Abstract
Extensive evidence exists regarding the efficacy and acceptability of medical abortion through 63 days since last menstrual period (LMP). In Mexico City’s Secretariat of Health (SSDF) outpatient facilities, mifepristone-misoprostol medical abortion is the first-line approach for abortion care in this pregnancy range. Recent research demonstrates continued high rates of complete abortion through 70 days LMP. To expand access to legal abortion services in Mexico City (where abortion is legal through 12 weeks LMP), this study sought to assess the efficacy and acceptability of the standard outpatient approach through 70 days in two SSDF points of service. One thousand and one women seeking pregnancy termination were enrolled and given 200 mg mifepristone followed by 800 μg misoprostol 24–48 hours later. Women were asked to return to the clinic one week later for evaluation. The great majority of women (93.3%; 95% CI: 91.6–94.8) had complete abortions. Women with pregnancies ≤8 weeks LMP had significantly higher success rates than women in the 9th or 10th weeks (94.9% vs. 90.5%; p = 0.01). The difference in success rates between the 9th and 10th weeks was not significant (90.0% vs. 91.2%; p = 0.71). The majority of women found the side effects (82.9%) and the use of misoprostol (84.4%) to be very acceptable or acceptable. This study provides additional evidence supporting an extended outpatient medical abortion regimen through 10 weeks LMP.
Résumé
De nombreuses données existent sur l’efficacité et l’acceptabilité de l’avortement médicamenteux jusqu’à 63 jours depuis la date des dernières règles (DDR). Dans les centres ambulatoires du Secrétariat de la santé de Mexico (SSDF), l’avortement médicamenteux sous mifépristone et misoprostol est la méthode de première intention pour l’interruption de grossesses de cet âge gestationnel. Des recherches récentes montrent des taux élevés d’avortement complet jusqu’à 70 jours après la DDR. Pour élargir l’accès à des services d’avortement légal à Mexico (où l’avortement est autorisé jusqu’à 12 semaines après la DDR), cette étude a tenté d’évaluer l’efficacité et l’acceptabilité de la méthode ambulatoire standard jusqu’à 70 jours dans deux centres du SSDF. Mille et une femmes souhaitant avorter ont été recrutées et ont reçu 200 mg de mifépristone suivis de 800 μg de misoprostol 24–48 heures plus tard. Elles ont été invitées à revenir au centre une semaine après pour évaluation. Chez la grande majorité des patientes (93,3% ; IC 95% : 91,6–94,8), l’avortement était complet. Les femmes enceintes ≤8 semaines après la DDR avait des taux de réussite sensiblement plus élevés que les femmes à 9 ou 10 semaines (94,9% contre 90,5% ; p = 0,01). La différence dans les taux de réussite entre la 9e et la 10e semaine était non significative (90,0% contre 91,2% ; p = 0,71). La majorité des femmes ont jugé les effets secondaires (82,9%) et l’utilisation du misoprostol (84,4%) très acceptables ou acceptables. Cette étude fournit des données supplémentaires soutenant un régime étendu d’avortement médicamenteux en ambulatoire jusqu’à 10 semaines après la DDR.
Resumen
Existe extensa evidencia respecto a la eficacia y aceptabilidad del aborto con medicamentos hasta los 63 días desde la fecha de la última menstruación (FUM). En las unidades de salud ambulatorias de la Secretaría de Salud del Distrito Federal de México (SSDF), el aborto con medicamentos inducido con mifepristona-misoprostol es el enfoque de primera línea en los servicios de aborto para esta etapa de la edad gestacional. Recientes investigaciones demuestran continuas altas tasas de aborto completo hasta concluidos los 70 días desde la FUM. Con el fin de ampliar el acceso a los servicios de interrupción legal del embarazo en el Distrito Federal (donde el aborto es legal hasta las 12 semanas desde la FUM), este estudio buscó evaluar la eficacia y aceptabilidad del enfoque ambulatorio estándar hasta los 70 días en dos puntos de entrega de servicios de la SSDF. Mil y una mujeres que buscaban interrumpir su embarazo fueron inscritas y administradas una dosis de 200 mg de mifepristona seguida de 800 μg de misoprostol, 24 a 48 horas después. Se les pidió a las mujeres que regresaran a la clínica una semana después para la evaluación. La gran mayoría de las mujeres (93.3%; 95% IC: 91.6–94.8) tuvo un aborto completo. Las mujeres con embarazos ≤8 semanas desde la FUM tuvieron tasas de eficacia significantemente más altas que las mujeres en la novena o décima semanas (94.9% vs. 90.5%; p = 0.01). La diferencia en las tasas de eficacia entre la novena y décima semanas no fue significante (90.0% vs. 91.2%; p = 0.71). La mayoría de las mujeres encontraron que los efectos secundarios (82.9%) y el uso de misoprostol (84.4%) eran muy aceptables o aceptables. Este estudio ofrece evidencia adicional que respalda un régimen de aborto con medicamentos extendido hasta concluidas las 10 semanas desde la FUM para usuarias ambulatorias.
Medical abortion using mifepristone and misoprostol is a safe and effective method of pregnancy termination that has been used by millions of women worldwide over the past 25 years. Extensive evidence regarding both efficacy and acceptability supports the outpatient use of medical abortion through 63 days since the last menstrual period (LMP).Citation1–6 In this pregnancy range, among the World Health Organization’s (WHO) recommended regimens is 200 mg mifepristone followed by 800 μg buccal misoprostol;Citation7 this protocol is routinely used for legal abortion in the outpatient facilities of the Secretariat of Health of Mexico City (SSDF),Citation6,8 where approximately 71% of women who obtain services have a medical abortion.Citation9
In Mexico, abortion is legal in the first trimester of pregnancy, and the first mifepristone product was registered there in 2011. At around the same time, a study of medical abortion through 63 days LMP was conducted among 1,000 women who sought services in several SSDF outpatient facilities, confirming the high efficacy and acceptability of the mifepristone–misoprostol regimen.Citation6 The findings facilitated the SSDF in establishing medical abortion with mifepristone and misoprostol as its first-line approach for abortion care. Due to the high demand for medical abortion and the advantages to service delivery it offers over aspiration (including reduced cost and in-clinic time), the Secretariat strives to offer outpatient medical abortion services to as many women seeking first trimester pregnancy termination as possible. A previous study of the characteristics of the women who have sought legal abortions in SSDF facilities found that they are diverse in age, marital status, and parity, and that a high percentage are Catholic.Citation10
A 2012 study in the United States using 200 mg mifepristone + 800 μg buccal misoprostol demonstrated that the same regimen can be used in women at 64–70 days LMP as in women at 57–63 days LMP, with comparable efficacy (92.8% and 93.5%, respectively) and satisfaction (88.3% vs. 87.4% satisfied or very satisfied).Citation11 In addition, the US National Abortion Federation guidelines now include outpatient mifepristone-misoprostol medical abortion through 70 days LMP.Citation12 As a next step in the process of extending the clinical protocol for legal abortion in SSDF facilities, this study sought to assess the efficacy and acceptability of medical abortion through 70 days (the 10th week) provided as part of routine clinical services.
Methodology
This was an open-label clinical study (both clinicians and participants knew the treatment being provided). Women seeking pregnancy termination were enrolled at two study sites: the outpatient service at the Hospital Materno Infantil Inguarán and the reproductive health clinic of a primary care clinic, Centro de Salud Beatriz Velasco de Alemán. Women were invited to join the study if they were eligible for medical abortion and had pregnancies up to 70 days LMP. Women interested in participating had to agree to undergo a surgical intervention (vacuum aspiration) if necessary, be willing to provide contact information for purposes of follow-up, have easy access to both a telephone and emergency transportation, and comply with the study protocol. All participants gave written informed consent. Ethical approval was granted by the Ethics, Biosecurity and Research Commission of the Secretariat of Health of Mexico City.
All participants were given the same regimen of 200 mg mifepristone (Zacafemyl, Intelligence Pharmaceutical Network) to swallow in the clinic followed by 800 μg misoprostol (4 × 200 μg; either Cytotec [Pfizer] or Cyrux [Llama Serral]) to take at home 24–48 hours later. Women were instructed to retain the misoprostol buccally (two pills inside each cheek) for 30 minutes before swallowing any remains. Analgesics were prescribed according to the standard of care at each facility, and participants were told to call the clinic with any questions or concerns. Each participating clinic was provided a mobile phone to facilitate communication with study participants.
Participants were asked to return to the clinic seven days after taking the mifepristone to assess abortion status. Women with ongoing pregnancies were recommended vacuum aspiration. Women with non-viable pregnancies, including persistent gestational sac, retained products of conception or bleeding were given the choice between an additional dose of 800 μg buccal misoprostol, expectant management, or vacuum aspiration. Those choosing either of the first two options were asked to return one week later for further follow-up. At that time women with a persistent non-viable pregnancy or substantial debris were offered vacuum aspiration. Prior to exiting the study, all women were asked a series of questions by a member of the on-site study team regarding their experiences with medical abortion.
The primary outcome of the study was method efficacy, defined as complete abortion without vacuum aspiration at any point. Additional outcomes included extent of bleeding, extent of pain and other side effects, rate of abortions needing further treatment, and acceptability. Based on the success rates in studies by Winikoff in 2008 (≤ 63 days LMP: 96.2%)Citation2 and in 2012 (57–63 days LMP: 93.5%; 64–70 days LMP: 92.8%),Citation11 a sample size of 456 women was deemed sufficient to show overall success of 95% +/— 2%. The sample size was increased to 1,000 to account for high rates of expected loss to follow-up and homogeneity between sites. Data were analysed using SPSS version 19 (IBM, Armonk, NY, USA) and STATA version 11 (StataCorp, College Station, TX, USA).
Findings
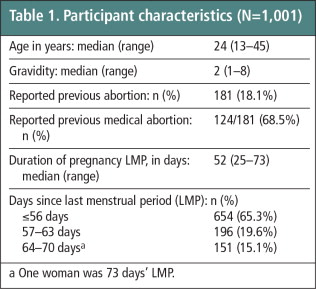
Between January and March 2012, 1,001 women were enrolled in the study. Of these, forty-one women were lost to follow-up and no follow-up data could be collected; therefore, they are only included in the analysis of participant characteristics and are excluded from further analyses. One woman with a pregnancy 73 days LMP was mistakenly enrolled in the study; her data are included in the analyses. Participant characteristics are presented in Table 1 . Around two-thirds (65.3%) of women in the study were ≤56 days LMP, one-fifth (19.6%) were 57–63 days LMP, and 15.1% were 64–70 days LMP.
Table 1 Participant characteristics (n = 1,001).Footnotea
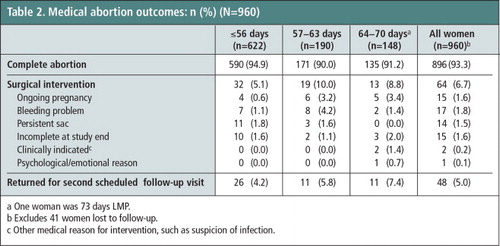
All but one study participant reported taking mifepristone and misoprostol as instructed; one woman said she took the misoprostol five days after the mifepristone. The great majority of women (93.3%; 95% CI: 91.6–94.8) had complete medical abortions without vacuum aspiration (Table 2). The reasons for the 64 vacuum aspirations included ongoing pregnancy (n = 15), bleeding problem (n = 17), persistent sac (n = 14), incomplete at study end (n = 15), clinically indicated intervention (n = 2) and psychological/emotional reasons (n = 1). Rate of complete abortion varied by study site: 98.5% vs. 92.0%.
Women at ≤8 weeks LMP (≤56 days) had significantly higher complete abortion rates than women in the 9th week (94.9% vs. 90%, p = 0.02), but statistical significance did not hold when comparing women ≤8 weeks LMP to those in the 10th week (94.9% vs. 91.2%, p = 0.12). The difference in complete abortion rates between the 9th and 10th weeks did not differ significantly (90.0% vs. 91.2%; p = 0.85). Twenty women (2.1%) attended another (non-study) medical facility for abortion-related care during the study after having received the two medications; these women were more likely to undergo surgical intervention than women who only received care at the study clinics (70.0% vs. 5.3%; p < 0.001). Seventy participants (7.0%) called the clinic with questions or concerns about the abortion process; women 10 weeks LMP were no more likely to call than women 9 weeks LMP (8.6% vs. 11.2%; p = 0.48; data not shown).
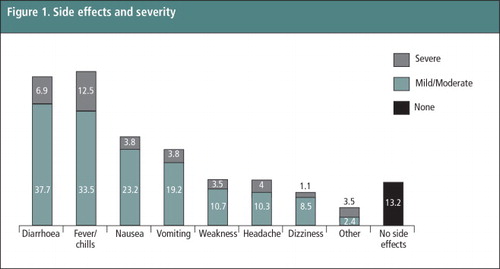
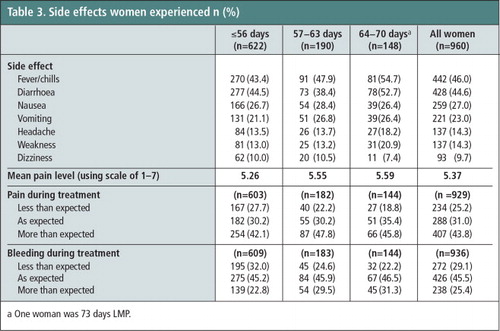
The most common side effects experienced during the abortion process included fever/chills (46.0%), diarrhoea (44.6%), nausea (27.0%), and vomiting (23.0%) (Table 3). Severity of side effects is presented in . Slightly over half of the women (56.2%) reported that the pain they experienced was less than or as expected, and three-fourths (74.6%) found the bleeding to be less than or as expected. Approximately the same proportion of women in the 10th week as in the 9th week reported having more pain than expected (45.8% vs. 47.8%, p = 0.74). Reported mean pain level (on a scale from 1–7) did not differ between women in the 9th and 10th weeks (5.59 vs. 5.55. p = 0.79) but was statistically significantly higher when women 9 and 10 weeks LMP were compared to those ≤56 days LMP (5.57 vs. 5.26; p = 0.003; data not shown).
Table 3 Side effects women experienced n (%).Footnotea
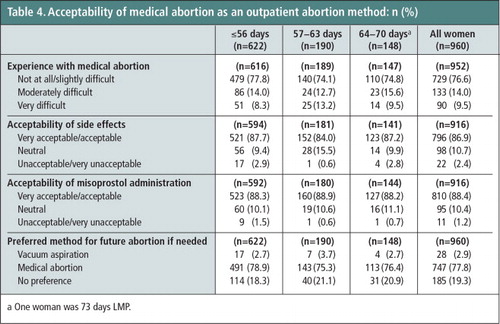
Three-fourths of the women (76.6%) found the medical abortion process to be not at all difficult or only slightly difficult, while 9.5% thought it was very difficult (Table 4). The majority of women (86.9%) found the side effects to be very acceptable or acceptable; only 2.4% thought they were unacceptable or very unacceptable. Most participants (84.4%) also found the mode of misoprostol administration to be very acceptable or acceptable, with only 1.2% finding the mode of administration unacceptable or very unacceptable. If another abortion is needed in the future, 77.8% of the women would again choose medical abortion; only 2.9% would choose vacuum aspiration and 19.3% had no preference. Best aspects of medical abortion as reported by participants included ease or convenience (26.0%), efficacy (24.4%), the quality of care received (11.7%), and privacy (8.5%). For 16.3%, there was no best aspect. Cramps/pain were the most commonly reported worst feature (28.8%). Over half (52.7%) of the women said that there was no worst aspect (data not shown).
Table 4 Acceptability of medical abortion as an outpatient abortion method: n (%).Footnotea
Discussion
This study demonstrated that outpatient medical abortion protocols can be extended through 70 days LMP with rates of efficacy and acceptability within the range seen in other studies with similar protocols.Citation11,13,14 The overall ongoing pregnancy rate of 1.6% in this study that included women through 70 days LMP was slightly higher than the 0.5% typically seen in studies among women ≤63 days.Citation2,6 However, a higher rate of ongoing pregnancies in the 9th week (3.2%) and 10th week (3.4%) was expected and concurs with the Winikoff et al. 2012 study, which reported ongoing pregnancy rates of 3.1% and 3.0%, respectively.Citation11 Compared to women with earlier pregnancies, the chance of an ongoing pregnancy following use of the medical abortion regimen among women in the 9th and 10th weeks increases from less than 1 woman per 100 to less than 4 women per 100. While the slightly higher rate of ongoing pregnancy might discourage some women from selecting this method of abortion, other women do not consider the drop in success to be a limiting factor. Outpatient medical abortion with this regimen for women 9 weeks LMP is recommended by the World Health OrganizationCitation7 and commonly used. That there is no incremental difference in the ongoing pregnancy rate when used by women 10 weeks LMP supports the expansion of outpatient medical abortion services to include this additional week of pregnancy. Each woman should be able to make an informed choice to select whichever method she thinks is best for her.
The overall success rate in this study is lower than in previous studies in Mexico City,Citation6 but it does not appear to be due to the inclusion of women with pregnancies of 64–70 days LMP since success dropped sharply in the 9th week. This may be due to chance, or in part to provider management, specifically non-study clinicians at other facilities. Clinical providers who do not offer medical abortion services will typically follow standard management of non-complicated incomplete abortion; in Mexico, these women are often hospitalized and treated with dilatation and curettage even if not clinically indicated. Among study clinicians, we cannot rule out the possibility of behavioural bias, as providers were not blinded to pregnancy duration. Heterogeneity among providers, that is, the differences in the way individual providers clinically manage a case, might also have contributed to site differences in success rate.
Women with pregnancies in the 9th and 10th weeks reported significantly higher mean pain scores than other study participants. However, the scores are so similar that they may not be clinically meaningful, that is, they may not call for different strategies in pain management. More importantly, the mean pain score of women in the 10th week was almost identical to that of women in the 9th week, further supporting the routine offering of outpatient medical abortion to women with pregnancies 64–70 days.
This study provides additional evidence to the published literature that supports extending outpatient medical abortion through 70 days (10th week) of pregnancy. In fact, based on these study results and the experience obtained through participating in this study, the SSDF has expanded outpatient care through 70 days as a part of routine care. Offering outpatient medical abortion as a treatment option for this population would further expand access to women, and if participation in this study is any indication, women 64–70 days LMP could represent as much as 15% of medical abortion users. In turn, this would be time- and resource-saving for providers and the health care system. It is possible that this medical abortion regimen could be an option for women beyond 70 days LMP as well, as it is unlikely that the rate of success would sharply plummet at 71 days LMP. Further research is necessary to explore efficacy later in the first trimester. In addition, as the pregnancy develops and becomes more recognizable upon expulsion at 11 and 12 weeks, it would be important to assess simultaneously the acceptability of outpatient medical abortion for women, as well as providers’ comfort with providing it, to determine the feasibility of this service delivery option.
Acknowledgments
The authors thank the staff at C.S. Beatriz Velasco Alemán and at H.M.I. Inguarán for their important work on this study, as well as the participants who made this analysis possible. We also thank Jennifer Britton for her invaluable assistance with data management and analysis.
Notes
a One woman was 73 days’ LMP.
a One woman was 73 days LMP.
b Excludes 41 women lost to follow-up.
c Other medical reason for intervention, such as suspicion of infection.
a One woman was 73 days LMP.
a One woman was 73 days LMP.
References
- J Blum, S Raghavan, R Dabash. Comparison of misoprostol-only and combined mifepristone–misoprostol regimens for home-based early medical abortion in Tunisia and Vietnam. International Journal of Gynecology & Obstetrics. 118: 2012; 166–171. 10.1016/j.ijgo.2012.03.039.
- B Winikoff, IG Dzuba, MD Creinin. Two distinct oral routes of misoprostol in mifepristone medical abortion: a randomized controlled trial. Obstetrics & Gynecology. 112: 2008; 1303–1310. 10.1097/AOG.0b013e31818d8eb4.
- P Goldstone, J Michelson, E Williamson. Early medical abortion using low-dose mifepristone followed by buccal misoprostol: a large Australian observational study. Medical Journal of Australia. 197: 2012; 282–286.
- S Raghavan, T Tsereteli, A Kamilov. Acceptability and feasibility of the use of 400 μg of sublingual misoprostol after mifepristone for medical abortion up to 63 days since the last menstrual period: evidence from Uzbekistan. European Journal of Contraception and Reproductive Health Care. 18: 2013; 104–111. 10.3109/13625187.2013.763225.
- H von Hertzen, NT Huong, G Piaggio, WHO Research Group on Postovulatory Methods of Fertility Regulation. Misoprostol dose and route after mifepristone for early medical abortion: a randomised controlled noninferiority trial. BJOG. 117: 2010; 1186–1196. 10.1111/j.1471-0528.2010.02636.x.
- M Peña, IG Dzuba, P Sanhueza Smith. Efficacy and acceptability of a mifepristone–misoprostol combined regimen for early induced abortion among women in Mexico City. International Journal of Gynecology & Obstetrics. 127: 2014; 82–85. 10.1016/j.ijgo.2014.04.012.
- World Health Organization. Safe Abortion: Technical and Policy Guidance for Health Systems. 2nd ed., 2012; WHO: Geneva. http://apps.who.int/iris/bitstream/10665/70914/1/9789241548434_eng.pdf.
- D Becker, C Díaz Olavarrieta. Decriminalization of abortion in Mexico City: The effects on women’s reproductive rights. American Journal of Public Health. 103: 2013; 590–593.
- Secretariat of Health of Mexico City. Information system of the Legal Termination of Pregnancy Program. Provisional data, June 2014.
- M Mondragón y Kalb, A Ahued Ortega, J Morales Velazquez. Patient characteristics and service trends following abortion legalization in Mexico City, 2007–10. Studies in Family Planning. 42: 2011; 159–166.
- B Winikoff, IG Dzuba, E Chong. Extending outpatient medical abortion services through 70 days of gestational age. Obstetrics & Gynecology. 120: 2012; 1070–1076. Doi: http://10.1097/AOG.0b013e31826c315f.
- National Abortion Federation. 2014 Clinical Policy Guidelines. 2014; NAF: Washington, DC. http://prochoice.org/education-and-advocacy/2014-clinical-policy-guidelines/.
- H Bracken, R Dabash, G Tsertsvadze. A two-pill sublingual misoprostol outpatient regimen following mifepristone for medical abortion through 70 days LMP: a prospective comparative open-label trial. Contraception. 89: 2014; 181–186. 10.1016/j.contraception.2013.10.018.
- AA Boersma, B Meyboom-de Jong, G Kleiverda. Mifepristone followed by home administration of buccal misoprostol for medical abortion up to 70 days of amenorrhoea in a general practice in Curaçao. European Journal of Contraception and Reproductive Health Care. 16: 2011; 61–66. 10.3109/13625187.2011.555568.