?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim
The aim of the current study is to detect CTCs in the blood of breast cancer females by the expression of Mammoglobin and Mucin-1 to evaluate their potential as diagnostic and prognostic markers of breast cancer and predictors of metastasis.
Subjects and methods
The study involved 50 patients and thirty controls. Fifty patients recently diagnosed with breast cancer were proved by fine needle aspiration cytology or core biopsy, all the patients were operated upon by modified radical mastectomy under general anesthesia after complete preoperative evaluation. From each subject a blood sample was drawn before surgery, 2 weeks after surgery and after 6 cycles of chemotherapy. Mononuclear cells were separated from 7.5 ml blood using a phicoll gradient. From these cells mRNA was extracted, and RT-PCR was carried out to detect Mammoglobin and Mucin-1 gene expression from CTCs. Also CA 15.3 was measured in all the samples.
Results
CTCs were detected in more than 80% of primary breast cancer patients at presentation, that percent decreased after surgery and after adjuvant chemotherapy. CTCs detection after surgery and after chemotherapy was significantly correlated with tumor size, grad, vascular invasion and metastasis. CTCs posed an almost two-fold risk of metastasis and significantly lower DFS time in positive than in negative patients. Detection of CTCs in peripheral blood after chemotherapy successfully predicted subsequent metastasis.
Conclusion
The hematogenous spread of tumor cells in patients with breast cancer may be an early phenomenon occurring before/apart from regional lymph nodes involvement. CTCs detection by RT-PCR could add to the initial evaluation of primary breast cancer patients. Also, adjuvant chemotherapy might eliminate or decrease occult tumor cells. But CTCs evaluation after adjuvant chemotherapy could predict metastasis and the presence of CTCs after chemotherapy reflect a higher potential for subsequent metastasis.
1 Introduction
Breast cancer is a major public health problem for women throughout the world. It is the most frequent cancer in women and the second leading cause of death.Citation1 In Alexandria, Egypt, breast cancer incidence was estimated to be 41.2% of all malignancies in females in 2007.Citation2
Some of the key decisions in the current management of primary breast cancer involve the need for prognostication; which is especially important in identifying patients whose prognosis is so favorable or patients whose prognosis is so poor with conventional treatment as to warrant consideration of more aggressive investigational therapies. The most useful and consistent of these prognostic factors are the number of positive axillary nodes, tumor size, poor differentiation, vascular invasion and estrogen and progesterone receptors.Citation3 In addition, breast cancer is a leading cause of mortality; primarily due to the failure of effective clinical detection and the treatment of metastatic diseases. Up to 30% of patients still succumb to the disease within the ten-year period following diagnosis.Citation4,Citation5 It remains difficult to accurately predict which patients will develop metastatic disease and over what time scale. The metastatic process is comprised of a series of sequential steps, including dissemination of cancer cells from the primary tumor into the blood stream (intravasation), survival in circulation, arrest and extravasation in a secondary site and initiation and maintenance of growth to form clinically detectable masses. Cancer cells must successfully complete all steps to form a metastatic tumor.Citation6–Citation8
Given the multistep nature of metastatic cascade, there should be several opportunities for early identification and therapeutic targeting of metastatic cells before they become a clinical problem. In fact, there is growing evidence that the presence of circulating tumor cells (CTCs) in the blood may be an important indicator of the potential for metastatic disease and poor prognosis.Citation9,Citation10
The ability to consistently detect, track and characterize rare CTCs in cancer patients holds tremendous promise in terms of identifying the potential for metastatic disease at very early stages, managing risk stratification in the adjuvant setting, monitoring response to treatment and monitoring disease recurrence. Identification of CTCs in blood depends on exploiting phenotypic differences between epithelial tumor cells and cells of hematopoietic origin using either tumor-type-specific markers or epithelial-specific markers.Citation10
The aim of the current study is to detect CTCs in the blood of breast cancer females by the expression of Mammoglobin and Mucin-1 to evaluate their potential as diagnostic and prognostic markers of breast cancer, and predictors of metastasis. In a preliminary study on 30 breast cancer patients that was carried out in our lab, the results were inclusive, so, this study was carried out with a larger cohort hoping to reach more conclusive results.
2 Subjects and methods
2.1 Subjects
The study was approved by the Ethics Committee and by the Institutional Review Board of the Medical Research Institute, Alexandria University, Egypt. All samples were collected with the patients’ informed written consent, and confidentiality of data was insured at all stages of the study.
For this prospective cohort study, fifty females recently diagnosed with breast cancer were randomly recruited from the Experimental and Clinical Surgery Department and Cancer Management and Research Department, Medical Research Institute, Alexandria University, during the period from September 2007 till October 2008. All patients were premenopausal females, diagnosed with primary breast cancer, as indicated by fine-needle biopsy or core biopsy. Eligibility criteria included confirmed diagnosis of breast cancer, no metastasis at the time of recruitment, no previous treatment, no previous malignancies and good cardiac, liver and kidney function. All patients were subjected to complete physical examination and a complete diagnostic evaluation to exclude the presence of distant metastases, consisting of chest X-rays, ultrasound of the liver, and a whole-body bone scan. Computed tomography scans and/or magnetic resonance imaging studies were performed if clinically indicated.
An informed written consent was taken from each patient. All patients were treated primarily with Modified Radical Mastectomy,Citation11 followed adjuvant FAC-based combination chemotherapy (6 cycles each one consisted of 5-flourouracil 500 mg/m2, adriamycin 50 mg/m2 and cyclophosphamide 500 mg/m2, given every 21 days). Patients who were positive for estrogen and progesterone receptors received hormonal therapy (Nolvadex tablets 10 mg, 2 tablets/day for 5 years) after chemotherapy.Citation12 Patients were clinically followed up for 48 months after completing chemotherapy.
In addition, thirty normal, healthy volunteer females of matched age as the patients group, were included as a control group.
2.2 Sampling
From each subject 10-ml venous blood samples were collected, once from control subjects and 3 times from group 1 patients (before surgery, 2 weeks after surgery and after 6 cycles of chemotherapy). 7.5 ml was processed using gradient centrifugation by Ficol-Paque Plus (Biochrom AG, Berlin, Germany) to obtain the buffy layer that would contain CTCs, if present, with other white blood cells. Cells were washed twice by PBS and then pelleted and stored. The remaining 2.5 ml of blood was left to clot then centrifuged to obtain serum. All cells and serum samples were stored at −80 °C until they were used.
2.3 RNA extraction
Total RNA was extracted from all cell samples using the SV Total RNA Isolation System (Promega Corporation, Madison, USA), the procedure was carried according to the manufacturer's instructions. Cells were thawed in lysis buffer, then diluted and centrifuged at 12,000–14,000×g for 10 min to remove the cell debris. RNA was precipitated from the cleared lysate using 95% ethanol and separated through spin columns, where RNA is washed and treated by DNase and washed again before it was eluted by 100 μl of nuclease-free water.
The amount of total RNA extracted was assessed spectrophotometrically using NanoDrop® ND-1000 UV–Vis Spectrophotometer to measure the optical density at 230, 260 and 280 nm. All samples included in the study had A260/A280 ratio ranging from 1.7 to 2.1.
2.4 RT-PCR for Mammoglobin and Mucin-1
Reverse transcription was carried out using Reverse Transcription System (Promega, Madison, USA) that used AMV Reverse Transcriptase and oligo (dT)15 primer to synthesize single-stranded cDNA from total RNA according to the manufacturer's instructions.
PCR was carried out using Go Taq®Green Master Mix (Promega Corporation, Madison, USA). Each PCR reaction mixture consisted of 12.5 μl PCR master mix; 1 μl of each amplification primer (400 pmol/μl) and 1 μg cDNA and the volume was brought to 25 μl by adding deionized water. The primers used for the amplifications were as follows; Mammoglobin F-5′-CGGATGAAACTCTGAGCAATGT and R-5′-CTGCAGTTCTGTGAGCCAAAG to produce a 108 bp fragment, Mucin-1 F-5′-ACTACTACCAAGAGCTG and R-5′-CTCATAGGATGGTAGGT to produce a 238 bp fragment, and β-actin as a house-keeping gene F-5′-ATGCCATCCTGCGTCTGGACCTGGC and R-5′-AGCATTTGCGGTGCGACATGGAGGG to produce a 607 bp fragment. Thermal cycling started by a first denaturation step of 4 min at 95 °C, followed by 40 cycles of 94 °C for 30 s, 64 °C for 30 s and 72 °C for 60 s and a final extention at 72 °C for 10 min. PCR products were then separated by gel electrophoresis, stained by ethedium bromide, visualized by UV and photographed.
2.5 Measurement of CA 15.3 by IRMA
CA 15.3 was measured in all serum samples collected by Immunoradiometric Assay (IRMA) using a commercially available kit (DIAsource ImmunoAssays S.A., Nivelles, Belgium). According to maneufacturer's instructions, 20 μl of each sample was diluted with 500 μl diluent and 50 μl of diluted sample or calibrator was dispensed into antibody-coated tubes. The tubes were aspirated and washed twice, then 50 μl of 125I-labeled anti-CA 15.3 was added to each tube, after incubated for 90 min/RT on shaker, tubes were aspirated and washed twice, and counted on a Gamma Counter. Sample concentrations were determined by interpolation from the standard curve.
2.6 Statistical analysis
Statistical analysis was performed using the SPSS (Statistical Package for the Social Sciences) software version 18.0 (SPSS Inc., Chicago, IL, USA).
2.6.1 Variables manipulation
The CA 15.3 was converted from a quantitative variable to a dichotomous variable using its median value among the controls as the cut off level criterion. Estrogen receptor (ER), progesterone receptor (PR), Mammoglobin, and Mucin-1 were also converted to dichotomous variables either negative or positive by combining several grades of positivity into positive.
2.6.2 Data analysis
For variables’ description, the percent was used for qualitative variables and the mean with the standard deviation for quantitative normally distributed variables, while the not normally distributed variables were described by the median and range. Testing the changes in percentage positives for the biochemical parameters at different times; before surgery, after 2 weeks of surgery and after 6 cycles of treatment was done using the Cochran's Q test. The relative risk with its 95% confidence interval was used to assess the risk of metastasis among those whose biochemical parameters were positive relative to those whose biochemical parameters were negative. Relative risk of value 1 indicates no risk and relative risk more than 1 indicates an increased risk. To be significant, the 95% confidence interval should not include 1.0. The cumulative proportion metastasis free surviving through the follow up duration was illustrated via the Kaplan–Meier survival curve, using the mean survival time and its 95% confidence interval as descriptive for the different survival times. Comparisons between the different survival distributions were done using the logrank test. The prognostic accuracy for the biochemical parameters before surgery, 2 weeks after surgery and 6 cycles after treatment was demonstrated by the different Receiver Operating Characteristic (ROC) Curves, the areas under the curves (AUCs) were indicators for the accuracy of the biochemical parameter. For the AUC to be significant its 95% lower confidence interval should be above .50. All tests were 2 sided and alpha was set at 0.05.
3 Results
3.1 Description of the study sample
A cohort of fifty breast cancer females with a mean age and standard deviation of 45 ± 4.6 years, the mean tumor size was 3.2 ± 1.7 cm. Half the cohort (50%) had moderately differentiated tumor while 48% had poorly differentiated tumor. The median number of lymph nodes metastasis was 2 ranging from 0 to 15. More than half the sample (58.0%) had vascular invasion and the majority were either stage 2 (56%) or stage 3 (40%). Positive estrogen receptor and positive progesterone receptor were each present in 84% of the cohort ().
Table 1 Description of the females cancer breast patients by clinic pathological parameters (n = 50).
3.2 Changes in Mammoglobin and Mucin-1 expression and CA 15.3 levels at different study points
represents the gel electrophoresis of the RT-PCR products showing the 108 bp band of Mammoglobin, 238 bp band of Mucin-1 and the 607 bp band of the house keeping gene β-actin. For Mammoglobin, 80% showed positive expression before surgery, 60% after surgery while 54% after treatment. These differences were statistically significant (Cochran's Q = 14.70, p = 0.001). Similarly, Mucin-1 expression was positive in 86% before surgery, in 84% after surgery, while in 54% after treatment. These differences were also statistically significant (Cochran's Q = 22.21, p < 0.001). Regarding CA 15.3, a reversed pattern was observed, where the positive percent was 20% before surgery, 24% after surgery, while 44% after treatment. These differences were also statistically significant (Cochran's Q = 13.78, p = 0.001 ().
Figure 1 Gel electrophoresis of RT-PCR products of Mammoglobin (108 bp), Mucin-1 (238 bp) and β-actin (607 bp).
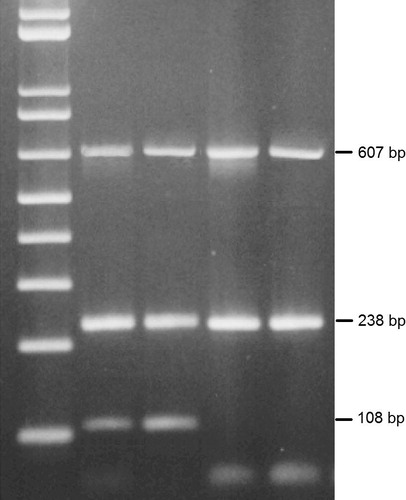
Table 2 Changes in the percentage expression of all parameters; before surgery, 2 weeks after surgery, & after 6 cycles of treatment.
3.3 Diagnostic potential of Mammoglobin, Mucin-1 and CA 15.3 for breast cancer
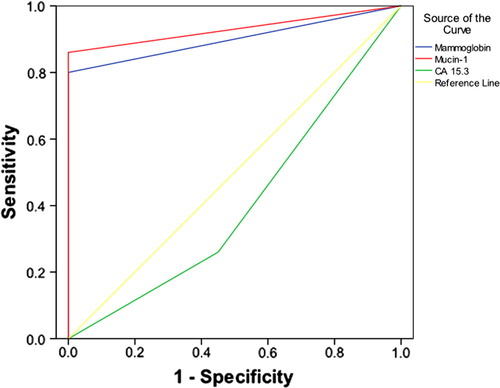
Expression of Mammoglobin and Mucin-1 before surgery proved a statistically significant diagnostic accuracy for breast cancer (p = 0.000 and 0.000, respectively) (). Mammoglobin had an AUC = 0.90, 95% at CI: 0.829, 0.971, with a corresponding sensitivity of 80% and specificity of 100%. While Mucin-1 had an AUC = 0.93, 95% at CI: 0.870, 0.990, with a corresponding sensitivity of 86% and specificity of 100%. While CA 15.3 was not of any diagnostic significance with an AUC = 0.263, 95% CI: 0.142, 0.384, the best cut off point was 12.5 ng/ml with a sensitivity of 42% and a specificity of 22%.
Figure 2 ROC curve of Mammoglobin, Mucin 1 and CA 15.3 as diagnostic markers for breast cancer before surgery.
Before surgery, neither Mammoglobin nor Mucin-1 expression showed a significant correlation with any of the clinical parameters involved in breast cancer. While after surgery and after chemotherapy, Mammoglobin and Mucin-1 were significantly correlated with vascular invasion, stage and metastasis. Only Mucin-1 correlated with tumor size and only Mammoglobin correlated with lymph node metastasis (). Other parameters including age, ER, PR and grade were not correlated with either Mammoglobin or Mucin-1 at any time. Mammoglobin and Mucin-1 were correlated with each other at all times. CA 15.3 did not show any correlation with any of the clinical parameters evaluated at any time.
Table 3 Correlations between Mammoglobin and Mucin-1, 2 weeks after surgery and after 6 cycles of chemotherapy with various clinical parameters.
3.4 The relative risks for metastasis estimated at different study points
Before surgery, the relative risk of metastasis was not significantly higher in Mammoglobin positive (RR = 1.6, 95% CI: 1.03, 2.48), Mucin-1 positive (RR = 1.3, 95% CI: .77, 2.30) and CA 15.3 positive (RR = 1.1, 95% CI: 0.59, 2.26). After 2 weeks of surgery, the relative risk of metastasis was significantly higher only in Mucin-1 positive (RR = 2.1, 95% CI: 1.52, 2.88) but no significant risk was detected either in Mammoglobin positive or in CA 15.3 positive (). After 6 cycles of treatment, the relative risk of metastasis was significantly higher both in Mammoglobin positive relative to Mammoglobin negative (RR = 1.8, 95% CI: 1.10, 3.04), and in Mucin-1 positive relative to Mucin-1 negative (RR = 1.8, 95% CI: 1.03, 2.79) meanwhile no significant risk was observed in CA 15.3 positive (RR = 1.7, 95% CI: 0.94, 2.91) ().
Table 4 The relative risk for breast cancer metastasis according to the biochemical parameters, 2 weeks after surgery.
Table 5 The relative risk for breast cancer metastasis according to the biochemical parameters, after 6 cycles of treatment.
3.5 Metastasis free survival based on Mammoglobin and Mucin-1 expression and CA 15.3 levels
About 46% of breast cancer patients developed metastasis by the end of follow up duration. The mean metastasis free survival time was 32.9 months (95% CI: 27.9, 37.9). Before surgery; the mean metastasis free survival time was 42.6 months (95% CI: 34.7, 50.5) in Mammoglobin negative compared to 30.4 months (95% CI: 24.7, 36.1) in Mammoglobin positive, though this apparent difference, the survival distributions were not statistically significantly different (logrank = 2.59, p = 0.108). For Mucin-1, the mean metastasis free survival time was 40.3 months (95% CI: 29.5, 51.1) in Mucin-1 negative compared to 31.7 months (95% CI: 26.2, 37.1) in Mucin-1 positive, but no significant difference was detected between both survival distributions (logrank = 0.74, p = 0.390). As regards the CA 15.3, the mean metastasis free survival time was 33.8 months, (95% CI: 28.4, 39.3) in CA 15.3 negative compared to 29 months, (95% CI: 17.2, 40.8) in CA 15.3 positive, but without statistical significant difference in the survival distributions (logrank = 0.26, p = 0.608).
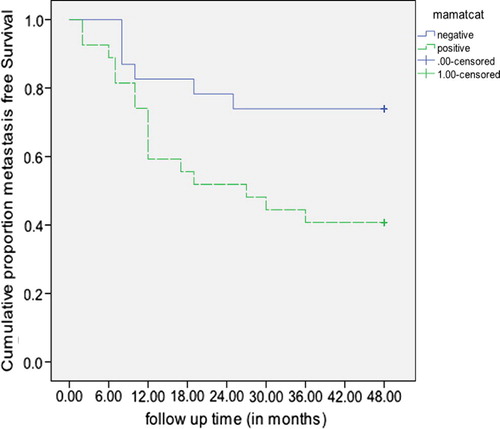
After surgery; the mean metastasis free survival time was 35.3 months (95% CI: 27.6, 43.1) in Mammoglobin negative compared to 31.1 months (95% CI: 24.6, 37.6) in Mammoglobin positive, but the difference in survival distributions was not statistically significant (logrank = 1.12, p = 0.291). The cumulative metastasis free survival distribution was statistically significantly higher in Mucin-1 negative relative to those that were Mucin-1 positive. (logrank = 5.90, p = 0.015) (The mean survival times were not computed as all Mucin-1 negative were censored) (). As regards, CA 15.3 the mean metastasis free survival time was 34.4 months (95% CI: 28.7, 40.1) in CA 15.3 negative compared to 28.1 months (95% CI: 18.0, 38.2) in CA 15.3 positive, however the difference in survival distributions was not statistically significant (logrank = 1.11, p = 0.292).
Figure 3 Metastasis free survival in breast cancer females negative and positive to Mucin-1, 2 weeks after surgery.
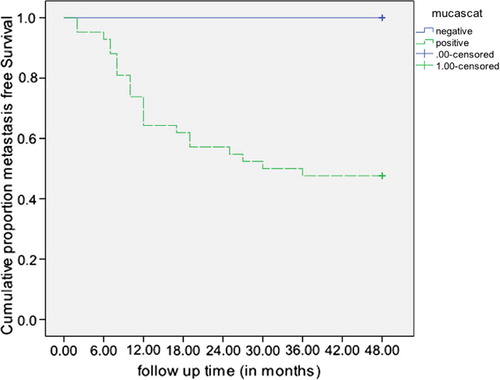
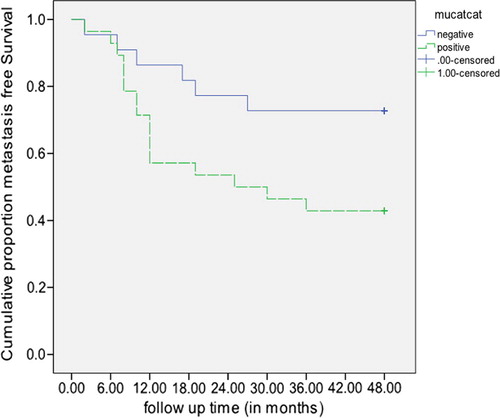
After 6 cycles of treatment; a statistically significant difference in the survival distributions was observed between Mammoglobin negative and Mammoglobin positive (logrank = 5.15, p = 0.023), with a mean survival time (38.9 months, 95% CI: 32.4, 45.3 vs 27.7 months, 95% CI: 20.8, 34.7 respectively) (). Likewise, a statistically significant difference in the survival distributions was detected between Mucin-1 negative and Mucin-1 positive (logrank = 4.17, p = 0.041), with a mean survival time (38.6 months, 95% CI: 32.0, 45.3 in Mucin-1 negative vs 28.3 months, 95% CI: 21.5, 35.1 in Mucin-1 positive) (). Meanwhile, the CA 15.3 did not prove a statistically significant difference in the survival distributions between those CA 15.3 negative and those positive (logrank = 2.95, p = 0.086), though the mean metastasis free survival time was 36.9 months, 95% CI: 30.5, 43.2 in CA 15.3 negative compared to 27.8 months, 95% CI: 20.3, 35.2 in CA 15.3 positive.
3.6 Evaluation of the accuracy of Mammoglobin and Mucin-1 expression and CA 15.3 levels
The prognostic accuracy for anticipating metastasis of none of the biochemical parameters was statistically significant either before or after 2 weeks of surgery. On the other hand, after 6 cycles of treatment, Mammoglobin and Mucin-1 proved a significant prognostic accuracy with an AUCs = 0.667, 95% at CI: 0.515, 0.820 and 0.649, 95% CI: 0.495, 0.804; respectively. While CA 15.3 was not of any prognostic significance with AUC = 0.635, 95% CI: 0.477, 0.792 ().
Figure 6 ROC curve for Mammoglobin, Mucin-1 and CA 15.3 as prognostic markers for metastasis in females with breast cancer after 6 cycles of chemotherapy.
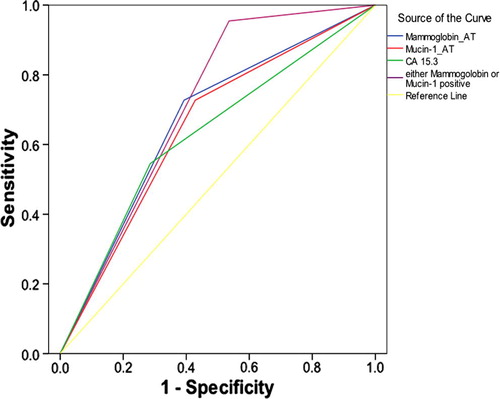
When constructing the ROC curve combining both Mammoglobin and Mucin-1, considering cases that are Mammoglobin positive and/or Mucin-1 positive as positive, the ROC curve demonstrated a statistically significant diagnostic accuracy (p = 0.01) with an AUC = 0.710; 95% CI: 0.566, 0.853; that was slightly higher than either Mammoglobin alone or Mucin-1 alone ().
4 Discussion
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in females worldwide, accounting for 23% (1.38 million) of the total new cancer cases and 14% (458,400) of the total cancer deaths in 2008. About half the breast cancer cases and 60% of the deaths are estimated to occur in economically developing countries.Citation4 However, despite optimal local and systemic adjuvant treatment, 30–40% of patients diagnosed with curable breast cancer eventually die of recurrent disease.Citation13 Therefore, improved techniques to both detect and treat metastatic breast cancer are needed.
It was previously thought that metastasis occurred late in disease progression; however, evidence has shown that metastasis may be an early event. This is supported by the fact that CTCs are found in patients with early breast cancer. A recent study showed that dissemination of tumor cells can occur at a pre-invasive stage of the primary tumor and that the presence of CTCs was independent of tumor size in early human breast cancer.Citation11 However, even though occult tumor dissemination may occur early, not all patients with detectable CTCs will develop overt metastases. These CTCs may be in a state of dormancy and the exact mechanism of transition to overt metastases is unclear.Citation14
For CTCs to be used as surrogates for metastasis, then accurate and reproducible techniques are needed for CTCs quantification. This is especially important when considering that CTCs concentration in peripheral blood can be as low as one per 105–107 cells.Citation15 It is generally thought that molecular techniques are more sensitive than the immunohistochemical techniques (IHC), and that the implementation of such techniques has greatly improved the ability to detect low numbers of breast cancer cells. The detection rate of RT-PCR was greater than that of IHC and the test detected CTCs in samples that were shown to be negative by IHC. However, studies showed that the detection of tumor cells in both bone marrow and blood varied from equal sensitivities for IHC and RT-PCR to 10 times more sensitivity using RT-PCR rather than IHC. The difference in the sensitivity depended upon the particular tumor marker being assayed.Citation16,Citation17
Our study aimed to evaluate using the expression of Mammoglobin, a tumor-derived antigen and Mucin 1, a tumor-associated antigen, to detect CTCs in blood of breast cancer females as diagnostic and prognostic markers and predictors of metastasis. Mammoglobin is a 93-amino acid protein belonging to the uteroglobin/Clara cell protein family of small epithelial secretory proteins, the secretoglobins.Citation18 It is expressed in normal breast epithelial cells and is specifically overexpressed in breast cancer. Mammoglobin was reported to be present in more than 80% of breast cancer cases. Thus, hMAG is promising for its potential diagnostic value as well as its prognostic indications in breast cancer, especially as it can be detected almost exclusively in breast cancer.Citation19,Citation20 The other gene, Mucin-1, is a member of the mucin family and encodes a membrane bound, glycosylated phosphoprotein. It has a core protein mass of 120–225 kDa which increases to 250–500 kDa with glycosylation.Citation21 Mucin-1 was found to be overexpressed in more than 90% of breast cancers in an underglycosylated form, which makes it not only a candidate for being a diagnostic and prognostic marker of breast cancer cells, but also a target for immunotherapy.Citation22
The study included a cohort of 50 females recently diagnosed with breast cancer; most of them were of high stage and high grade. CA 15.3 was elevated in a small proportion of cases (20%), while Mammoglobin and Mucin expression was observed in the majority of cases (80% and 86%; respectively). That reflects that CTCs detection in blood could be a much better diagnostic tool than the conventional tumor markers that are currently in use. In another study that was conducted to detect CTCs in patients of relatively higher stage breast cancer, their results were comparable to ours where they found that expression of CTC genes correctly identified 71% of the patients with invasive breast cancer, and 86% of the node-positive patients at presentation.Citation23 Furthermore, expression of CTC genes reflected the impact of surgery and of chemotherapy by the significant decrease in the percent of patients that are still positive, especially Mammoglobin expression.
There have been few studies regarding CTCs in early breast cancer. The reported CTC positivity rate has ranged from 9.4% to 48.6% depending on the method used and the patient group.Citation24–Citation29 The positivity rates were so much lower than our study, most probably because they studied low-grade breast cancer patients, while the majority of patients in the current study were of high stage. Although CTDs dissemination in blood is supposed to be an early event in metastasis, its dissemination in primary breast cancer is not well studied. However, it might not be an early event, and is most probably correlated with tumor invasiveness or early metastasis. Also the method used for the detection and the gene whose expression is measured is another important factor.
Studies have tried to identify primary tumor characteristics that would predict the presence of CTCs. A recent study by Krishnamurthy et al. looked at CTCs in stage 1 and 2 breast cancer patients and found that the presence of CTCs was independent of lymph node status, tumor grade, tumor size, and receptor status.Citation28 This is in contrast with early findings of the SUCCESS trial, that reported a positive correlation between lymph nodes status and CTCs.Citation29 Our results were in agreement with those of Krishnamurthy et al., where Mammoglobin and Mucin-1 expressions before surgery were present in the majority of patients and did not correlate with any of the clinical parameters. That is probably because the tumor mass was still present and was seeding more tumor cells into the circulation. From model systems, it was estimated that about 106 tumor cells/g tumor tissues are shed daily into the blood, although such model calculations might overestimate the number actually shed in vivo.Citation30,Citation31 Recent studies also showed that intraoperative shedding of tumor cells into the circulation may take place.Citation32,Citation33 However, blood is only a temporary compartment for tumor cells, and a significant part of circulating tumor cells do not survive. It was reported that a large number of circulating tumor cells in patients with breast cancer are apoptotic and therefore might be unable to settle in secondary organs.Citation34 After surgery, when the tumor mass was removed, the presence of living tumor cells in the circulation was significantly correlated with tumor size, vascular invasion, stage and subsequent metastasis, but not with receptor status, grade and lymph node metastasis.
One goal of CTC detection is to correlate it to disease progression and response to treatment as a prognostic marker. Regarding CTCs prognostic role in breast cancer, the presence of Mucin-1 expression after surgery posed a doubled risk of metastasis in Mucin-1 positive than in negative patients, while after chemotherapy, the expression of either Mammoglobin or Mucin-1 posed a 1.8 relative risk of metastasis than in negative patients. This demonstrates that the presence of CTCs after surgery of after treatment with adjuvant chemotherapy could be associated with worse prognosis. This was further confirmed when considering DFS, where the same profile was noticed. Patients that were Mucin-1 positive after surgery had a significantly shorter disease free survival time compared to Mucin-1 negative patients, while after chemotherapy, patients who showed the expression of Mammoglobin or Mucin-1 had significantly shorter disease free survival time compared to negative patients (27.7 vs 38.9 months for Mammoglobin and 28.3 vs 38.6 months for Mucin-1).
Our results were consistent with most of the published data, though there is a great inconsistency regarding tumor response. Several studies involving early breast cancer patients have shown that the presence of CTCs is associated with a worse prognosis, early clinical relapse and disease-related death.Citation27,Citation35,–Citation38 In other studies no significant correlation was found between CTC detection and the primary tumors response to neoadjuvant therapy and no correlation was found between CTC response and tumor response.Citation24,Citation39,Citation40 Though data is inconsistent regarding tumor response, most studies have found that the presence of CTCs does predict early relapse. The SUCCESS trial found that pretreatment CTC detection was associated with reduced disease-free survival as well as overall survival, while post treatment CTC detection was only associated with reduced disease-free survival.Citation29
The prognostic accuracy for anticipating metastasis of CTCs detected in peripheral blood by the expression of Mammoglobin or Mucin-1 after 6 cycles of chemotherapy proved to be significant with AUCs = 0.667 and 0.649; respectively. However, due to the heterogenous nature of breast cancer, many reported that the simultaneous measurement of multiple markers significantly improved the prognostic accuracy of CTCs. Combining Mammoglobin and Mucin-1 expressions for CTC detection resulted in improved AUC becoming 0.71, however, the increase was not huge, it was significant.
It was not surprising that in the current study, CA 15.3 was not of any diagnostic or prognostic significance and did not correlate with any of the clinical parameters of the disease and could not predict metastasis. Currently, CA 15.3, which detects a soluble form of Mucin-1 protein, is the most widely used serum marker in patients with breast cancer. Its main use is for monitoring therapy in patients with metastatic disease. In this setting, CA 15.3 should not be used alone but in conjunction with diagnostic imaging, clinical history and physical examination.Citation41 It may also be used in the postoperative surveillance of asymptomatic women who have undergone surgery for invasive breast cancer. In this setting, serial determination can provide median lead-times of 5–6 months in the early detection of recurrent/metastatic breast cancer. It is unclear however, whether administering systemic therapy based on this lead-time improves patient outcome. The main limitation of CA 15.3 as a marker for breast cancer is that serum levels are rarely increased in patients with early or localized disease. CA 15.3 increases in some cases only when metastasis has already happened, probably before clinical symptoms become visible, but as such it is not of any prognostic value in primary breast cancer.Citation42,Citation43
In conclusion, the data reported in the present study indicate that the hematogenous spread of tumor cells in patients with breast cancer may be an early phenomenon occurring before/apart from regional lymph nodes involvement. CTCs detection by RT-PCR could add to the initial evaluation of primary breast cancer patients. Also, adjuvant chemotherapy might eliminate or decrease occult tumor cells. But CTCs evaluation after adjuvant chemotherapy could predict metastasis and the presence of CTCs after chemotherapy reflect a higher potential for subsequent metastasis. As understanding of the biology of the breast cancer continues to improve, treatment of the disease continues to change, although the ultimate goal of the treatment remains improved survival, increasing emphasis is now put on less morbid treatments, and improves quality of life. Detection of the disease or metastasis remains our goal for achieving good control of the disease.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine
Available online 12 July 2012
References
- Howlader NNA, Krapcho M, Neyman N, Aminou R, Waldron W, et al. editors. SEER Cancer Statistics Review, 1975–2008. 2010, National Cancer Institute: Bethesda.
- Alexandria Cancer Registry, Medical Research Institute, Alexandria University, Egypt. 2007.
- H.J.BursteinJ.R.HarrisM.MorrowMalignant tumors of the breastV.T.DeVitaT.S.LawrenceS.A.RosenbergCancer: principles and practice of oncology8th ed.2008Lippincott Williams & WilkinsPhiladelphia16061654
- A.JemalR.SiegalE.WardY.HaoJ.XuT.MurrayCancer statisticsCA Cancer J Clin5820087196
- B.FisherJ.H.JeongS.AndersonJ.BryantE.R.FisherN.WolmarkTwenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiationN Engl J Med3472002567575
- E.RuoslahtiHow cancer spreadsSci Am27519967277
- A.F.ChambersA.C.GroomI.C.MacDonaldDissemination and growth of cancer cells in metastatic sitesNat Rev Cancer22002563572
- K.PantelR.H.BrakenhoffDissecting the metastatic cascadeNat Rev Cancer42004448456
- C.Alix-PanabieresV.MullerK.PantelCurrent status in human breast cancer micrometastasisCurr Opin Oncol192007558563
- S.DawoodM.CristofanilliIntegrating circulating tumor cell assays into the management of breast cancerCurr Treat Options Oncol820078995
- R.SainsburyBreastR.M.KirkM.C.WinsletGeneral surgical operations5th ed.2006Churchill Livingstone
- C.M.HaskellBreast cancerD.A.CasciatoManual of clinical oncology6th ed.2004Lippincott Williams and WilkinsUSA
- B.WeigeltJ.L.PeterseL.J.Van't VeerBreast cancer metastasis: markers and modelsNat Rev Cancer52005591602
- H.GravesB.J.CzernieckiCirculating tumor cells in breast cancer patients: an evolving role in patient prognosis and disease progressionPathol Res Int20112011621090
- M.Alunni-FabbroniM.T.SandriCirculating tumour cells in clinical practice. Methods of detection and possible characterizationMethods502010289297
- A.SchoenfeldK.H.KrugerJ.GommH.D.SinnettJ.C.GazetN.SacksThe detection of micrometastases in the peripheral blood and bone marrow of patients with breast cancer using immunohistochemistry and reverse transcriptase polymerase chain reaction for keratin 19Eur J Cancer331997854861
- J.A.López-GuerreroP.B.GilabertE.B.GonzálezM.A.Sanz AlonsoJ.P.PérezA.S.TalensUse of reverse- transcriptase polymerase chain reaction (RT-PCR) for carcinoembryonic antigen, cytokeratin 19, and maspin in the detection of tumor cells in leukapheresis products from patients with breast cancer: comparison with immuno cytochemistryJ Hematother8419995361
- J.NiM.Kalff-SuskeR.GentzJ.SchagemanM.BeatoJ.KlugAll human genes of the uteroglobin family are localized on chromosome 11 q 12.2 and form a dense clusterAnn New York Acad Sci92320002542
- P.N.SpanE.WaandersP.MandersJ.J.HeuvelJ.A.FoekensM.A.WatsonMammaglobin is associated with low-grade, steroid receptor-positive breast tumors from postmenopausal patients, and has independent prognostic value for relapse-free survival timeJ Clin Oncol222004691698
- B.K.ZehentnerD.CarterMammaglobin: a candidate diagnostic marker for breast cancerClin Biochem372004249257
- M.A.HollingsworthB.J.SwansonMucins in cancer: protection and control of the cell surfaceNat Rev Cancer420044560
- R.SinghD.BandyopadhyayMUC1: a target molecule for cancer therapyCancer Biol Ther62007481486
- M.M.ReinholzA.NibbeL.M.JonartK.KitzmannV.J.SumanJ.N.IngleEvaluation of a panel of tumor markers for molecular detection of circulating cancer cells in women with suspected breast cancerClin Cancer Res11200537223732
- S.RiethdorfV.MüllerL.ZhangT.RauS.LoiblM.KomorDetection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant Gepar-Quattro trialClin Cancer Res16201026342645
- S.ApostolakiM.PerrakiA.PallisV.BozionelouS.AgelakiP.KanellouCirculating HER2 mRNA-positive cells in the peripheral blood of patients with stage I and II breast cancer after the administration of adjuvant chemotherapy: evaluation of their clinical relevanceAnn Oncol182007851858
- F.C.BidardC.MathiotS.DelalogeE.BrainS.GiachettiP.de CremouxSingle circulating tumor cell detection and overall survival in non metastatic breast cancerAnn Oncol212010729733
- M.IgnatiadisG.KallergiM.NtouliaM.PerrakiS.ApostolakiM.KafousiPrognostic value of the molecular detection of circulating tumor cells using a multimarker reverse transcription-PCR assay for cytokeratin 19, mammaglobin A, and HER2 in early breast cancerClin Cancer Res14200825932600
- S.KrishnamurthyM.CristofanilliB.SinghJ.ReubenH.GaoE.N.CohenDetection of minimal residual disease in blood and bone marrow in early stage breast cancerCancer116201033303337
- B.K.RackC.SchindlbeckU.AndergassenA.SchneeweissT.ZwingersM.LichteneggerUse of circulating tumor cells (CTC) in peripheral blood of breast cancer patients before and after adjuvant chemotherapy to predict risk for relapse: the SUCCESS trialJ Clin Oncol1820101003
- T.P.ButlerP.M.GullinoQuantitation of cell shedding into efferent blood of mammary adenocarcinomaCancer Res351975512516
- Y.S.ChangE.di TomasoD.M.McDonaldR.JonesR.K.JainL.L.MunnMosaic blood vessels in tumors: frequency of cancer cells in contact with flowing bloodProc Natl Acad Sci USA9720001460814613
- M.G.DenisM.TessierB.DrenoP.LustenbergerCirculating micrometastases following oncological surgeryLancet3471996913
- P.EschwègeF.DumasP.BlanchetV.Le MaireG.BenoitA.JardinHaematogenous dissemination of prostatic epithelial cells during radical prostatectomyLancet346199515281530
- G.MéhesA.WittE.KubistaP.F.AmbrosCirculating breast cancer cells are frequently apoptoticAm J Pathol15920011720
- S.ApostolakiM.PerrakiG.KallergiM.KafousiS.PapadopoulosA.KotsakisDetection of occult HER2 mRNA-positive tumor cells in the peripheral blood of patients with operable breast cancer: evaluation of their prognostic relevanceBreast Cancer Res Treat1172009525534
- P.WülfingJ.BorchardH.BuergerS.HeidlK.S.ZänkerL.KieselHER2-positive circulating tumor cells indicate poor clinical outcome in stage I to III breast cancer patientsClin Cancer Res12200617151720
- N.XenidisM.IgnatiadisS.ApostolakiM.PerrakiK.KalbakisS.AgelakiCytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancerJ Clin Oncol27200921772184
- P.FerroM.C.FranceschiniB.BacigalupoP.DessantiE.FalcoV.FontanaDetection of circulating tumour cells in breast cancer patients using human mammaglobin RT-PCR: association with clinical prognostic factorsAnticancer Res30201023772382
- M.IgnatiadisN.XenidisM.PerrakiS.ApostolakiE.PolitakiM.KafousiDifferent prognostic value of cytokeratin-19 mRNA positive circulating tumor cells according to estrogen receptor and HER2 status in early-stage breast cancerJ Clin Oncol25200751945202
- J.Y.PiergaF.C.BidardC.MathiotE.BrainS.DelalogeS.GiachettiCirculating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trialClin Cancer Res14200870047010
- M.J.DuffySerum tumor markers in breast cancer: are they of clinical value?Clin Chem522006345351
- M.J.DuffyD.EvoyE.W.McDermottCA 15–3: uses and limitation as a biomarker for breast cancerClin Chim Acta411201018691874
- R.MolinaV.BarakA.van DalenM.J.DuffyR.EinarssonM.GionTumor markers in breast cancer–European Group on Tumor Markers recommendationsTumour Biol262005281293