Abstract
Background
This study was designed to evaluate the effect of intravenous dexmedetomidine infusion in patients undergoing major abdominal surgery on stress response markers as plasma interleukin-6, cortisol and blood glucose level. It also assessed its effect on recovery profile and postoperative pain.
Methods
Thirty adult ASA I–III patients admitted to the surgery department of the Alexandria Main University Hospital scheduled for elective major abdominal surgery under general anaesthesia were included. They were randomly classified into two equal groups of 15 patients each, dexmedetomidine group (Group D) received intravenous dexmedetomidine infusion and placebo group (Group P) received intravenous infusion of normal saline. Haemodynamic parameters were recorded intra- and postoperatively. Interleukin-6, cortisol and blood glucose levels were measured. Recovery profile, postoperative pain score and analgesic requirement postoperatively were assessed.
Results
Heart rate and mean arterial pressure were significantly lower in group D relative to group P during most of the intra- and postoperative periods. Postoperatively, the levels of interleukin-6, cortisol and blood glucose were significantly lower in group D relative to group P. Recovery time was longer in group D than group P but with no significant difference. Postoperative pain score was significantly less in group D relative to group P during the early postoperative period with smaller amount of analgesic requirements in group D.
Conclusion
Dexmedetomidine is safe and effective in blunting the postoperative rise of the proinflammatory cytokine interleukin-6 and resulted in lower levels of markers of stress response to surgery as cortisol and blood glucose. Dexmedetomidine also reduces the postoperative pain score without delaying recovery from anaesthesia.
1 Introduction
The stress response to surgery is a major neuroendocrine and cytokine response to surgical trauma, characterized by increases in catecholamine and steroid hormones, with predictable metabolic consequences.Citation1 This stress response has been considered as the homeostatic defense mechanism, important for the body for adaptation and developing resistance to the noxious insults. Such exaggerated physiological changes in patients with coexisting diseases is always life threatening. If the stress response is prolonged, the continuous hyper-metabolic state may result in exhaustion of essential components of the body causing loss of weight, fatigue, decreased resistance, delayed ambulation and increased morbidity and mortality.Citation2
The cytokine cascade activated in response to surgical trauma consists of a complex biochemical network with diverse effects on the injured host. Cytokines are immune mediators that direct the inflammatory response to sites of injury and infection and are essential for wound healing. An exaggerated production of proinflammatory cytokines from the primary site of injury, however, can manifest systemically as haemodynamic instability or metabolic derangements.Citation3
Circulating interleukin-6 (IL-6) level appears to be proportional to the extent of tissue injury during an operation. IL-6 stimulates the acute-phase reaction, which enhances the innate immune system and protects against tissue damage.Citation4
Excitation of the hypothalamus during stress results in the secretion of ACTH which in turn initiates sudden increase in cortisol level. The metabolic effects of cortisol are directed to overcome the stressful state. Cortisol has widespread effects on the metabolism and utilization of glucose, amino acid and fatty acids in hepatic and extra-hepatic tissues. The cortisol causes rapid mobilization of amino acids and fat from their cellular stores, making them immediately available both for energy and synthesis of other compounds including glucose needed by different tissues.Citation5
Alpha-2 receptors are a subgroup of noradrenergic receptors that mediate the function of the sympathetic nervous system. The alpha-2 receptor is probably the body's most important presynaptic receptor; its activation results in reduction in norepinephrine release, which can be used therapeutically to induce sympatholysis.Citation6
Alpha-2 agonists including clonidine and dexmedetomidine have seen extensive use. Dexmedetomidine differs from clonidine in two important respects: significantly greater (8x) affinity for the alpha-2 receptor and is easier to titrate.Citation6 Dexmedetomidine is constituted from the dextro-rotatory isomer of medetomidine.Citation7
Dexmedetomidine is the first marketed sedative to make use of highly selective alpha-2 agonist activity. Sedation with dexmedetomidine differs in several important ways from sedation with other agents. First, unlike commonly used sedatives such as propofol or midazolam, dexmedetomidine produces an “interactive” form of sedation, in which patients may be aroused easily with stimulation, and are cooperative once aroused. Second, dexmedetomidine has analgesic properties and may significantly reduce concomitant opioid use. Third, dexmedetomidine is accompanied by virtually no respiratory depression at clinically relevant doses. Finally, dexmedetomidine has predictable sympatholytic effects.Citation6
Because of its sympatholytic properties, dexmedetomidine was initially developed as an anaesthetic premedication, with the goal of attenuating the sympathetic response to perioperative stresses such as laryngoscopy and intubation.Citation8
In addition to sedative effects, dexmedetomidine has significant analgesic qualities and has been labelled as “analgesia-sparing” by the FDA. Analgesia with dexmedetomidine is mediated primarily through interaction at alpha-2a within the spinal cord, where drug activity attenuates nociceptive signal transduction. The actual mechanism of action appears to involve an interaction with opioid receptors, and although dexmedetomidine alone has been documented to reduce pain, the effect when given jointly with opioids may be additive or synergistic.Citation9 Dexmedetomidine provides intense analgesia during the postoperative period and reduces the total number of post-surgical patients requiring opioids with a corresponding reduction in opioid-associated side effects.Citation10 Because dexmedetomidine has no depressant effects on ventilation, its analgesic effect may offer a significant advantage for patients at risk for respiratory decompensation.Citation11
The aim of the present work was to study the effect of intravenous dexmedetomidine infusion on haemodynamic changes, plasma level of interleukin-6, cortisol, blood glucose level, recovery profile and postoperative pain in patients undergoing major abdominal surgery.
2 Methods
2.1 Patients
The present double blind study was carried out on 30 adult patients admitted to the surgery department of the Alexandria Main University Hospital belonging to American Society of Anaesthesiologists (ASA) physical status grade I–III, scheduled for elective major abdominal surgery under general anaesthesia. After approval of the medical ethics committee, informed written consent was obtained from all patients.
Patients were randomly classified using closed envelope technique into two equal groups according to the drug infused intraoperatively. Dexmedetomidine group (Group D); received loading dose of intravenous dexmedetomidine infusion of 1 μg/kg IV over 10 minutes followed by maintenance dose of 0.5 μg/kg/hr till the end of surgery and placebo group (Group P); received intravenous infusion of normal saline 0.9 % over 10 minutes followed by continuous infusion till the end of surgery.
All patients received the same anaesthetic technique. Standard monitoring was established. Before induction of general anaesthesia, a thoracic epidural catheter was placed.
2.2 Measurements
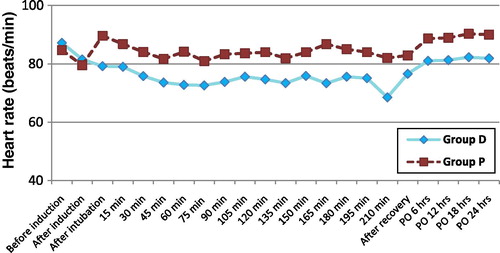
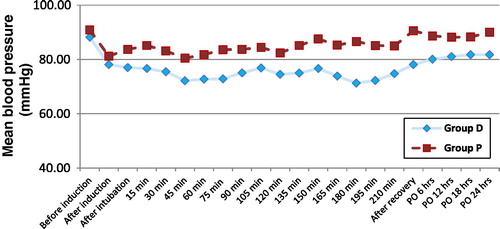
Heart rate (HR) and mean arterial pressure (MAP) were recorded at the following times: before induction, after induction, after intubation, intraoperatively every 15 minutes till the end of surgery, after complete recovery and postoperatively every 6 hours during the first 24 hours.
Plasma levels of interleukin-6, plasma cortisol level and blood glucose level had been measured before induction, after complete recovery and first day postoperatively at 8:00 am.
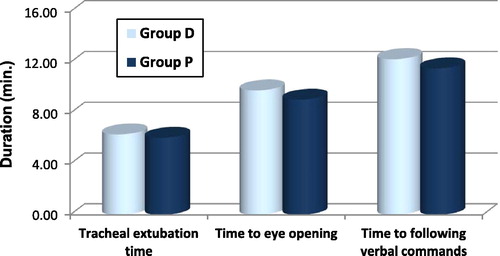
Recovery profile had been assessed by measuring tracheal extubation time, time to eye opening, and time to following verbal commands. These durations start from end of surgery and discontinuation of inhalational anaesthetics.Citation12
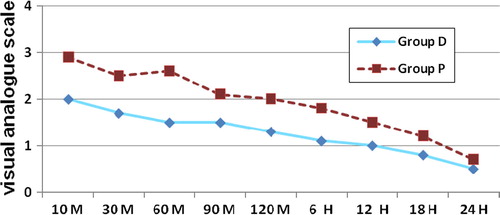
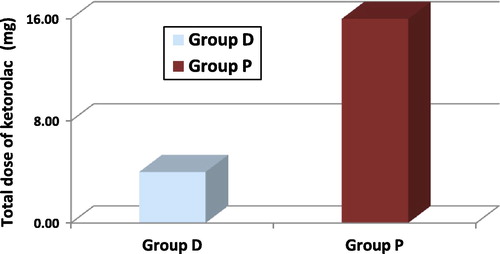
Assessment of postoperative pain using visual analogue scale (VAS) in which each patient was asked to estimate his pain on vertical VAS 0–10 cm where (0) is marked as no pain and (10) is marked as the worst pain ever felt.Citation13 This was recorded at 10, 30, 60, 90 and 120 minutes and at 6, 12, 18 and 24 hours postoperatively. Total dose of ketorolac given for each patient in the first 24 hours was recorded.
2.3 Statistical analysis
Data was analysed using SPSS statistical package version 15 for Microsoft Windows. Numerical data was expressed as mean ± standard deviation. Comparison between the two groups was done using parametric or non-parametric t-test. The level of significance adopted for this study was P <0.05.
3 Results
There were no statistically significant differences between the two groups as regards age, sex, body weight and durations of surgery and anaesthesia as shown in .
Table 1 Demographic and operative data of dexmedetomidine group and placebo group.
Baseline values of HR and MAP were comparable in both groups. Intraoperatively, the HR and MAP were significantly lower in group D relative to group P after intubation and during most of the intra- and postoperative periods as shown in and .
There was no statistically significant difference between the two groups as regards the basal level of blood glucose, interleukin-6, and cortisol. After complete recovery and at the first day postoperatively, they increased in both groups but with significantly lower value in group D relative to group P as shown in .
Table 2 Comparison between the two studied groups regarding changes of plasma interleukin-6, cortisol and blood glucose.
Recovery profile was assessed by measuring tracheal extubation time, time to eye opening and time to following verbal commands which were longer in group D relative to group P but with no statistically significant difference between both groups as shown in .
Postoperative pain score using VAS was significantly less in group D relative to group P during 10, 30 and 60 minutes postoperatively. During the other postoperative study periods, the VAS was less in group D but with no significant difference between both groups as shown in . Postoperative ketorolac requirement was significantly less in group D relative to that in group P as shown in .
4 Discussion
The stress response to surgery is the name given to the hormonal and metabolic changes following injury or trauma. A normal, balanced, well controlled inflammatory response in previously healthy patients almost always results in an uneventful recovery. However, some patients may develop an exaggerated high or insufficient response. A suppressed response or prolonged illness may result in compromised organ function that requires exogenous support.Citation14
Dexmedetomidine is an α2-adrenergic agonist which is the pharmacologically active dextroisomer of medetomidine.Citation15 The centrally acting α2-adrenergic agonists including dexmedetomidine activate receptors in the medullary vasomotor centre, reducing norepinephrine turnover and decreasing central sympathetic outflow, resulting in alterations in sympathetic function. Additional effects result from the central stimulation of parasympathetic outflow and inhibition of sympathetic outflow from the locus cereleus in the brainstem. The activation of α2-adrenergic receptors in the dorsal horn of the spinal cord inhibits the release of substance P, a nociceptive mediator, resulting in primary analgesic effects as well as potentiating of opioid-induced analgesia.Citation16
The present study was designed to evaluate the effect of intravenous dexmedetomidine infusion on stress response markers in major abdominal surgery as plasma interleukin-6, cortisol and blood glucose level. It also assessed its effect on recovery profile and postoperative pain.
All patients were scheduled for elective major abdominal surgery under general anaesthesia. They were divided into one of two groups according to the type of infusion. Dexmedetomidine group (Group D) whose patients received intravenous dexmedetomidine infusion and placebo group (Group P) whose patients received intravenous infusion of normal saline. Patients were followed up in the surgical ICU for postoperative care.
In the present study, the basal readings of HR and MAP were within normal physiological ranges and with no significant difference between both groups. HR and MAP were significantly lower but with haemodynamic stability, in group D relative to group P during most of the intra- and postoperative periods. Dexmedetomidine was able to blunt the increase in HR and MAP associated with endotracheal intubation in group D.
In agreement with the present study, Jaakola et al.Citation17 showed that dexmedetomidine attenuated the increase in HR and MAP during intubation. Lawrence et al.Citation18 found that a single dose of dexmedetomidine before induction of anaesthesia attenuated the haemodynamic response to intubation and extubation.
Postoperatively, the levels of interleukin-6, cortisol and blood glucose increased in both groups but were significantly lower in group D relative to group P.
Aho et al.Citation19 found that patients receiving dexmedetomidine had significantly lower intraoperative cortisol levels as compared with those who did not receive the drug before surgery. This supports the finding of the present study that dexmedetomidine administration resulted in lower levels of stress response markers to surgery.
In agreement with the present study, Uyar et al.Citation20 found that plasma concentration of cortisol and glucose had increased significantly in the placebo group, than in the dexmedetomidine group. Interestingly, Mukhar et al.Citation21 found that dexmedetomidine did inhibit the hyperglycaemic response to surgery significantly more than placebo, and this may reflect attenuation of the sympatho-adrenal response.
In contrast to the present study, Aantaa et al.Citation22 measured cortisol level in patients undergoing minor gynaecologic surgery and found that it was equally increased in saline and dexmedetomidine group.
The results of the present study indicated that proinflammatory cytokine production (IL-6) was stimulated in the postoperative period following major abdominal surgery. Dexmedetomidine attenuated the stress response and so suppressed the postoperative rise of IL-6 which was significantly higher in group P relative to group D. IL-6 is a main proinflammatory cytokine produced as early as two to four hours after tissue damage. IL-6 level has a direct relation to the severity of inflammation and tissue injury. One mechanism of stimulation of IL-6 release is via the intracellular cyclic adenosine monophosphate (cAMP) concentrations.Citation23 Dexmedetomidine stimulates the postsynaptic α2-adrenergic receptors resulting in inhibitory feedback and decreased activity of adenylyl cyclase enzyme.Citation24
In the present study, dexmedetomidine provides sedation without delaying recovery from anaesthesia. Dexmedetomidine is associated with what has been termed “arousable sedation.” Patients receiving dexmedetomidine can typically respond to commands when lightly aroused.
Turan et al.Citation25 found that dexmedetomidine improved extubation conditions but did not prolong recovery in patients presenting for craniotomy. Norimasa et al.Citation26 studied the recovery profile from dexmedetomidine as a general anaesthetic adjuvant in patients undergoing lower abdominal surgery. They concluded that postoperative cognitive function was not affected by dexmedetomidine administration.
Basar et al.Citation27 concluded that a single dose of 0.5 μg/kg of dexmedetomidine given preoperatively led to significant sedation with no change in recovery scores.
In the present study, VAS for pain score was less in group D relative to group P and postoperative ketorolac requirement was significantly less in group D relative to that in group P.
Dexmedetomidine provides sedation and analgesia with no accompanying respiratory depression.Citation28 In addition, it has an opioid-sparing effect.Citation29 The analgesic, sedative/hypnotic and anxiolytic properties of dexmedetomidine make this drug potentially useful for painful surgical procedures.Citation30
In agreement with the present study, Gurbet et al.Citation31 found that dexmedetomidine resulted in reduced need for rescue sedation. They found that continuous infusion of dexmedetomidine during abdominal surgery significantly reduces the amount of patient controlled analgesia with morphine without affecting time to extubation. Dholakia et al.Citation32 found that patients in the dexmedetomidine group were administered less narcotics during their hospital stay, and these patients were discharged home sooner than patients in the control group.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 17 December 2011
References
- S.C.O’RiainD.J.BuggyM.J.KerinInhibition of the stress response to breast cancer surgery by regional anesthesia and analgesia does not affect vascular endothelial growth factor and prostaglandin E2Anesth Analg100200512441249
- I.VelickovicJ.YanJ.A.GrossModifying the neuroendocrine stress response. Seminars in anaesthesiaPerioper Med Pain2120021625
- E.LinS.E.CalvanoS.F.LowryInflammatory cytokines and cell response in surgerySurgery12722000117126
- S.F.LowryE.E.LinS.E.CalvanoMediators of Inflammation and InjuryJ.A.NortonP.S.BarieR.R.BollingerA.E.ChangS.F.LowryS.J.MulvihillH.I.PassR.W.ThompsonSurgery2nd ed.2008Springer ScienceNew York7599 Chap. 4
- T.AsohH.TsujiC.ShirasakaY.TakeuchiEffect of epidural analgesia on metabolic response to major upper abdominal surgeryActa Anaesthesiol Scand271983233237
- S.B.VictorJordenTungAveryDexmedetomidine: clinical update seminars in anesthesiaPerioper Med Pain212002265274
- E.KuuselaO.VainioA.KasitinenSedative, analgesic, and cardiovascular effects of levomedetomidine alone and in combination with dexmedetomidine in dogsAm J Vet Res6242001616621
- H.ScheininM.L.JaakolaS.SjovaliIntramuscular dexmedetomidine as premedication for general anesthesia. A comparative multicenter studyAnesthesiology786199310651075
- C.A.FairbanksL.S.StoneK.F.KittoAlpha-c adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergyJ Pharmacol Exp Ther30012002282290
- R.M.VennC.J.BradshawR.SpencerPreliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unitAnaesthesia54199911361142
- P.TalkeR.ChenB.ThomasThe hemodynamic and adrenergic effects of perioperative Dex infusion after vascular surgeryAnesth Analg9042000834839
- Y.GünesM.GündüzD.ÖzcengizDexmedetomidine-remifentanil or propofol-remifentanil anesthesia in patients undergoing intracranial surgeryNeurosurg Quart1522005122126
- C.H.KindlerC.HarmsF.AmslerThe visual analog scale allows effective measurement of preoperative anxiety and detection of patients’ anesthetic concernsAnesth Analg902000706712
- E.RiversB.NguyenS.HavstadEarly goal-directed therapy in the treatment of severe sepsis and septic shockN Engl J Med345200113681377
- R.VirtanenJ.M.SavolaV.SaanoCharacterization of selectivity, specificity, and potency of medetomidine as an alpha 2-adrenoceptor agonistEur J Pharmacol1501998914
- L.E.NelsonJ.LuT.GuoThe alpha 2-adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effectsAnesthesiology982003428436
- M.L.JaakolaT.A.Ali-MelkkiläJ.KantoA.KallioH.ScheininM.ScheininDexmedetomidine reduces intraocular pressure, intubation responses and anaesthetic requirements in patients undergoing ophthalmic surgeryBr J Anaesth681992570575
- C.J.LawrenceS.De LangeEffects of a single pre-operative dexmedetomidine dose on isoflurane requirements and perioperative haemodynamic stabilityAnaesthesia521997736744
- M.AhoM.ScheininA.M.LehtinenIntramuscularly administered dexmedetomidine attenuates hemodynamic and stress hormone responses to gynecologic laparoscopyAnesth Analg751992932939
- A.S.UyarH.YagmurdurY.FidanDexmedetomidine attenuates the hemodynamic and neuroendocrinal responses to skull-pin head-holder application during craniotomyJ Neurosurg Anesthesiol2032008174179
- A.M.MukhtarE.M.ObayahA.M.HassonaThe use of dexmedetomidine in pediatric cardiac surgeryAnesth Analg10320065256
- R.AantaaJ.KantoM.ScheininA.KallioH.ScheininDexmedetomidine, an α2-adrenoceptor agonist, reduces anesthetic requirements for patients undergoing minor gynecologic surgeryAnesthesiology731990230235
- Y.NaitoS.TamaiS.KohResponses of plasma adrenocorticotropic hormone, cortisol, and cytokines during and after upper abdominal surgeryAnesthesiology771992426431
- C.Correa-SalesC.NacifK.ReidInhibition of adenylate cyclase in the locus cereleus mediates the hypnotic response to an alpha-2 agonist in the ratJ Pharmacol Exp Ther263199210461050
- G.TuranA.OzgultekinC.TuranE.DincerG.YukselAdvantageous effects of dexmedetomidine on haemodynamic and recovery responses during extubation for intracranial surgeryEur J Anaesthesiol252008816820
- O.NorimasaK.KotaroS.KazuhiroY.YutakaM.EijiRecovery profiles from dexmedetomidine as a general anesthetic adjuvant in patients undergoing lower abdominal surgeryAnesth Analg1076200818711874
- H.BasarS.AkpinarN.DoganciThe effects of preanesthetic, single-dose dexmedetomidine on induction, hemodynamic, and cardiovascular parametersJ Clin Anesth202008431436
- P.TalkeE.LoboR.BrownSystemically administered alpha 2-agonist-induced peripheral vasoconstriction in humansAnesthesiology9920036570
- M.L.JaakolaM.SalonenR.LehtinenH.ScheininThe analgesic action of dexmedetomidine – a novel α2-adrenoceptor agonist – in healthy volunteersPain461991281285
- T.Z.GuoJ.Y.JiangA.E.ButtermannM.MazeDexmedetomidine injection into the locus ceruleus produces antinociceptionAnesthesiology841996873881
- A.GurbetE.Basagan-MogolG.TurkerF.UgunF.N.KayaB.OzcanIntraoperative infusion of dexmedetomidine reduces perioperative analgesic requirementsCan J Anesth532006646652
- C.DholakiaG.BeversteinM.GarrenC.NemergutJ.BoncykJ.C.GouldThe impact of perioperative dexmedetomidine infusion on postoperative narcotic use and duration of stay after laparoscopic bariatric surgeryJ Gastrointest Surg11200715561559