
- Frontline. Breast Implants on Trial. At: <www.pbs.org/wgbh/pages/frontline/implants/cron.html. >. Accessed 7 May 2010.
- D Zuckerman. Reconstruction after breast implantation after mastectomy. Archives of Surgery. 114(10): 2006; 714–715.
- P Lieberman, D Zuckerman. Do breast implants cause disease?. At: <www.breastimplantinfo.org/what_know/meta82000.html. >. Accessed 7 May 2010.
- NN Dubler, A Schissel. Women, breasts, and the failure of informed consent. JAMWA. 55(5): 2000; 255–260.
- Breast implants. Official Journal of the European Union 2004:C43E/363–366. At: <http://eur-lex.europa.eu/LexUriServ/site/en/oj/2004/ce043/ce04320040219en03630366.pdf>. Accessed 3 May 2010.
- Ceatus Media Group LLC. Consumer Guide to Plastic Surgery. What does breast augmentation cost. At: <www.yourplasticsurgeryguide.com/breast-augmentation/cost.htm. >. Accessed 7 May 2010.
- Breast reconstruction before and after pictures, reconstruction before and after photos. At: <www.smartplasticsurgery.com/reconstruction/photos.html. >. Accessed 7 May 2010.
- Breast reconstruction advantages. At: <www.breastimplantsafety.org/BreastReconstruction/index.php. >. Accessed 7 May 2010.
- Testimony of Anne Stansel before the FDA. At: <www.breastimplantinfo.org/per_stories/annes.html. >. Accessed 7 May 2010.
- JH Rowland, KA Desmond, BE Meyerowitz. Role of breast reconstructive surgery in physical and emotional outcomes among breast cancer survivors. Journal of National Cancer Institute. 92(17): 2000; 1422–1429.
- Marina Equipment Leasing. Los Angeles gummy bear implants. At: <www.gummybearbreastimplants.com/cohesive-gel-implants.cfm. >. Accessed 7 May 2010.
- Einstein Industries DocShop. Cohesive silicone “gummy bear” breast implants – Type of breast implants. At: <www.docshop.com/education/cosmetic/breast/augmentation/implants/cohesive-gel. >. Accessed 7 May 2010.