Abstract
Background
Due to increasing problems of inadequate and unreliable medical treatments for Cryptosporidium enteritis, alternative therapies are being sought.
Objective
The current study was designed to evaluate the prophylactic and therapeutic efficacy of Allium sativum (garlic) against Cryptosporidium infection in experimentally infected immunocompetent and immunosuppressed mice.
Methods
Forty eight male Swiss albino mice were divided equally into control and experimental groups. Each group was further subdivided into four equal subgroups; two immunosuppressed and two immunocompetent. Cryptosporidial oocysts were isolated from human stools, and were used to infect the mice. The experimental subgroups received garlic orally two days before infection or one day following infection, and continued daily till the end of the study. Two weeks following garlic administration, mice stools were examined for counting the cryptosporidial oocysts, then the animals were sacrificed; their small intestines were processed and were examined for detection of the pathological lesions and for counting of the parasites. Also, myeloperoxidase (MPO) activity was measured in jejunal sections.
Results
The results showed that the infected immunosuppressed subgroups of mice; showed a statistically significant increase in the number of cryptosporidial oocysts in stool and ileal sections, as well as an increase in the MPO activity when compared to the corresponding immunocompetent subgroups. Garlic successfully eradicated the Cryptosporidium oocysts from stool and intestinal sections of the infected immunocompetent subgroup of mice receiving garlic two days before the infection. Besides, the oocysts were significantly reduced in all other infected experimental subgroups in comparison to the corresponding infected control subgroups. The intestinal sections of all subgroups received garlic before or after the infection, revealed a more or less normal architecture. Reduction in the level of MPO activity was also detected in all experimental subgroups.
Conclusion
Our findings suggest that garlic is a convenient prophylactic and a promising therapeutic agent for cryptosporidial infection.
1 Introduction
Allium sativum (A. sativum) or garlic has been used as both food and medicine in many cultures for thousands of years, dating at least as far back as the time that the Giza pyramids were built. It has been recognized not only as a spice but also as a substance which exerts a control on microorganisms.Citation1–Citation3
A. sativum is remarkable for a number of potentially active chemical constituents. It contains seventeen amino acids as arginine, at least 33 organosulphate compounds as aliin and allicin, eight minerals (germanium, calcium, copper, iron, potassium, magnesium, selenium and zinc), enzymes as allinase, and the vitamins A, B1 and C. The physiological activity of dietary A. sativum is attributed to allicin (diallyl thiosulphinate), which is one of the organosulphate compounds found in the bulb. It is responsible for the anti-microbial properties and the characteristic flavor of fresh garlic.Citation2–Citation4
Ancient Egyptians realized the benefits of garlic; its medical and magical powers were described on the walls of ancient temples and on papyri dating to 1500 BC. In recent times, garlic has been shown to have multiple beneficial effects such as antimicrobial, antithrombotic, hypolipidemic, hypoglycemic and antitumor activities.Citation4 Lately, garlic has widely been used to treat intestinal parasites. The antihelminthic effect of garlic has been a matter of interest of researchers. Their results showed that treatment with garlic evoked a significant reduction in the worm load.Citation1,Citation2,Citation5–Citation7 In addition, garlic has been used successfully in a single uncontrolled study in China applied on 20 AIDS patients to treat Cryptosporidium.Citation8 Moreover, garlic compounds were purified and tried as complementary medicine in the management of leishmaniasis.Citation9 Thus, because many of the microorganisms susceptible to garlic extract are medically significant, garlic holds a promising position as a broad-spectrum therapeutic agent.Citation10
Cryptosporidiosis is a parasitic disease caused by Cryptosporidium, a protozoan parasite in the phylum Apicomplexa. Despite not being identified until 1976, it is one of the most common waterborne diseases and is found worldwide. It affects the intestine of mammals and is typically an acute short-term infection. It spreads through the fecal–oral route, often through contaminated water. The parasite is transmitted by environmentally hardy microbial cysts (oocysts) that, once ingested, exist in the small intestine and result in an infection of intestinal epithelial tissue. The main symptom is self-limiting diarrhea in people with intact immune systems. In immunocompromised individuals, such as AIDS patients, the symptoms are particularly in the form of severe dehydration, electrolyte imbalances, malnutrition, wasting, and eventual death. Cryptosporidium is the organism most commonly isolated in HIV positive patients presenting with diarrhea.Citation11–Citation14
Gastrointestinal inflammation caused by the intestinal parasites is often accompanied by functional disturbances, marked changes in the structure and chemical content of the intestine together with biochemical changes as changes in the myeloperoxidase (MPO) activity. MPO is the most abundant enzyme in neutrophils and monocytes; it is the focus of inflammatory pathologies. MPO is believed to be involved in augmenting the cytotoxic activity of H2O2 and O2 to kill a variety of micro-organisms. Thus, in case of intestinal parasitic infection, increase in MPO activity level is an indicator for intestinal inflammation.Citation15,Citation16
There is a history of inadequate and unreliable treatments for Cryptosporidium enteritis. Against this background, certain antiparasitic agents such as paromomycin, nitazoxanide and azithromycin are sometimes used, but they usually have only temporary effects and sometimes relapses happened. Currently, the best approach is to improve the immune status in immunodeficient individuals, for example, by using antiviral therapy in patients with AIDS and supportive treatment for symptoms.Citation17–Citation20
Because of the great need to develop new anti-cryptosporidial agents, trials were designed to test the potency of traditional medicinal plants for treating cryptosporidiosis.
Therefore, the present study aimed at investigating the antiparasitic effectiveness of A. sativum (garlic) as a natural component in the prevention, as well as treatment of Cryptosporidium infections in experimentally infected immunocompetent and immunosuppressed mice.
2 Materials and methods
2.1 Parasites
Stool samples were collected from 20 immunosuppressed patients with chronic diarrhea, in the Alexandria University Hospital and Fever Hospital from Haematology Department and Renal Dialysis Unit, from July to August 2011. Informed consents were obtained from the patients. The stool samples were transferred to the Parasitology Department to be screened by different techniques for the presence of intestinal protozoa. All samples were microscopically screened by direct smear, iodine smear; Sheather's sugar floatation technique and modified Ziehl–Neelsen acid fast stain (MZN), aiming to identify cases of Cryptosporidium ().Citation21 Three out of the 20 examined samples proven to be Cryptosporidium by the conventional diagnostic techniques, were pooled, and were used for the completion of the study. The parasites were isolated by Lumb's technique. In short, each stool sample was mixed with 10 ml of distilled water, and then filtered through coarse sterile gauze. The homogenate was then centrifuged at 2500×g for 5 min. The supernatant fluid was discarded, and the sediment was washed twice in 1 ml of phosphate buffer saline (PBS), with centrifugation at 13,000×g for 2 min. After repeated washing followed by centrifugation, fecal debris was totally eliminated.Citation22 Cryptosporidial oocysts were preserved in 2.5% potassium dichromate solution and stored at 4 °C until use for infection.Citation23 Just before use, the cryptosporidial oocysts were washed three times in distilled water to remove the potassium dichromate, centrifuged at 1500×g for 10 min, and the organisms were counted with a hemocytometer. Suspension containing the required concentration for the infection (104 oocysts/ml) was prepared by dilution of the organism in the appropriate amount of distilled water.Citation24 This study was approved by the Ethics Committee of Alexandria University.
2.2 A. sativum (garlic)
Garlic was administrated to the experimental animals as crude juice. The crude extract was prepared as follows: Fresh garlic bulbs were separated, peeled, and washed with distilled water. After drying, about 500 g of garlic bulbs were crushed in a blender until a uniform consistency was achieved. The resulting paste was diluted with distilled water to obtain a 1 g/ml aqueous solution. Raw garlic juice was aliquoted and was stored at −20 °C until use.Citation3,Citation7,Citation25 Working solution was made from the stock solution by dilution with distilled water. The selected dose for the present work was 50 mg/kg body weight.Citation7
2.3 Experimental animals
Animals used in this work were male Swiss albino mice, aged three to five weeks, weighing 20–25 g. They were housed in well ventilated cages with perforated covers, supplied with standard pellet food and water. Bedding was changed everyday. The mice were allowed to adapt to the laboratory environment for one week before the experiment,Citation26 and their stools were examined by direct wet saline smear, iodine and Sheather's sugar flotation method to exclude the presence of parasites.Citation21 This animal study was approved by the Ethics Committee of Alexandria University.
Forty eight mice were divided into two groups; control group (Group I) and experimental group (Group II). The control group (24 mice) was further subdivided into four equal subgroups (six mice each); normal non infected group (subgroup Ia), immunosuppressed group (subgroup Ib), infected group (subgroup Ic), infected immunosuppressed group (subgroup Id). Each of the previously mentioned subgroups was further subdivided into other two equal subgroups (1 and 2). The experimental group (24 mice) was further subdivided into four equal subgroups (six mice each). The first two subgroups, infected (subgroup IIa) and infected immunosuppressed (subgroup IIb) received garlic two days before the infection, and continued daily for 12 days post infection. The other two subgroups, infected (subgroup IIc) and infected immunosuppressed (subgroup IId) received garlic one day after the infection, and continued daily for two weeks. Thus, the duration and the dose of administration of garlic as prophylaxis and as treatment were equal; 50 mg/kg body weight for two weeks.
Each mouse of the immunosuppressed subgroups was immunosuppressed with cyclophosphamide (endoxan), using two doses of 70 mg/kg intraperitoneally, given a week apart. The last dose was given 48 hours before infection.Citation27
Each infected mouse of subgroup Ic, subgroup Id and Group II was inoculated orally with the isolated Cryptosporidium oocysts at a dose of 104 oocysts/mouse,Citation24 and each mouse of Group II received garlic orally as a crude juice in a dose of 50 mg/kg body weight.Citation7 Ingestion was performed by gastric gavage, using a 23-gauge needle tipped with plastic tubing.Citation7,Citation24
2.4 Evaluation of the garlic efficacy in mice was done by
| • | Survival rate of both immunocompetent and immunosuppressed subgroups of mice. | ||||
| • | Undiluted stool samples from all subgroups of mice were stained by MZN and examined by microscopy to count Cryptosporidium oocysts at the same day of sacrifice in ten fields of oil immersion lens, and the mean counts were considered.Citation21 | ||||
| • | Using over dose of ether, the sacrifice of animals was achieved 12 days post infection for the subgroups Ia1, Ib1, Ic1, Id1, IIa and IIb. On the other hand, 15 days post infection; the subgroups Ia2, Ib2, Ic2, Id2, IIc and IId were sacrificed. Small intestines were dissected, fixed in 10% formalin, embedded in paraffin sections and stained by hematoxylin and eosin stain (H&E). The intestinal sections were examined for the detection of pathological changes and for the counting of the parasites in ten fields of oil immersion lens, and the mean counts were calculated.Citation28 | ||||
| • | Myeloperoxidase activity (MPO activity) was measured in extracts of full thickness sections (200–300 mg) of mouse jejunum in all subgroups of miceCitation29 at the day of sacrifice. Tissue samples were weighed and homogenized with hexadecyltrimethylammonium bromide (HTAB) buffer (0.5% HTAB in 50 mM phosphate buffer, pH 6.0, 4 °C). The homogenates were freeze-thawed three times and then centrifuged at 35,000g for 30 min. The pellets were discarded, and the supernatants were assayed for soluble protein and for MPO activity. MPO activity was measured by adding 0.1 ml supernatant to 2.9 mol reaction buffer (50 mM phosphate buffer, pH 6.0, containing 0.167 mg/ml o-dianisidine hydrochloride and 0.0005% hydrogen peroxide). After 1 min, the change in absorbency at 460 nm was measured by the spectrophotometer. One unit of MPO activity was defined as that degrading 1 μmol of peroxidase per minute at 25 °C. The MPO activity was expressed per milligram of protein. Soluble protein in the tissue supernatant was assayed using Lowry's method.Citation30 | ||||
2.5 Calculations
Mean and standard deviation were calculated for Cryptosporidium oocysts in stool samples and intestinal sections, and for the intestinal MPO activity. Furthermore, t-test was applied in order to compare between each experimental subgroup receiving garlic and its corresponding control subgroup. In addition, t-test was used to compare between the immunocompetent and the immunosuppressed subgroups of mice (P values less than 0.05 were considered significant and less than 0.001 were considered highly significant), according to Knapp and Miller (1992).Citation31
3 Results
3.1 Survival rate of mice
The number of dead animals were recorded throughout the study and revealed that, only one immunosuppressed mouse of subgroup Id (infected immunosuppressed group) and one immunocompetent mouse of the subgroup IIc (experimentally infected mice by Cryptosporidium receiving garlic one day following the infection) got death. Besides, all other mice were still alive till the end of the study. So, the survival rate recorded in this study was 95.8% for both immunocompetent and immunosuppressed groups of mice.
3.2 Fecal count of the parasites
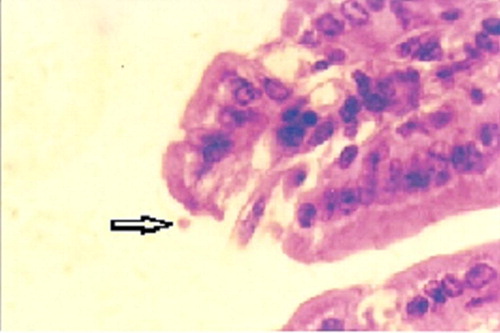
In the control group infected by Cryptosporidium, the oocysts were best visualized by MZN stain as spherical pink organisms, 4–6 μm in diameter () with a mean number of (4.74 ± 5.19) in subgroup Ic1, (6.25 ± 2.62) in subgroup Id1, (4.93 ± 4.07) in subgroup Ic2 and (6.88 ± 7.67) in subgroup Id2 ().
Table 1 Mean fecal count of Cryptosporidium oocysts in mice given garlic before and after the infection.
Garlic successfully eradicated the Cryptosporidium oocysts from the stools of the infected subgroup of mice (subgroup IIa) receiving garlic two days before the infection. Besides, the oocysts were greatly diminished in all other experimental subgroups; in subgroup IIb, subgroup IIc and subgroup IId, with a mean number of 1.13 ± 2.08, 1.77 ± 1.13 and 2.15 ± 4.71 respectively. There was a significant decrease in the number of cryptosporidial oocysts in all experimental subgroups as compared to the corresponding infected control subgroups. However, there was a statistically significant increase in the number of cryptosporidial oocysts in the stool samples of infected immunosuppressed subgroups of mice (Id1, Id2, IIb and IId) as compared to the corresponding immunocompetent subgroups (Ic1, Ic2, IIa and IIc) (). No parasites have been detected in the stool samples of subgroups Ia1 and Ia2 (normal) or subgroups Ib1 and Ib2 (immunosuppressed).
3.3 Parasite counts and histopathological changes in small intestinal sections
Following sacrifice of mice, the ileal sections of the Cryptosporidium infected control subgroup (Ic) and the Cryptosporidium infected immunosuppressed subgroup (Id) stained with H&E, revealed the presence of large numbers of Cryptosporidium oocysts on the luminal surface of the epithelium lining the villi, as small rounded organisms (), with a mean count of 5.83 ± 2.28 for subgroup Ic1, 7.14 ± 6.01 for subgroup Id1, 5.51 ± 6.15 for subgroup Ic2 and 6.92 ± 2.74 for subgroup Id2 (). The infected sections showed altered mucosal architecture, with shortening, blunting and widening of the intestinal villi ().
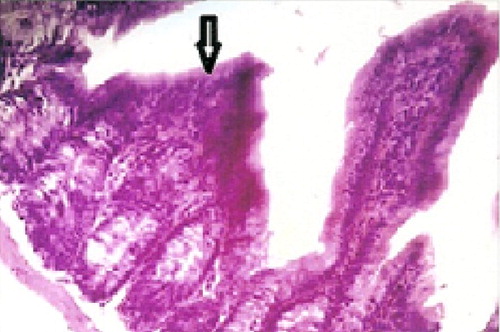
Table 2 Mean count of Cryptosporidium oocysts in the intestinal sections of mice given garlic before and after the infection.
The parasite was completely eradicated from the intestine of subgroup IIa. However, ileal sections of mice of subgroups IIb, IIc and IId showed reduced number of Cryptosporidium oocysts to 1.76 ± 2.12, 1.89 ± 21.4 and 2.53 ± 3.18 respectively. The differences between the experimental and the corresponding infected control subgroups were statistically significant. Besides, there was a statistically significant increase in the number of cryptosporidial oocysts in the ileal sections of infected immunosuppressed subgroups of mice (Id1, Id2, IIb and IId) as compared to the corresponding immunocompetent subgroups (Ic1, Ic2, IIa and IIc) (). It was noticed that the intestinal sections of all subgroups received garlic before or after the Cryptosporidium infection, revealed a more or less normal architecture. No parasites and no pathological changes have been detected in the intestinal sections of subgroups Ia1 and Ia2 (normal) or subgroups Ib1 and Ib2 (immunosuppressed).
The intestinal MPO activity showed a statistically significant increase at the day of sacrifice in the subgroups Ic1, Ic2, Id1 and Id2 in comparison to its level in Ia1, Ia2, Ib1 and Ib2, which indicates the presence of intestinal inflammation. In subgroups IIa and IIb, there was a statistically significant drop in its level when compared to subgroups Ic1 and Id1. Furthermore, subgroups IIc and IId showed a statistically significant decrease when compared with the corresponding control subgroups; Ic2 and Id2. In contrast the MPO activity showed a statistically significant increase in immunosuppressed infected mice (Id1, Id2, IIb and IId) when compared with the corresponding immunocompetent subgroups (Ic1, Ic2, IIa and IIc respectively) ().
Table 3 Level of myeloperoxidase activity in μ/mg protein in intestinal sections of different subgroups of mice.
4 Discussion
Explosive, chronic, fatal, non bloody diarrhea is considered a very serious management problem in immunosuppressed patients as well as in normal persons in both developed and developing countries. Pathogenic intestinal protozoa represent the main causes of this diarrhea, among which Cryptosporidium produces regularly occurring outbreaks throughout the world.Citation32,Citation33 Most of the immunodeficient patients got failure of the available drugs used for the treatment, besides the multiple adverse effects that they produced.Citation34 Thus, new drugs against this parasite became consequently urgently needed.
The search for bioactive plants which can be used as non-conventional anti-parasitic treatment has received considerable attention in recent times because of the increasing worldwide development of resistance to chemical drugs in parasitic populations. However, scientific evidence to validate the use of plants remains limited.Citation35 Thus, this study was oriented to evaluate the protective and curative capacity of garlic against Cryptosporidium in experimental mice.
In this study, the allicin, which has antimicrobial effect, was obtained from crushed fresh garlic bulbs as mentioned by Ankari and Mirelman (1999), Sasaki et al. (1999) and Lemar et al. (2002).Citation36–Citation38
The dose selected for the present work was 50 mg/kg body weight.Citation7 Riad et al. (2009) supposed that this dose is equivalent to the daily amount of garlic recommended for an average human to maintain good health (∼4 g).Citation7
In this study, immunosuppression of experimental animals was induced by cyclophosphamide (endoxan) at two doses of 70 mg/kg each. The choice of this drug was depending on its capability for immunosuppression of both humoral and cell-mediated immune mechanisms.Citation27,Citation39 The recorded survival rate of all groups of mice in this work was 95.8%. This high percentage was coincided with Camenga et al. (1974),Citation39 who suggested that the intraperitoneal injection of cyclophosphamide at the given dose in this study produced no or minimal deaths in normal bald mice. The cause of death of the immunosuppressed mouse of subgroup Id was certainly related to the immunosuppression situation. In contrast the cause of death of the immunocompetent mouse of the subgroup IIc is still not apparent and may be referred to animal housing.
In the present study, light microscopic examination of infected intestinal sections by Cryptosporidium revealed the presence of altered mucosal architecture, with shortening, blunting and widening of the intestinal villi. Connor et al. (1993)Citation40 demonstrated the same structural abnormalities in the villi.
In this work, garlic successfully eradicated the Cryptosporidium oocysts from stool and intestinal sections of the infected immunocompetent subgroup of mice (subgroup IIa) receiving garlic two days before the infection and continued for two weeks, and it is associated with normal intestinal architecture. Besides, the cryptosporidial oocysts were greatly diminished in all other subgroups. This impressive effect of garlic was similarly obtained in the in vitro study on the cestode, Hymenolepis nana using garlic extract, in which lethal effects had been shown on the worms (Soffar and Mokhtar, 1991).Citation1 Moreover, Fareed et al. (1996),Citation8 demonstrated that when garlic was used for the treatment of HIV patients with chronic diarrhea and confirmed cryptosporidiosis, complete remission occurred in some patients and partial remission occurred in the others. In addition, the strong prophylactic effect of garlic when it was given before the infection could be supported by Riad et al. (2009).Citation7 They demonstrated that treating mice with garlic before schistosomal infection evoked a highly significant reduction in the mean worm count as compared to the mice giving garlic post-infection. Moreover, garlic efficacy was the highest in the group treated with garlic before and after bilharzial infection.
In this study, the intestinal MPO activity showed statistically significant increase at the day of sacrifice in both infected immunocompetent and infected immunosuppressed subgroups (Ic1, Ic2, Id1 and Id2) in comparison to the corresponding non infected control subgroups (Ia1, Ia2, Ib1 and Ib2) which indicate intestinal inflammation. This is also reported by Venkova et al. (2000) and Khan et al. (2002),Citation16,Citation41 who suggested that the MPO activity is a reliable index of inflammation intensity in mucosa, submucosa and smooth muscle tissues. This increase in MPO activity is coincided with the pathological changes detected in the intestinal villi. On the other hand, there was a statistically significant drop in the level of MPO activity in subgroups receiving garlic before the infection (IIa and IIb), and a further statistically significant drop in treated subgroups by garlic after the infection (IIc and IId), when compared to the corresponding control subgroups, which indicate remission of the intestinal inflammation. This agreed with the amelioration of the intestinal pathology detected in all experimentally infected subgroups.
The infected immunosuppressed subgroups of mice; showed a statistically significant increase in the number of cryptosporidial oocysts in stool and ileal sections, as well as an increase in the MPO activity when compared to the corresponding immunocompetent subgroups. This result could be attributed to the immunodeficient situation that flared up the infection.
The efficacy of garlic in the prophylaxis and treatment of experimental cryptosporidiosis could be explained by different mechanisms. Adetumbi et al. (1986)Citation42 suggested that the blockage of lipid synthesis may be an important component of the antimicrobial activity of garlic. Moreover, Kyo et al. (1997), Sutton and Haik (1999) and El Shenaway et al. (2008),Citation6,Citation43,Citation44 reported the enhancement of phagocytosis and an increase in natural killer cell activity which promote the immune system function, and strengthen the body's defense mechanism during the duration of treatment by garlic. Furthermore, Masamha et al. (2010),Citation3 reported that A. sativum, disrupts the normal physiological functions of the parasite like mobility, food absorption, and reproduction.
Garlic has been consumed for several thousand years without any adverse long-term effects, suggesting that modest quantities of garlic (two to three chewed fresh cloves of garlic, or one tablespoon of garlic oil daily) produce no risks to normal individuals. However, some undesirable effects could associate the high dose of garlic. Garty (1993),Citation45 stated that consuming too much garlic can result in halitosis, stomachache, allergic reactions, and if garlic is applied externally, a burning sensation of the skin may occur. Also, Fareed et al. (1996),Citation8 reported that the major side effect was a strong garlic smell and taste. Studies have shown that sipping milk at the same time as consuming garlic can significantly neutralize bad breath. Also, if garlic is taken in a hot water with honey, it will be a bit more palatable.Citation46
Based on our results, it can be concluded that garlic has good efficacy as a prophylactic and a promising therapeutic agent against Cryptosporidium and therefore validates the traditional use of the plant in parasitic infections. It is recommended that further investigations be carried out on the applications of garlic as a complementary medicine in the management of cryptosporidiosis.
5 Source of support
Financial support by Parasitology Department, Faculty of Medicine, Alexandria University, Alexandria, Egypt.
Acknowledgements
I appreciate the technical assistance provided by Assistant Professor Iman Diab for the Biochemistry work which was supported by the Biochemistry Department, Faculty of Medicine, Alexandria University.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 18 January 2012
References
- S.A.SoffarG.M.MokhtarEvaluation of the antiparasitic effect of aqueous garlic (Allium sativum) extract in hymenolepiasis nana and giardiasisJ Egypt Soc Parasitol211991497502
- E.AyazI.TürelA.GülO.YilmazEvaluation of the antihelmintic activity of garlic (Allium sativum) in mice naturally infected with Aspiculuris tetrapteraRec Pat Anti-Inf Drug Discov32008149152
- B.MasamhaC.T.GadzirayiI.MukutirwaEfficacy of Allium sativum (garlic) in controlling nematode parasites in sheepInter J Appl Res Vet Med82010161169
- M.ThomsonM.AliGarlic [Allium sativum]: a review of its potential use as an anti-cancer agentCurr Cancer Drug Targ320036781
- E.H.Abdel-RahmanO.M.KandilK.N.Abdel MegeedComparative studies of lethal effects of Bacillus thuringiensis, Allium sativum and Nerium oleander on Trichostrongylidae parasitesEgypt J Zool3019986579
- G.A.SuttonR.HaikEfficacy of garlic as an anthelminthic in donkeysIsrael J Vet Med5419996678
- N.H.A.RiadH.A.TahaY.I.MahmoudEffects of garlic on albino mice experimentally infected with Schistosoma mansoni: A parasitological and ultrastructural studyTrop Biomed2620094050
- G.FareedM.ScolaroW.JordanN.SandersC.ChessonM.SlatteryThe use of a high-dose garlic preparation for the treatment of Cryptosporidium parvum diarrheaInt Conf AIDS111996288
- B.W.WabwobaC.O.AnjiliM.M.NgeiywaP.K.NgureE.M.KigonduJ.IngongaExperimental chemotherapy with Allium sativum (Liliaceae) methanolic extract in rodents infected with Leishmania major and Leishmania donovaniJ Vector Borne Dis472010160167
- M.A.AdetumbiB.H.LauAllium sativum (garlic) – a natural antibioticMed Hypoth121983227237
- R.W.GoodgameR.M.GentaA.C.WhiteC.ChappellIntensity of infection in AIDS-associated cryptosporidiosisJ Inf Dis1671993704709
- R.FayerC.A.SpeerJ.P.DubeyThe general biology of CryptosporidiumR.FayerCryptosporidium and Cryptosporidiosis1997CRC PressBoca Raton, FL142
- T.K.GraczykR.FayerR.KnightB.Mhangami-RuwendeJ.M.TroutA.J.Da SilvaMechanical transport and transmission of Cryptosporidium parvum oocysts by wild filth fliesAm J Trop Med Hyg632000178183
- P.R.MurrayK.S.RosenthalM.A.PfallerMedical Microbiology5th ed.2005Elsevier Inc.Philadelphia
- M.DhimanJ.G.Estarda-FrancoJ.M.PandoF.J.Ramirez-AguilarH.SparttS.Vazquez-CorzoIncreased myeloperoxidase activity and protein nitration are indicators of inflammation in patients with chagas’ diseaseClin Vaccine Immunol162009660666
- W.I.KhanP.A.BlennerhassetA.K.VargheseS.K.ChowdhuryP.OmstedY.DengIntestinal nematode infection ameliorates experimental colitis in miceInfect Immun70200259315937
- H.V.SmithG.D.CorcoranNew drugs and treatment for cryptosporidiosisCurr Opin Infect Dis172004557564
- A.R.MeamarM.RezaianS.RezaieCryptosporidium parvum bovine genotype oocysts in the respiratory samples of an AIDS patient: efficacy of treatment with a combination of azithromycin and paromomycinParasitol Res982006593595
- B.D.KirkpatrickC.L.SearsCryptosporidiosisL.GoldmanD.AusielloCecil Medicine23rd ed.2007Saunders ElsevierPhiladelphia, PA371
- G.GargalaDrug treatment and novel drug target against CryptosporidiumParasite152008275281
- L.S.GarciaD.A.BrucknerMacroscopic and microscopic examination of fecal specimensDiagnostic Medical Parasitology3rd ed.1997AMS pressWashington D.C.608649
- R.LumbJ.SwiftC.JamesK.PapanaoumT.Mukh-erjeeIdentification of the microsporidian parasite, Enterocytozoon bieneusi in faecal samples and intestinal bio-psies from an AIDS patientInt J Parasitol231993793801
- A.M.KhalifaM.M.El TemsahyI.F.Abou El NagaEffect of ozone on the viability of some protozoa in drinking waterJ Egypt Soc Parasitol312001603616
- M.R.GaafarEffect of solar disinfection on viability of intestinal protozoa in drinking waterJ Egypt Soc Parasitol3720076586
- J.M.BurkeA.WellsP.CaseyJ.E.MillerGarlic and papaya lack control over gastrointestinal nematodes in goats and lambsVet Parasitol1592009171174
- Y.El-FakhryA.AchbarouI.DesportesD.MazierEncephalitozoon intestinalis: Humoral responses in interferon-y receptor knockout mice infected with a Microsporidium pathogenic in AIDS patientsExp Parasitol891998113121
- D.SherwoodK.W.AngusD.R.SnodgrassS.TziporiExperimental cryptosporidiosis in laboratory miceInfect Immun381982471475
- R.A.B.DruryE.A.WallingtonCarleton's Histological Technique5th ed.1980Oxford University PressOxford, New York, Toronto
- B.GreenwoodJ.M.PalmerNeural integration of jejunal motility and ion transport in nematode-infected ferretsAm J Physiol2711996G48G55
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem1931951265275
- R.G.KnappM.C.MillerClinical epidemiology and biostatistics1992Williams and WilkinsBaltimore
- P.S.MaCryptosporidiosis, isosporiosis and microsporidiosisInt Conf AIDS51989192
- M.J.G.FarthingTreatment options for the eradication of intestinal protozoaNat Clin Pract Gastrenterol Hepatol32006436445
- Y.M.MiaoB.G.GazzardReviews in opportunistic infectious management of protozoal diarrhea in HIV diseaseHIV Med12000194199
- Hoste H, Torres-Acosta JF, Alonso-Diaz MA, Brunet S, Sandoval-Castro C, Adote SH. Identification and validation of bioactive plants for the control of gastrointestinal nematodes in small ruminants. Proceedings of 5th International Workshop: Novel Approaches to the Control of Helminth Parasites of Livestock 2008: 56–72.
- S.AnkariD.MirelmanAntimicrobial properties of allicin from garlicMicrob Infect11999125129
- J.SasakiT.KitaK.IshitaH.UchisawaH.MatsueAntibacterial activity of garlic powder against Escherichia coli O-157J Nutr Sci Vitaminol (Tokyo)451999785790
- K.M.LemarM.P.TurnerD.LioydGarlic (Allium sativum) as an anti-Candida agent: a comparison of the efficacy of fresh garlic and freeze-dried extractsJ Appl Microbiol932002398405
- D.L.CamengaN.NathansonG.A.ColeCyclophosphamide-potentiated West Nile viral encephalitis: relative influence of cellular and humoral factorsJ Infect Dis1301974634641
- B.A.ConnorD.R.ShlimJ.V.ScholesJ.L.RayburnJ.ReidyR.RajahPathological changes in the small bowel in nine patients with diarrhea associated with coccidian like bodyAnn Intern Med1191993377382
- K.VenkovaS.T.DunnA.M.AdesinaB.GreenwoodNeuromuscular dysfunction in the jejunum and colon of human leukocyte antigen B27 transgenic ratsAm J Physiol29320006066
- M.AdetumbiG.T.JavorB.H.LauAllium sativum (garlic) inhibits lipid synthesis by Candida albicansAntimicrob Agents Chemoth301986499501
- E.KyoN.UdaM.KakimotoK.YokoyamaM.UshijimaI.SumiokaAnti-allergic effects of aged garlic extractPhytomedicine41997335340
- N.S.El ShenawyM.F.SolimanS.I.ReyadThe effect of antioxidant properties of aqueous garlic extract and Nigella sativa as anti-schistosomiasis agents in miceRev Inst Med Trop São Paulo5020082936
- B.Z.GartyGarlic burnsPediatrics911993658659
- A.T.FleischauerL.ArabGarlic and cancer: a critical review of the epidemiologic literatureJ. Nutr13120011032S1040S