Abstract
Background
CD44 is a polymorphic family of cell surface proteoglycans and glycoproteins implicated in cell-to-cell and cell-to-matrix adhesion interactions and tumor metastasis.
Aim
This study was designed to histopathologically examine and immunohistochemically detect the expression of CD44v6 in differentiated thyroid carcinomas, and its association with clinicopathologic parameters.
Methods
Forty different thyroid lesions constituted the material of this study. Cases were divided into a malignant (n = 30) and a non-malignant group (n = 10). Immunostaining was manually performed using CD44v6 mouse monoclonal antibody.
Results
CD44v6 expression was significantly higher in malignant compared to non-malignant lesions. This significance was not maintained when cases were categorized as neoplastic and non-neoplastic. Female sex and patients age <45 years were significantly associated with higher CD44v6 expression in the malignant group. Papillary thyroid carcinoma (PTC) showed higher CD44v6 expression than follicular thyroid carcinoma (FTC). CD44v6 immunopositivity did not significantly associate with the microscopic variant of PTC and FTC. Also, tumor size failed to relate significantly with CD44v6 expression within the subtypes of PTC and FTC. However, female sex, patient age <45 years, and tumor size between 1-4cms associated with significantly higher CD44v6 expression in the PTCs when compared to non-malignant lesions. In FTCs no significant relationship was observed between CD44v6 immunopositivity and patients’ age, sex, or tumor size. In the non-malignant group no significant relationship was found between CD44v6 immunopositivity and patients’ sex, or age. The background non-neoplastic thyroid tissue was CD44v6 negative in all cases of both groups except for 4 cases of PTCs.
Conclusion
Deregulated expression of CD44v6 occurs in differentiated thyroid carcinomas and in benign thyroid nodules. Thus, further studies are warranted to investigate the diagnostic utility of CD44v6 expression in the differentiation of benign from malignant thyroid lesions. Also, the predictive value of CD44v6 deregulated expression in PTC and the occurrence of nodal metastasis needs further verification.
1 Introduction
Thyroid tumors are the most common malignancies of the endocrine system.Citation1 In Egypt thyroid cancer represents 1.2% of cancers in males and 2.2% of cancers in females, and ranks the 10th most common cancer site among females.Citation2
CD44 is a polymorphic family of immunologically related integral membrane glycoproteins and proteoglycans, implicated in cell-cell and cell-matrix adhesion,Citation3–Citation6 lymphocyte activation and homing,Citation7 cell migration, and tumor metastasis.Citation3–Citation7 CD44 is encoded by a gene located on 11p13 chromosome and consists of at least 20 exons. Polypeptide isoforms of CD44 are produced by alternative splicing of at least 10 of the 20 exons during mRNA processing.Citation7,Citation8 The simplest CD44 isoform (CD44 standard or CD44s) does not contain any additional exon product and is expressed by hematopoietic cells and most of the epithelial cells.Citation7,Citation9
Alternations in the composition of CD44 protein and of its isoforms are associated with neoplastic transformation and metastasis in a number of different tissues. So, increased expression of CD44 has been implicated in melanoma metastasis,Citation7,Citation10 pancreatic adenocarcinomas, colorectal carcinomas,Citation7,Citation11 non-Hodgkin lymphomas, breast and lung carcinomas.Citation7,Citation12 On the other side, decreased expression of CD44v6 is related to tumor recurrence and unfavorable outcome in poorly differentiated squamous cell carcinoma,Citation7,Citation12 laryngeal carcinomas, superficial bladder carcinomaCitation7,Citation13 and prostate carcinomas.Citation7,Citation14,Citation15
CD44 variant isoforms are also expressed in normal and neoplastic thyroid tissues, but it is unclear whether they have any prognostic value in differentiated thyroid carcinomas,Citation7,Citation14 which include papillary and follicular carcinomas and their subtypes.Citation7,Citation8
The aim of this study was to histopathologically examine and immunohistochemically detect the expression of CD44v6 in papillary and follicular thyroid carcinomas, in addition to evaluating the expression of CD44v6 in benign thyroid lesions to evaluate any possible alteration in its expression. Also we associated CD44v6 expression in the different studied thyroid lesions with clinicopathologic parameters.
2 Methods
The present study included a total of 40 cases representing different thyroid lesions both neoplastic and non-neoplastic. Clinical data including patients’ age, sex, clinical presentation, and type of operation (total, subtotal or hemithyroidectomy) were obtained by reviewing the pathology request forms. The study was approved by the Alexandria University, Faculty of Medicine Research Ethics Committee.
One paraffin block representative of the lesion with adequate material suitable for immunohistochemical (IHC) studies was selected for every case. The formalin fixed paraffin-embedded (FFPE) tissue blocks were cut into 5 μm thick sections that were reevaluated without the knowledge of any previous diagnosis. As a first step, hematoxylin and eosin (H&E) stained sections were assessed to diagnose all cases. The presence of background lesions e.g. Hashimoto's thyroiditis in cases of PTC or degenerative changes in cases of adenomas were noted.
2.1 Immunohistochemistry
Five micron-thick sections, of FFPE tissue blocks were cut and mounted on coated slides. Tissue sections were deparaffinized in standard xylene and rehydrated in a graded alcohol series (100% to 70%) followed by incubation in Hydrogen Peroxide Block for 10-15 minutes, to block the endogenous peroxidase activity (EPA) to reduce nonspecific background staining. For CD44v6 immunostaining heat induced antigen retrieval was done in microwave oven using sodium citrate buffer (0.01 M Na-citrate monohydrate, pH 6.0) for 10 min, then allowed to cool down to room temperature. Tissue sections were immunostained for CD44 using mouse monoclonal antibody, (CD44v6, clone VFF-7 that specifically recognizes an epitope encoded by exon v6 on the variant portion of human CD44) provided by Bender Medsystems, Vienna, Austria diluted at 1:350 and incubated overnight at 4 °C in a humidified chamber. The antigen-antibody reaction was visualized by Thermo scientific UltraVision LP Detection System. Immunohistochemical reactions were developed with diaminobenzidine. Sections were counterstained with Harrris hematoxylin and covered by a coverslip using Canada balsam. All immunostains were manually processed with the utilization of appropriate positive and negative controls for each batch of slides.
2.2 Quantification of immunostaining
The immunostained slides were then evaluated and scored by two pathologists, independently without knowledge of any clinical data. The percentage of positive cells was counted in 10 high power fields (HPF) using semiquantitative method: 0 = negative; 1+ = less than 25% positive staining; 2+ = 25–50% positive staining; 3+ = greater than 50% positive area. The intensity of the stained cells was recorded as weak, moderate or strong but was not scored. Only continuous membranous immunoreactivity was considered positive. Neither cytoplasmic staining alone nor punctate membrane staining was considered positive.Citation7
2.3 Statistical analysis of the data
Data were analyzed using SPSS software package version 18.0 (SPSS, Chicago, IL, USA).Citation16 Quantitative data were expressed using minimum, maximum, mean, standard deviation, median and inter-quartile range as the distribution was abnormal. The qualitative data were expressed in frequency and percent. Qualitative data were analyzed using Chi-square test also exact tests such as Fisher exact and Likelihood Ratio were applied to compare groups. Quantitative data were analyzed using Mann Whitney test to compare between any two groups. Spearman correlation was used and regression equation was determined if there was significant correlation. Scatter plot was drawn to present the correlation and regression. Agreement of the different predictives with the outcome was used and expressed in sensitivity, specificity, positive predictive value, negative predictive value, and accuracy of the CD44v6 where histomorphologic diagnosis was considered as the gold standard. Sensitivity was defined on the basis of thyroid cancer detection using immunostaining for CD44v6 [no. positive/(true positive + false negative)]. Specificity was defined on the basis of benign thyroid lesions detection [no. negative/(true negative + false positive)]. Positive and negative predictive values were computed as no. positive/ (true positive + false positive) and no. negative/true negative + false negative), respectively. p-Value was assumed to be significant at 0.05.
3 Results
3.1 Clinical data
The present study included 40 cases of different thyroid lesions both neoplastic and non-neoplastic. The forty cases were divided into two major groups: malignant and non-malignant. Group 1 included 30 cases of papillary and follicular thyroid carcinomas, whereas group 2 included 10 cases of different benign thyroid lesions. The age of the patients ranged between 14 and 70 years (mean ± SD 44.18 ± 13.25 years). Nineteen cases were < 45 years and 21 cases were ⩾ 45 years. Six cases were males (15%) and 34 cases (85%) were females.
3.2 Histopathological examination
Histopathological examination of the H&E stained sections revealed 24 (60%) papillary thyroid carcinoma cases (PTC), 6 cases (15%) follicular thyroid carcinoma (FTC), 4 cases (10%) multinodular colloid goiter, 3 cases (7.5%) adenoma of the thyroid and 3 cases (7.5%) Hashimoto's thyroiditis.
3.3 Group 1; Malignant cases (n = 30)
Twenty-four out of the 30 cases (80%) in this group were females and 6 cases (20%) were males.
3.4 Papillary thyroid carcinomas
Nineteen cases (79.2%) were females and 5 cases (20.8%) were males. The patients’ age in thirteen cases (54.2%) was <45 years and in 11 cases (45.8%) was ⩾45 years.
The 24 PTC were microscopically subtyped according to De Lellis et al.Citation2 into: 8 cases (33.3%) conventional (or classic) type of PTC, 6 cases (25%) micropapillary variant of PTC carcinoma, 7 cases (29.2%) follicular variant of PTC (FVPTC), 2 cases (8.3%) encapsulated variant of PTC, and 1 case (4.2%) tall or columnar cell variant of PTC.
A significant relationship between the microscopic subtype of PTC and tumor size was noted for tumors with diameter <1 cm as well as for tumor size between 1 and 4 cms; (p < 0.001 for both diameters). This significant association was not maintained for tumor size > 4 cm, (p = 0.091). Within the 24 PTC cases no significant association was observed between tumor size and patients’ age (p = 0.340), patients’ sex (p = 0.145), and TSH level (p = 0.422).
3.5 Follicular thyroid carcinoma
Five cases (83.3%) were females and one case (16.7%) was a male. The age range was 38–62 years (mean ± SD 53.33 ± 8.82 years). The tumor size ranged from 1 to 3 cm (mean ± SD 2.08 ± 0.80 cm).
The six cases of follicular thyroid carcinoma were microscopically subtyped according to De Lellis et al.Citation2 into: two cases (33.3%) minimally invasive FTC and four cases widely invasive FTC (66.7%); among these four cases a case was diagnosed as Hürthle cell carcinoma.
3.6 Group 2 (non-malignant cases)
All 10 cases in this group were females. Three cases were adenomas (one microfollicular, one mixed micro and macrofollicular and the third was a Hürthle cell adenoma), three cases were Hashimoto's thyroiditis and four cases were multinodular colloid goiters.
3.7 CD44v6 immunohistochemical staining of tissue sections
CD44v6 positive immunostaining revealed significantly higher expression in the malignant (80% of cases CD44v6 positive) versus the non-malignant (30% of cases CD44v6 positive) cases; (p = 0.006), . This statistical significance was not maintained when cases were categorized as neoplastic (differentiated thyroid carcinomas, and adenoma cases) and non-neoplastic.
Table 1 CD44v6 immunoreactivity in the malignant and non-malignant cases.
Statistical analysis between both groups (groups 1 and 2) indicated that female sex, patients’ age <45 years and thyroid stimulating hormone (TSH) ⩾1.8 uLU/ml were associated with significantly higher levels of CD44v6 immunopositivity in malignant compared to non-malignant lesions; (p = 0.015, p = 0.017, and p = 0.048, respectively).
The sensitivity and the specificity of CD44v6 positive immunoreactivity in malignant versus non-malignant cases were 80% and 70%, respectively. Positive and negative predictive values were 88.89% and 53.85%, respectively.
3.8 CD44v6 expression in PTC cases (n = 24)
Twenty out of the 24 cases (83.3%) of PTC included in this study stained positive for CD44v6. Eleven cases (45.8%) showed membranous immunopositivity in >50% of tumor cells population and were given score 3, 5 cases (20.8%) showed immunopositivity in 20–50% of tumor cells population and were given score 2, and the remaining 4 cases (16.7%) showed positive staining in <25% of tumor cell population and were given score 1. and show the CD44v6 immunoreactivity in the different subtypes of PTC.
Figure 1 (A) Conventional (classic) PTC showing intense membranous immunopositivity, (CD44v6, ×400). (B) Follicular variant of PTC showing follicles with strong membranous immunostaining, (CD44v6, ×200). (C) Encapsulated variant of PTC, score 3; with capsular fibroblast serving as positive internal control, (CD44v6, ×100). (D) Columnar cell variant of PTC, score 3, (CD44v6, ×100). (E) Higher power of the previous case showing strong cytoplasmic membrane immunoreactivity (CD44v6, ×400).
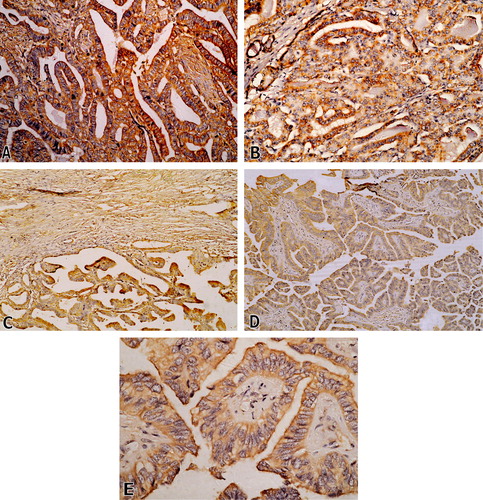
Table 2 CD44v6 immunoreactivity in the different subtypes of PTC.
The staining intensity varied between strong (14 cases; 58.4%), moderate (5 cases; 20.8%), and weak (5 cases; 20.8%). Also, intratumoral variability of staining intensity was noted, and characteristically the strongest intensity was seen at the tumor border and invasive edges.
Out of 24 cases of PTC; only nine cases were submitted with cervical lymphadenectomy. Four out of those nine cases (44.4%) showed nodal metastatic deposits that were also immunostained using CD44v6 and revealed positive strong and diffuse immunoreactivity in all four cases.
The background thyroid tissue showed positive CD44v6 immunoreactivity in four out of the 24 PTC cases, three cases were score 2 with moderate to weak intensity (two cases of conventional PTC, and one case of FVPTC), and the fourth case (micropapillary variant of PTC) was score 1 with focal and weak immunostaining. The background thyroid tissue was totally negative in the remaining 20 cases.
In the present study, papillary thyroid carcinoma showed significantly higher CD44v6 expression compared to non-malignant lesions, (p = 0.005). CD44v6 immunopositivity did not associate with PTC microscopic subtype, (p = 0.147). Also, the extent of CD44v6 expression (score) did not associate with PTC microscopic subtypes. CD44v6 immunopositivity within the different subtypes of PTC showed a significant association with patients’ age only for age group ⩾45 years; (p = 0.038), and not in the age group <45 years. No statistically significant relationship was found between CD44v6 immunostaining and sex within the different subtypes of PTC.
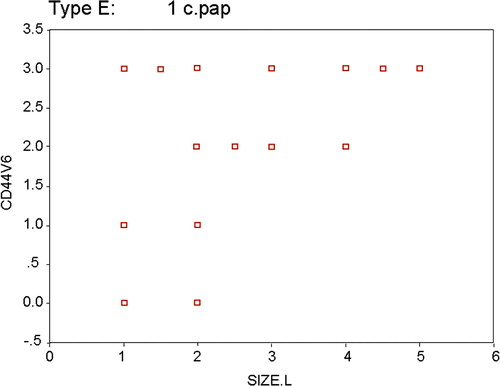
Statistical analysis showed that female sex, patients’ age <45 years and tumor size ranging between 1 cm and 4 cm were associated with significantly higher levels of positive CD44v6 immunostaining in PTC compared to non-malignant lesions; (p = 0.011, p = 0.022 and p = 0.017, respectively). There was a significant positive and moderate correlation between CD44v6 immunostaining and the largest tumor size (rho = 0.536, p = 0.007), and 31.4% of the changes in CD44v6 could be explained by variations in the largest tumor size as R2 = 0.314. The CD44v6 value could be predicted by determining the largest tumor size according to the regression equation as for each unit change in the largest tumor size the CD44v6 increased by 0.515 ().
Figure 2 Scatter plot for the relation between CD44v6 and largest tumor size among papillary thyroid carcinoma cases. Rho = 0.536*, p = 0.007; R = 0.561, R2 = 0.314 CD44v6 = 0.734 + 0.515 × largest tumor size t = 3.175*, p = 0.004.
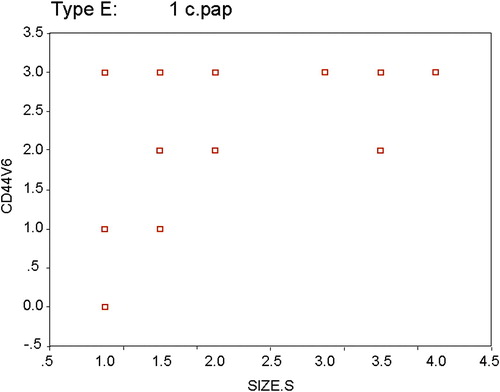
Also there was a significant positive and moderate correlation between CD44v6 and the smallest tumor size (rho = 0.633, p = 0.001), and 33.2% of changes in CD44v6 could be explained by variations in the smallest tumor size as R2 = 0.332. The CD44v6 value could be predicted by determining the smallest tumor size according to regression equation as for each unit change in the smallest tumor size the CD44v6 increased by 0.714 ().
Figure 3 Scatter plot for the relation between CD44v6 and smallest tumor size among papillary thyroid carcinoma cases. Rho = 0.633*, p = 0.001; R = 0.576, R2 = 0.332, CD44v6 = 0.619 + 0.714 × smallest tumor size, t = 3.304*, p = 0.003.
The sensitivity and the specificity of CD44v6 in papillary thyroid carcinoma versus non-malignant lesions were 83.3% and 70.0%, respectively. Positive and negative predictive values were 86.96% and 63.6%, respectively.
3.9 CD44v6 expression in follicular carcinoma cases
Four out of six FTC cases included in the present study showed CD44v6 positive immunoreactivity. Three cases were score 3 (one case minimally invasive FTC and two cases widely invasive FTC), and the fourth case was given score 1 (widely invasive FTC), ().
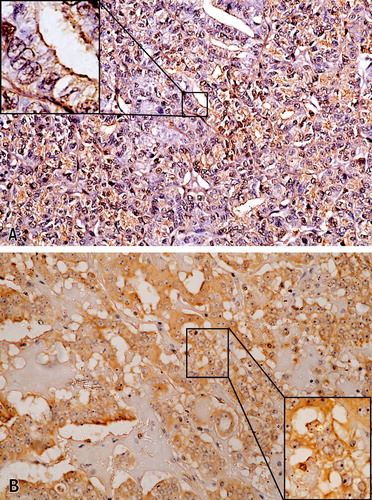
Figure 4 (A) Follicular thyroid carcinoma, score 3, (CD44v6, ×200). The inset highlights the membranous staining (CD44v6, ×1000). (B) Hürthle cell carcinoma, score 3 (CD44v6, ×200), and the inset demonstrates the positive membrane staining (CD44v6, ×400).
The sensitivity and the specificity of CD44v6 positive immunostaining in follicular thyroid carcinoma versus non-malignant lesion were 66.67% and 70.00%, respectively. Positive and negative predictive values were 57.14% and 77.78%, respectively.
3.10 CD44v6 expression in the non- malignant group
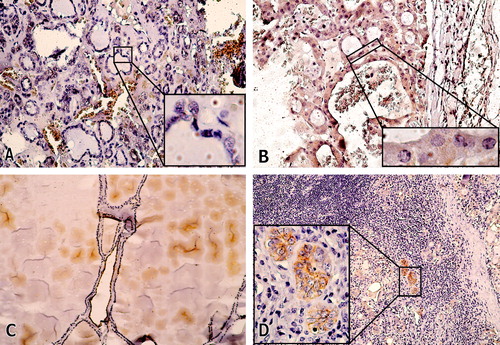
In the non-malignant group CD44v6 positive immunostaining was detected in only three out of 10 cases (30%). All three cases of benign thyroid adenomas were CD44v6 negative, Fig. (, ). Two out of 4 multinodular goiters and one out of three Hashimoto's thyroiditis showed CD44v6 immunopositivity and were given scores 3, 2, and 1, respectively, and , respectively).
Figure 5 (A) Microfollicular adenoma negative for CD44v6 (CD44v6, ×200). The inset demonstrates negative staining of neoplastic thyroid follicles, (CD44v6, ×1000). (B) Hürthle cell adenoma, negative for CD44v6, (×200), with absence of the membramous staining in neoplastic cells seen in the inset, (CD44v6, ×1000). (C) Multinodular goiter, score 2, (CD44v6, ×200). (D) Hashimoto's thyroiditis, showing focal moderate to strong immunoreactivity of the Hürthle cells, score 1 (CD44v6×100), that is highlighted in the inset (CD44v6, ×400).
In the non-malignant group no statistically significant relationship was found between CD44v6 immunopositivity and patients’ age, patients’ sex, or TSH level.
4 Discussion
CD44 exists as a standard molecule and as multiple isoforms (v1–v10).Citation6,Citation17,–Citation19 CD44v6 exists in proliferating thyroid cells, and is upregulated in carcinomas.Citation4,Citation19 Several studies reported that PTCs, adenomas and multinodular goiters exhibit an increase in CD44 mRNA isoforms, that distinguish them from the histologically normal thyroid tissue,Citation4,Citation7,Citation8 while others concluded that the majority of PTCs overexpress CD44 in contrast to normal follicular cells and non-papillary carcinoma thyroid lesions.Citation20,Citation21 In differentiated thyroid carcinoma it is still unclear whether the deregulated expression of CD44 has any prognostic value.Citation7,Citation14
The current study was undertaken to analyze CD44v6 expression in differentiated thyroid carcinomas and to correlate CD44v6 immunoreactivity to clinicopathological parameters.
Although some studies reported CD44v6 to be a marker of deregulated thyrocyte proliferation that is overexpressed in proliferating benign and malignant thyroid lesions,Citation7,Citation22,Citation23 yet in agreement with Li et al.Citation24 in this study CD44v6 expression was significantly higher in malignant compared to non-malignant lesions. This statistical significance was not maintained when cases were categorized as neoplastic and non-neoplastic, which implies that although CD44v6 is highly expressed in neoplastic lesions, yet it cannot differentiate neoplastic from non neoplastic thyroid lesions.
Our results showed that female sex, and patients’ age < 45 years showed significantly higher CD44v6 expression in malignant compared to non-malignant cases, yet, this statistical significance was also not maintained when cases were classified as neoplatic and non-neoplastic.
Within the malignant group, and in agreement with others,Citation25 CD44v6 expression was higher in PTC compared to FTC, yet, this difference did not reach statistical significance. The prevalance of CD44v6 in PTC was previously suggested to be due to differences in the genetic background of both tumors.Citation25 However, when compared to non-malignant lesions, PTC revealed significantly higher CD44v6 overexpression, which may support the suggestion that CD44 immunoreactivity may be of value in confirming the diagnosis of borderline fine-needle aspirates, as stated previously by Ross et al.Citation20 and Pazaitou-Panayiotou et al.Citation26
CD44v6 overexpression did not associate with the PTC subtype. Also, tumor size failed to relate significantly with CD44v6 expression when comparing the subtypes of PTC. However, female sex, patients’ age < 45 years and tumor size ranging between 1 cm and 4 cm were associated with significantly higher levels of positive CD44v6 immunostaining in PTC compared to non-malignant lesions. Similarly, Hamam et al.Citation27 reported that CD44v6 expression was significantly higher in younger than older patients, although, others reported absence of association between CD44 immunopositivity and tumor size, patients’ age and sex.Citation24,Citation28,Citation29
Papillary thyroid carcinoma is considered to be an indolent form of cancer with an excellent prognosis, yet occasional cases may behave in an aggressive manner.Citation20,Citation30 CD44 was implicated to facilitate lymph node metastasis,Citation20,Citation31,Citation32 yet whether its expression in PTC, predicts disease progression, and explains the propensity for this tumor to involve regional lymph nodes is still under investigation.Citation20
In this study, we were not able to fully investigate the role of CD44v6 overexpression in PTC in the development of regional nodal metastasis. However, it was noted that the nodal metastatic deposits of PTC failed to stimulate a significant lymphoid tissue reaction. This may result from the lymphocyte-like surface molecule expression that appears as a characteristic of this tumor.Citation20 Thus, further evaluation of CD44 expression in thyroid papillary carcinoma as a predictive marker for cervical nodal metastasis is strongly warranted.
CD44v6 overexpression was detected in 66.7% of the studied FTCs. Conversely, the three studied follicular adenomas were CD44v6 negative. This goes in agreement with studies that reported FTC to highly express CD44v6 compared to follicular adenomaCitation19,Citation33 to the degree that they proposed that CD44v6 might be a useful diagnostic tool for the differentiation of FTC from follicular adenoma on thyroid fine needle aspiration. CD44 negativity in adenomas may be due to the shedding of ectodomainCitation34–Citation36 or failure of recognition of the receptor by the antibody due to post-translational changes which alter the 3-dimensional conformation of the protein.Citation28,Citation37
In our study, CD44v6 negativity in adenoma cases goes with previous reports that the expression of CD44v6 in follicular adenomas ranges from 0% to 58.33%.Citation21,Citation24,Citation29,Citation33,Citation38 We report CD44v6 immunopositivity in 50% of multinodular goiters, which is within the expression range reported by some studies.Citation4,Citation7,Citation22,Citation38 Conversely, other researchers reported the absence of CD44 in multinodular goiter.Citation20,Citation21 As regards Hashimoto's thyroiditis we report a CD44v6 expression rate that goes in agreement with others,Citation7,Citation22 and the relative relation between Hashimoto thyroiditis and thyroid cancer may explain the CD44 expression in the former.Citation7 Conversely, Chieng et al.Citation21 reported that CD44v6 was not expressed in this lesion.
Similar to others,Citation4,Citation20,Citation22,Citation33,Citation38 we report that the background non-neoplastic thyroid tissue was CD44v6 negative in all cases of both groups except for four cases of PTCs that revealed moderate to weak CD44v6 immunostaining of the background thyroid parenchyma. Conversely, background positivity was explained by Ermak et al.Citation4 and Mackay et al.Citation39 by the release of soluble factors from the tumor resulting in the expression of CD44v6 in the normal adjacent thyroid tissue.
Most of the differentiated thyroid carcinomas (PTC and FTC), in opposition to non-malignant thyroid lesions and normal thyroid follicular cells, overexpress CD44v6. This suggests that deregulated expression of this adhesion molecule may play a role in the pathogenesis and better biological behavior of differentiated thyroid carcinomas. The usefulness of CD44 in confirming the diagnosis of PTC on borderline fine needle aspirates, and its utility in predicting cervical nodal metastatsis needs to be investigated on a larger number of cases. Also, evaluating the role of CD44v6 in differentiating follicular adenoma from follicular carcinoma in routine surgical pathology should be investigated.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 24 May 2012
References
- J.K.C.ChanM.HirokawaH.EvansE.D.WilliamsY.OsamuraB.CadyR.A.DeLellisR.V.LloydP.U.HeitzC.EngWHO classification of tumours: pathology, genetics of tumours of endocrine organs2004IARC PressLyon, Francep.98–103
- National Cancer Institute, Cairo University, Egypt. Cancer Registry 2002–2003. NCI 2002-03: 1–75.
- H.PontaL.ShermanP.A.HerrlichCD44: from adhesion molecules to signalling regulatorsNature Rev Mol Cell Biol420033345
- G.ErmakG.GerasimovK.TroshinaT.JenningsL.RobinsonJ.S.RossDeregulated alternative splicing of CD44 messenger RNA transcripts in neoplastic and nonneoplastic lesions of the human thyroidCancer Res55199545944598
- U.GünthertCD44: a multitude of isoforms with diverse functionsCure. Top Microbiol Immunol18419934763
- C.UnderhillCD44: the hyaluronan receptorJ Cell Sci1031992293298
- A.KiziridouA.PantidouC.H.DestouniT.ToliouImmunohistochemical expression of CD44 in thyroid gland lesionsArch Oncol111200358
- G.ErmakT.GenningsL.RobinsonJ.S.RossJ.FiggeRestricted patterns of CD44 variant exon expression in human papillary thyroid carcinomaCancer Res56199610371042
- E.DaraiF.Walker-CombrouzeA.FauconnierP.MadelenatF.PotetJ.-Y.ScoazecAnalysis of CD44 expression in serous and mucinous borderline tumours of the ovary: comparison with cystadenomas and overt carcinomasHistopathology321998151159
- S.RegauerA.OttBeham.A.BergholdCD44 expression in sinonasal melanomas: is loss of isoform expression associated with advanced tumour stage?J Pathol1871999184190
- D.CoppolaM.HyacintheL.FuA.CantorR.KarlJ.MarcetCD44v6 expression in human colorectal carcinomaHum Pathol291998627635
- D.HudsonP.SpeightF.WattAltered expression of CD44 isoforms in squamous-cell carcinomas and cell lines derived from themInt J Cancer661996457463
- V.TomaD.HauriU.SchmidD.AckermannR.MaurerG.AlundFocal loss of CD44 variant protein expression is related to recurrence in superficial bladder carcinomaAm J Pathol155199914271432
- J.P.BöhmL.K.NiskanenR.T.PirinenK.KiralyJ.K.KellokosiKiMoisioReduced CD44 standard expression is associated with tumor recurrence and unfavourable outcome in differentiated thyroid carcinomaJ Pathol1922000321327
- M.A.NoordzijG.J.Van SteenbruggeN.S.VerkaikF.H.SchroderT.H.Van der KwastThe prognostic value of CD44 isoforms in prostate cancer patients treated by radical prostatectomyClin Cancer Res31997805815
- E.LeslieJ.GeoffreyM.JamesStatistical analysis. Interpretation and uses of medical statistics4th ed.1991Oxford Scientific Publications411416
- G.ScreatonM.BellD.JacksonF.CornelisU.GerthJ.BellGenomic structure of DNA encoding the lymphocyte homing receptor CD44 reveals at least 12 alternatively spliced exonsProc Natl Acad Sci USA8919921216012164
- H.PontaD.WainwrightP.HerrlichMolecules in focus. The CD44 protein familyInt J Biochem Cell Biol301998299305
- J.MarutaH.HashimotoH.YamashitaH.YamashitaS.NoguchiImmunostaining of Galectin-3 and CD44v6 using fine-needle aspiration for distinguishing follicular carcinoma from adenomaDiag Cytopathol312004392396
- J.S.RossA.D.del RosarioB.SandersonH.X.BuiSelective expression of CD44 cell-adhesion molecule in thyroid papillary carcinoma fine-needle aspiratesDiagn Cytopathol141996287291
- D.ChhiengJ.RossB.McKennaCD44 immunostaining of thyroid fine-needle aspirates differentiates thyroid papillary carcinoma from other lesions with nuclear grooves and inclusionsCancer Cytopathol811997157162
- A.BartolazziA.GasbarriM.PapottiG.BussolatiT.LucanteA.KhanApplication of an immunodiagnostic method for improving preoperative diagnosis of nodular thyroid lesionsLancet357200116441650
- S.FischerS.L.AsaApplication of Immunohistochemistry to Thyroid NeoplasmsArch Pathol Lab Med1322008359372
- L.LiL.LinJ.QiuExpression of cell adhesion molecule (CD44V6) in thyroid tumors and its significance [abstract]Zhonghua Nei Ke Zhi40102001677680
- M.K.McLeodM.E.EastR.E.BurneyJ.K.HarnessN.W.ThompsonHashimoto's thyroiditis revisited: the association with thyroid cancer remains obscureWorld J Surg121988509516
- Pazaitou-Panayiotou K, Mygdakos N, Boglou K, kiziridou A, Chrisoulidou A, Destouni C. The immunocytochemistry is a valuable tool in the diagnosis of papillary thyroid cancer in FNA's using liquid-based cytology. J Oncology doi:10.1155/2010/963926.
- Hamam SM, Ahmed KS, Younis LK, El- Kayal SA, Hassan M. In vitro expression of CD44 v6 in non-metastatic and metastatic colorectal carcinoma. Honours (thesis) Alexandria: Alexandria University;1999.
- S.J.WangG.WongA.M.de HeerW.XiaL.Y.W.BourguignonCD44 variant isoforms in head and neck squamous cell carcinoma progressionLaryngoscope119200915181530
- W.K.F.SeelentagU.GünthertP.SaremaslanE.futoM.PfaltzP.U.HeitzCD44 standard and variant isoform expression in human epidermal skin tumors is not correlated with tumor aggressiveness but down-regulated during proliferation and de-differentiationInt J Cancer (Pred. Oncol.)691996218224
- V.A.LiVolsiPapillary neoplasms of the thyroid. Pathologic and prognostic featuresAm J Clin Pathol971992426434
- U.GünthertM.HofmannW.RudeyS.ReberM.ZöllerI.HaussmannA new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cellsCell6519911324
- R.ArchK.WirthM.HofmannH.PontaS.MtzkuP.HerrlichParticipation in normal immune responses of a metastasis-inducing splice variant of CD44Science2571992682685
- J.GuT.DaaK.KashimaS.YokoyamaI.NakayamaS.NoguchiExpression of splice variant of CD44 in thyroid neoplasms derived from follicular cellsPathol Int481998184190
- A.AfifyL.C.LynneL.HowellCorrelation of cytologic examination with ELISA assays for hyaluronan and soluble CD44v6 levels in evaluation of effusionsDiagn Cytopathol352007105110
- M.KajitaY.ItohT.ChibaH.MoriA.OkadaH.KinohMembrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migrationJCB15352001893904
- K.N.SugaharaT.HirataH.HayasakaR.SternT.MuraiM.MiyasakaTumor cells enhance their own CD44 cleavage and motility by generating hyaluronan fragmentsJ Biol Chem2819200658615868
- H.MatsukiK.YonezawaK.ObataK.IwataH.NakamuraY.OkadaMonoclonal antibodies with defined recognition sequences in the stem region of CD44 detection of differential glycosylation of CD44 between tumor and stromal cells in tissueCancer Res63200382788283
- A.GasbarriM.P.MarteganiF.Del PreteT.LucanteP.G.NataliA.BartolazziGalectin-3 and CD44v6 isoforms in the preperative evaluation of thyroid nodulesJ Clin Oncol17199934943502
- F.MackayH.LoetscherD.StueberG.GehrW.LesslauerTumor Necrosis Factor a (TNFa)-induced cell adhesion to human endothelial cells is under dominant control of one TNF receptor type, TNF-R55J Exp Med177199312771286