 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To determine the cellular immune response in Bell's palsy (BP) and its prognostic value in relation to clinical and electrophysiological findings.
Methods
Twenty patients with BP were subjected to: Facial nerve paralysis assessment according to House–Brackmann (H&B) grading system, bilateral facial nerve conduction study with electroneurography (ENoG) quotient calculation, blink reflex, and needle electromyography (EMG) for the affected side; one week from the onset. Before the start of medical treatment, peripheral blood mononuclear cell subsets were analyzed to reveal the percentage of total T cells (CD3+), T helper/inducer cells (CD4+), T cytotoxic (CD8+) and B cells (CD19+). Patients were followed up by H&B, ENoG and needle EMG up to 3 months from the onset (end point). Fifteen age and sex matched healthy control subjects for the electrophysiological study and laboratory tests were included.
Results
The percentages of CD3+, CD4+ & CD8+ were significantly depressed in BP patients than in control. CD19+ percentage did not show significant difference between them. On follow up, H&B revealed significant improvement. Neither electrophysiological parameters nor ENoG showed significant difference between initial and follow up assessments. Initial CD4+ percentage correlated negatively with disease duration. While Initial CD8+ percentage correlated positively with follow up compound muscle action potential (CMAP) amplitude of orbicularis oris muscle and ENoG of orbicularis oculi and nasalis muscles. Initial CD19+ percentage correlated negatively with follow up H&B and R1 & R2 responses of follow up blink reflex. Initial CD3+ percentage did not correlate with any of the follow up measures.
Conclusion
Decreased percentage of peripheral blood CD3+, CD4+ & CD8+ in BP patients emphasizes the role of cell mediated autoimmune pathogenesis in the acute stage of the disease. These cells have a prognostic significance for prediction of the disease duration and outcome. Analysis of T lymphocytes subsets may provide an additional parameter to differentiate patients with favorable from those with poor prognosis.
1 Introduction
Bell's palsy (BP) is primarily an acute 7th cranial nerve paralysis that cannot be attributed to central or peripheral lesions, systemic infections or inflammatory conditions.Citation1 Several mechanisms have been proposed for the etiology of BP including ischemia, autoimmune reaction and viral infection.Citation2–Citation6 Herpes simplex 1 virus (HSV-1) was proved to be a cause of BP by several studies.Citation4,Citation5,Citation7,Citation8 It is thought to persist as a latent infection. Reactivation of a dormant HSV-1 within the geniculate ganglion (GG) and anterograde transport to facial nerve termini with subsequent inflammation and entrapment of the facial nerve at the meatal foramen was proposed as a pathogenic mechanism in BP.Citation1,Citation9,–Citation12 Reactivation of a latent virus can be triggered by surgical trauma, upper respiratory tract infection, fever, dental extraction, emotional distress, exposure to cold, metabolic disorders and immunosuppression.Citation8,Citation13 Among the lymphocytes, the role of CD8+ T cells is established where the reduced number of these cells is among the factors that facilitate HSV-1 reactivation.Citation14 Prevention of reactivation requires a complex interplay among the virus, neurons and immune response. An accumulating evidence supports the view that CD8+ effector T cells employ both lytic granule (perforin)- and IFN γ-dependant effector mechanisms in maintaining HSV-1 latency.Citation12 The exact role and timing of contribution of lymphocyte subsets during HSV-1 reactivation is still uncertain. On the other hand, a number of case reports have linked recent primary oral HSV-1infection with unilateralCitation15 or bilateralCitation16 facial paralysis. In primary HSV-1infection a strong immune response evolves composed of virus neutralizing antibodies and antiviral CD4+ and CD8+ T cell response which efficiently inhibits virus replication with induction of virus-specific immunity by the adaptive immune system.Citation17,Citation18
A concern of both patients with BP and their physicians is when and how facial paralysis will be completely resolved.Citation19 The underlying pathology of the facial nerve is the major prognostic indicator in such patients i.e. demyelination favors a good prognosis whereas axonal degeneration has a bad one. Accordingly, it is assumed that the underlying immune response will determine the severity of inflammation and nerve damage and hence the prognosis of BP. Since lymphocytes are migratory cells i.e. their distribution in the body reflects the rate at which they enter and depart particular sites as well as their local replication, thus these emigrating cells eventually are carried into the bloodstream and traffic among organs according to their interaction with the target tissue.Citation20 Therefore, in a clinical setting as BP, it seems convenient to study the peripheral blood lymphocytes subsets which reflects their dynamic activity and hence the competence of the immune system. In this context, several studies investigated the lymphocyte subsets in BP with controversial results.Citation21–Citation23 Therefore, this study aimed at identifying the pattern of peripheral blood lymphocyte subsets in BP patients compared to healthy controls and their relation to disease prognosis as assessed by functional and electrophysiological outcome measures.
Study design: case-control prospective study.
2 Subjects and methods
Twenty patients; 11 males (55%) and 9 females (45%) with unilateral BP were included in the study. Their ages ranged between 7 and 55 years (mean ± SD: 28.9 ± 15.2). The diagnosis of BP was determined according to criteria described by TavernerCitation24: sudden onset, unilateral, complete or partial paralysis of facial muscles; absence of symptoms and signs attributable to nervous system diseases; absence of symptoms and findings attributable to ear and posterior fossa lesions as well as history of trauma to the head or surgery in the face or the parotid region, diabetes mellitus, infiltrative disease and history of irradiation to the neck. Patients with a previous history of Bell's palsy were excluded from the study.
The study also included 15 age and sex matched healthy control subjects (without a previous history of Bell's palsy) for the electrophysiological study and laboratory tests. The control group was composed of 8 (53.3%) males and 7 (46.7%) females with an age range of 9–53 years (mean 26.1 ± 10.9).
All patients were subjected to the following:
| 1. | History taking including; demographic data: name, age, sex, occupation, present history: mode of onset, duration of the disease, herpetic eruptions. | ||||
| 2. | Full neurological examination.Citation25 | ||||
| 3. | Examination of facial muscles and their grading according to facial nerve grading system of House-Brackman (HB).Citation26 | ||||
| 4. | Electrophysiological examination was performed at 10 to15 days of the disease onsetCitation27 which included the following: | ||||
| a) | Bilateral facial nerve motor conduction studies (for patient and control groups):Citation28 | ||||
The facial nerve was stimulated below the ear anterior to the mastoid. The active recording electrode was placed over the frontalis, orbicularis occuli (OOc), nasalis and orbicularis oris (OOr) muscles. The reference electrode was placed over the standard corresponding point. The ground electrode was placed at the wrist. The compound muscle action potential (CMAP) latency and amplitude were recorded for each studied muscle. In the patients’ group, the ratio of the amplitude of CMAP on the affected side to that on the healthy side was calculated as a percent (electroneurographic quotient i.e. ENoG quotient). Electroneurography served as an index of degree of nerve involvement.Citation29Regarding the control group, calculation of ENoG quotient was carried out according to the following formula:
| b) | Blink reflex:Citation28 | ||||
Recording was performed from both sides simultaneously using 2 channels. Recording settings were: sweep speed 10 ms/division, gain 200 μV/division and analysis time 100 ms.
| 5. | Needle electromyography (EMG) of OOc and OOr muscles of the affected side was carried out after 10–15 days of the onset.Citation30 Patients with axonopathy should have both EMG findings of axonal degeneration in the acute stage (denervation potentials and decreased recruitment) and ENoG less than the mean-2SD of that of the control group, otherwise demylination is considered.Citation28 | ||||
| 6. | EDTA blood samples were obtained from all patients and control group before the start of medical treatment. Peripheral blood lymphocyte subsets were analyzed with flow cytometry (Becton–Dickinson, Erembodegem, Belgium) and monoclonal antibodies labeled with either fluorescein isothiocyanate or phycoerythrin (CD3/FITC, CD19/PRE, CD4/FITC and CD8/PRE) to reveal the percentage of total T cells (CD3+), B cells (CD19+), T helper/inducer cells (CD4+) and T cytotoxic cells (CD8+). | ||||
All patients were treated within the first 72 h of symptoms onset, after obtaining blood sample, with oral acyclovir (400 mg five times daily) and oral prednisolone (at a dose of 40–60 mg daily)Citation31 that was gradually withdrawn.
Patients were followed up by HB grading system, facial nerve motor conduction studies, ENoG, needle EMG and assessment of T-cells percentage on recovery or up to 3 months of the onset (as an end point).Citation32 The study was approved by the Local Ethics Committee, Faculty of Medicine, Alexandria University. All patients and control groups were informed about the nature of the study and written acceptance consent was signed by each participant.
Statistics: Description of the sample was expressed as frequencies and percentages. Distribution of all variables was evaluated using Skewness test. All variables were normally distributed except for the initial and follow up latency and amplitude of the affected OOc and OOr muscles respectively as well as the initial and follow up peripheral blood CD3+ and CD19+. For normally distributed variables, Student t test was used to compare between patients and controls, while paired t test was used for initial and follow up comparisons. For other variables, Mann–Whitney test was used for comparison between patients and controls and Wilcoxon test for initial and follow up comparison. Correlation between variables was examined by Spearman's test. SPSS v 11.0 was used to perform the statistical analysis. Cut off values were calculated as the mean ± 2SD of controls for electrophysiological parameters. P is significant if ⩽0.05.
3 Results
No statistical significant difference in age was found between patients and controls (t = 0.578, P = 0.568). Eleven patients (55%) had right side BP and 9 patients (45%) had left side lesion. None of the patients complained of hyperacusis or disturbed taste sensation i.e. all had distal facial nerve affection. The triggers for the disease as stated by the studied patients were exposure to cold, emotional stress and a preceding upper respiratory tract infection. Mean disease follow up duration was 5.36 ± 4.78 weeks. According to HB grading system; 5 patients (25%) were grade II, 3 patients (15%) were grade III, 5 patients (25%) were grade V, and 7 patients (35%) were grade VI at initial evaluation. On follow up evaluation, 7 patients (35%) were grade I, 5 patients (25%) were grade II, 2 patients (10%) were grade III, 3 patients (15%) were grade V, and 3 patients (15%) were grade VI. shows the comparison between patients and controls regarding the ENoG findings as well as the ENoG cut off values (mean of the control value-2SD) of the studied facial muscles. There was a statistically significant decrease of the ENoG of frontalis, nasalis and OOr of patients compared to controls. (p ⩽ 0.05) ().
Figure 1 Compound muscle action potential recorded from (a) frontalis, (b) orbicularis occuli, (c) nasalis (d) orbicularis oris muscles of patient's healthy (upper 2 traces) and affected sides (lower 2 traces).
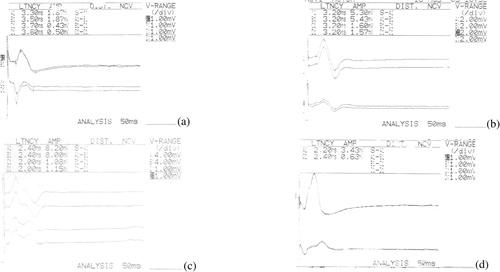
Table 1 Comparison of the electroneurography of the facial muscles between patients and controls.
shows the mean values of initial and follow up HB grading system. A statistical significant improvement was found in HB grading system at follow up evaluation (P = 0.001). It shows also the mean amplitude and latency of the facial nerve CMAP recorded from the frontalis, OOc, nasalis, OOr muscles of the affected side among the studied patients at initial and follow up evaluation. Neither the mean latency nor the mean amplitude of the recorded CMAP showed a statistically significant difference on comparing the initial to the follow up assessment. Similarly, ENoG did not show significant difference between initial and follow up values.
Table 2 Comparison of initial and follow up values of House and Brackmann grading system, affected facial nerve CMAP latency and amplitude, electroneurography and blink reflex responses among patients.
Individual data analysis revealed that 5 patients (25%) had demylination pathology (they recovered completely before 3 months) and 15 patients (75%) had axonal lesion based on cut off values of ENoG and EMG findings as well as the follow up HB grading system. shows the mean values of peripheral CD3+, CD4+, CD8+ and CD19+ mononuclear cell subsets among patients and control groups at initial evaluation. The initial percentage of CD3+, CD4+ & CD8+ subsets was significantly depressed in BP patients group compared to age matched control subjects (p ⩽ 0.05). However, the percentage of CD19+ subset did not show a significant difference between patients and control groups (p = 0.69).
Table 3 Comparison of peripheral blood mononuclear cell subsets between patients and control groups.
Moreover, comparison between BP patients with demyelinating pathology and those with axonal lesion regarding the mean initial percentage of peripheral blood T cells revealed no statistical significant difference (CD 4+; t = −0.6, p = 0.558, CD8+; t = 1.596, p = 0.138 and CD3+; Z = −0.681, p = 0.496), while there was a statistically significant increase in the mean initial percentage of CD19+ in patients with demyelination compared to those with axonopathy (Z = −2.632, p = 0.008). Spearman's correlation revealed a negative correlation between the initial percentage of CD4+ subset with disease follow up duration (r = −0.882, P = 0.02). Initial percentage of CD8+ subset correlated positively with follow up CMAP amplitude of OOr muscle (r = 0.841, P = 0.04) on the affected side and positively with follow up ENoG of OOC and nasalis muscles (r = 0.894, P = 0.04 for each). Initial percentage of CD19+ subset correlated negatively with follow up HB grading system (r = −0.807, P = 0.003) and negatively with latencies of ipsilateral R1, R2 and contralateral R2 responses of follow up blink reflex on the affected side (r = −1.0, P = 0.001 for each). Initial CD3+ percentage did not correlate with any of the follow up measures.
4 Discussion
Among the proposed mechanisms underlying BP which may be directly relevant to the immune system are the reactivation of HSV-1Citation1,Citation9,–Citation12 and the less commonly considered; the autoimmune mechanism.Citation3,Citation21 In the present study, the studied BP patients were assumed to have reactivation of a latent herpes virus as a probable pathogenic mechanism. None of the studied patients was suffering from primary herpes infection (or any other illness) at the time of presentation of BP which ruled out the possibility of the primary or recent HSV-1 infection with concomitant BP. In the context of autoimmunity, Abramsky et al. suggested that in BP there is in vivo sensitization of lymphocytes to self protein called neuritogenic basic protein and concluded that an immunological process similar to Guillian-Barr syndrome (GBS) occurs in BP and even the latter could represent a mononeuritic variant of GBS.Citation3 In the present study neither specific viral nor specific neural markers were assessed, therefore, both mechanisms will be adopted to explain the results of the study.
Our study revealed a decrease of the mean percent of the components of cellular immunity (CD8+, CD4+ and Total CD3+) among the studied patients while CD19+ percent (B cells) was slightly increased in patients though not statistically significant compared to control. The interactions between viruses and the host immune system are not only complex but also critical in determining the outcome of infection and prevention strategies.Citation33 In the target tissue (GG in BP) where the virus resides in a latent state, infiltrating virus-specific CD8+ T cells (which provide constant immunosurveillance)Citation34 inhibit HSV-1 reactivation.Citation35–Citation37 Although the role of CD4+ T cells in viral latency was not well highlighted, yet it was shown that the lack of CD4+ T cell's help of CD8+ cells during the primary infection leads to a transient inability to control the latent virus with reduced functional avidity of ganglionic HSV specific CD8+ cells.Citation34,Citation38 Under certain circumstances HSV-1 reactivates with peripheral shedding of the infectious virus.Citation39 Stress-induced HSV-1 reactivation results from a transient compromise of CD8+ T cells surveillance.Citation40 Thus, it appears that stressful stimuli virtually impair the T cell function in the target tissue (not assessed in this study) and in the peripheral circulation (assessed in this study) leading to decrease in their percent in the peripheral circulation. This may explain our results (decreased components of cell mediated immunity). In addition, the decline in the percentage of the immune cells in blood may reflect the attempts of the cells to control viral reactivation which led to their migration from the blood to the target.Citation41 However, the possibility that decreased percentage of these cells may result from virus-T cells exhaustion and/or dysregulation cannot be excluded.Citation42 In this context, the concept of the relationship between facial paralysis and immune suppression was adopted by some authors.Citation43,Citation44
In the present study, comparing the initial T cell percentage among patients with demyelination and those with axonal degeneration revealed that both groups had comparable values of T cells but patients with demyelination had a significant increase in the initial percentage of B cells which may account for the relatively favorable prognosis of such patients. Although the role of B cells was not well addressed in HSV-1 latency and reactivation, yet some studies demonstrated the beneficial role of B cells in controlling the persistent viral infection.Citation45,Citation46 Weck et al. observed that γ herpes virus latency was regulated by B cells and that the majority of the persistently infected B-cell deficient mice died between 100–200 days post infection, whereas the normal control mice maintain the virus in a latent state.Citation45 Thus, the humoral responses act in concert with cellular immunity to control primary and latent viral infections.Citation47 Therefore, it can be assumed that patients with demyelinating facial nerve pathology (with increased percent of CD19+) had a less inflammation, less edema around the nerve, less compression and hence the possibility of axonal degeneration is less. This deduction was supported by the negative correlation between CD19+ in patients group and the follow up HB grading score emphasizing the role of CD19+ in this context. Furthermore, the CD19+ percent was negatively correlated with the follow up latencies of R1 and ipsilateral R2 of the blink reflex in the affected side. Both correlations suggest that B cells appear to be a decisive factor with respect to the prognosis of BP.
Studying the changes of the immune system in patients with BP was a point of interest for many researches with controversial results regarding the findings and interpretations.Citation7,Citation21,Citation32,Citation48,Citation49 Jonsson et al. analyzed T lymphocytes and their subsets in 20 BP patients and found a decrease in the total T cell percentage and T helper cells (CD4+) (as the present study). But unlike ours they found no change in the percent of the cytotoxic T cells (CD8+). After 4–6 weeks these depressions were restored to normal levels.Citation48 In our study all increased on follow up but reaching a significant level only for the total percent of CD3+ (data not shown). In another study performed by Aveil et al. the results were similar to ours.Citation21 Also Vedeler et al. found a significant decrease of the mean percent of the active and total T cells in the first week in BP patients among whom 8 patients showed a decreased percent of T suppressor cells and all returned to the normal values after 3 months of the disease onsetCitation49On the other hand, Kaygusuz et al. studied the role of viruses and cellular immune response in idiopathic facial palsy. They demonstrated that the percent of CD4+(similar to our results) and CD4+/CD8+ ratios was lower while the percent of natural killer (NK) cells and CD8+ percent was higher than controls (unlike our results) and CD19+ percent was normal (similar to the present study)Citation7They suggested that this pattern was similar to viral reactivation. Alternatively, Tekgul et al. found decreased CD19+ cells (unlike ours) and decreased CD8+ T cells in children with BP. They suggested that the underlying mechanism was an autoimmune process resulting from decreased viral clearance due to decreased CD19+ as well as delayed viral clearance due to viral induced CD4+ lymphopenia with subsequent autoimmune process.Citation32 Thus it can be concluded from these studies (including our study) that there is no stereotyped consistent cellular immune response among BP patients and no consensus about the underlying operating mechanism(s). These differences may be attributed to the variability in the designs of these studies. However, this conflict may have its implication on management of those patients.
Although the studied patients exhibited significant improvement of their mean follow up HB score, yet the ENoG, blink reflex and CMAP of the facial muscles improved but not to a significant level. In several studies, histological analysis of facial nerve in BP revealed a mixed lesion of various nerve injuries i.e. intact, demyelinated and degenerated nerves.Citation50–Citation52 It was also found that inflammation of the nerve initially leads to reversible neurapraxia but ultimately Wallarian degeneration occursCitation10Thus, it could be assumed that the recovery of the demyelinated fibers enhanced by medical treatment with or without a possible regeneration of the degenerated fibers (if the conditions were favorable and the pathology was axonotemesis), all of which can improve the function sizably particularly if demyelination is the predominant pathology. On the contrary, improvement of CMAP and ENoG depends solely on regeneration of the degenerated fibers which may take a longer time and need optimal tissue conditions to take place. Hence, rapid and sizable improvement of CMAP and ENoG is unlikely compared to HB. This assumption is confirmed and can be explained by the significant improvement of HB score in patients with axonopathy on follow up assessment (data not shown, P = 0.014). Moreover, the inherent nature of ENoG makes it susceptible to variabilities including impedance, electrode location, skin resistance, different distribution of muscle mass and the patient's tolerance level to the stimulus intensity.Citation27,Citation53 Therefore its results should be interpreted cautiously. The discrepancy between the improvement of HB and ENoG was further confirmed by the lack of correlations between the follow up HB and ENoG among patients.
Blink reflex has the advantage over ENoG and facial nerve conduction in being able to examine the proximal part of the facial nerve in BP.Citation28 However, there was no significant improvement on comparing its initial and follow up parameters. This can be explained on the same basis as ENoG i.e. the concept of a mixed nerve lesion in BP. It is to be noted that some patients who were grade VI on HB had initial normal latencies of the blink reflex. This may be explained by the presence of few intact nerve fibers which could convey impulses but were not sufficient to perform functional movement. Our results regarding ENoG were consistent with that of Honda et al. who found that the ENoG recovery tended to be delayed compared to clinical recovery in BP.Citation51 Also in another study ENoG failed to provide accurate information regarding the prognosis or the rate of recovery in BP as it had no correlation with the probability of recovery in such patientsCitation54However, unlike our results R1 of the blink reflex paralleled the recovery of facial paralysis well.Citation51,Citation55 In this study, patients were divided into those with demyelination, and those with axonal degeneration of the facial nerve according to the initial electrophysiological study which was confirmed later on by improved HB grading on follow up. Four patients with demyelination recovered within 7 to 10 days, while the remaining one recovered completely after 8 weeks. It is to be noted in this study that there was uneven involvement of the facial nerve branches as far as the pathology is concerned (judged by the EMG and ENoG) i.e. some patients showed features of axonopathy in some muscles and demyelination in others and even one patient with demyelinating lesion and who recovered completely after 10 days, had a significant decrease in ENoG of the lower face muscles namely, nasalis and OOr. This implies that ENoG may give false positive results denoting axonal degeneration (particularly in absence of EMG abnormalities).
Some follow up electrophysiological parameters were correlated with the initial percentage of the immune cells in peripheral blood of the studied patients signifying some prognostic value of these cells. The initial CD8+ T cells percentage was positively and significantly correlated with the follow up values of OOr CMAP amplitude and the ENoG of nasalis and OOc muscles of the affected side. This emphasizes the role of CD8+ cells in clearing the virus after reactivation (a window of opportunity for the immune system)Citation56and improving inflammation and hence promoting recovery of the nerve i.e. increasing the number of functioning fibers and hence increasing the ENoG and CMAP. Also the initial CD4+ T cells were significantly and negatively correlated with the disease duration implying that increased percentage of CD4+ cells will promote recovery by virtue of its role in controlling viral reactivation or autoimmunity as proposed mechanisms for BP and thus shorten the disease duration. From this study it can be concluded that decreased percentage of the peripheral blood CD3+, CD4+ & CD8+ subsets in BP patients together with increased B subset in patients with demyelination emphasize the role of cell mediated and humoral immunity in the pathogenesis of BP. These cells have some prognostic significance regarding disease duration and facial nerve recovery. Analysis of T lymphocytes subsets (especially B cells) may provide an additional parameter to differentiate patients with favorable from those with poor prognosis.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine
Available online 29 March 2012
References
- S.C.GilbertBell's palsy and herpes virusesHERPES9320027073
- P.P.DevrieseCompression and ischemia of the facial nerveActa Otolaryngol (Stockh)771974108118
- O.AbramskyC.WebbD.TeitelbaumR.A.AmonCellular immune response to peripheral nerve basic protein in idiopathic facial paralysis (Bell's palsy)J Neurol Sci26119751320
- N.HatoImmunological determinants and the spread of viral infection in facial nerve paralysis induced by herpes simplex virus in miceNippon Jibiinkoka Gakkai Kaiho.9941996544551
- J.SchirmP.S.MulkensBell's Palsy and herpes simplex virusAPMIS105111997815823
- Z.UnluA.AslanB.OzbakkalogluO.TungerS.SurucuogluSerologic examinations of hepatitis, cytomegalovirus, and rubella in patients with Bell's palsyAm J Physical Med Rehabil82120032832
- I.KaygusuzA.GodekmerdanE.KeleT.KarlidaSinasiYalcinMucahitYildizA.TazegulThe role of viruses in idiopathic peripheral facial palsy and cellular immune responseAm J Otolaryngol2562004401406
- S.MurakamiM.MizobuchiY.NakashitoT.DoiN.HatoN.YanagiharaBell's palsy and herpes simplex virus: identification of viral DNA in endoneurial fluid and muscleAnn Intern Med124119962730
- J.J.TownsendP.K.CollinsPeripheral nervous system demeylination with herpes simplex virusJ Neuropathol Exp Neurol4541986419425
- N.J.HollandG.M.WeinerRecent developments in Bell's palsyBMJ3292004553557
- T.LinderW.BossatD.BodmerBell's palsy and herpes simplex virus fact or mysteryOto Neurotol2612005109113
- S.DivitoT.L.CherpesR.L.HendricksA triple entente; virus, neurons and CD8+ T cells maintain HSV-1 latencyImmunol Res361-32006119126
- A.LannelloO.DebbecheE.MartinL.H.AttalahS.SamaraniA.AhmadViral strategies for evading antiviral cellular immune responses in the hostJ Leukocyte pathol7920061635
- T.LuiK.M.KhannaX.P.chenD.J.FinkR.L.HendricksCD8+ T cells can block herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neuronsJ Exp Med1911200014591466
- P.GroutBell's palsy and herpes simplexBMJ21977829830
- D.Q.SantosK.K.AdourBilateral facial paralysis related to sexually transmitted herpes simplex: clinical course and MRIOtolaryngo Head Neck Surg10831993298303
- M.DaheshiaL.T.FeldmanB.T.RouseHerpes simplex virus latency and the immune responseCurr Opin Microbiol11998340345
- C.M.PrestonRepression of viral transcription during herpes simplex virus latencyJ Gen Virol812000119
- M.UshinoK.KondoN.TakeuchiH.TojimaT.YamaguchiK.KagaPrediction of the prognosis of Bell's palsy using multivariate analysesOtol Neurotol2920076972
- T.G.ParslowLymphocytes and lymphoid tissueT.G.ParslowD.P.StitesA.I.TerrJ.B.ImbodenMedical Immunology10th ed.2001Appleton & LangeNew York, Chicago, San Francisco, Lisbon, London, Madrid, Mexico City, Milan, New Delhi, San Juan, Seoul, Singapore, Sidney, Toronto4060
- A.AvielE.OstfeldR.BursteinG.MarshakZ.BentwichPeripheral blood T and B subpopulations in Bell's palsyAnn Otol Rhinol Laryngol922 pt 11983187191
- M.Manos-PujolE.BuendiaM.MestreR.JimenezE.GilJ.P.MenenM.Manos-GonzalboCellular immune abnormalities in patients with recurrent Bell's palsyClin Otolaryngol1241987283287
- L.JonssonO.SjobergL.ThomanderActivated T cells and Leu-7+ cells in Bell's palsyActa Otolaryngol1051-21988108113
- D.TavernerTreatment of Bell's palsyLancet21971168
- DonaghyMichaelClinical diagnosisDonaghyMichaelBrain's diseases of the nervous system.11th ed2001Oxford University pressOxford, New York255
- T.L.YenC.L.DriscellA.K.LalwaniSignificance of House and Brackmann facial nerve grading global score in the setting of differential facial nerve functionOto Neurotol2412003118122
- R.L.HsiehC.W.WuL.Y.WangW.C.LeeCorrelates of degree of nerve involvement in early Bell's palsyBMC Neurology920092226
- D.C.PrestonB.E.ShapiroElectromyography and neuromuscular disorders. Clinical- electrophysiological correlation2nd ed.2007Elsevier, Butterworth- HeinemannPhiladelphia, Pennsylvania
- D.DumitruN.E.WalshL.D.PorterElectrophysiologic evaluation of the facial nerve in Bell's palsyAm J Phys Med Rehabil6741988137144
- H.J.LeeJ.A.DelisaSurface anatomy for needle electromyography: head and neckHurleyRobertManual of nerve conduction study and surface anatomy for needle electromyography4th ed.2005Lippincott Williams & WilkinsPhiladelphia
- F.M.SullivanI.R.SwanP.T.DonnanM.J.MorrisonB.H.SmithB.McKinstryEarly Treatment with prednisolone or acyclovir in Bell's PalsyN Engl J Med357200715981607
- H.TekgulM.PolatG.SardarogluT.IkizogluM.YalazN.KutukculerS.GokbenLymphocyte subsets in Bell's palsy; immune pathogenesis and outcome predictionPediatr Neurol3142004258260
- J.MillsViral infectionsT.G.ParslowD.P.StitesA.I.TerrJ.B.ImbodenMedical Immunology10th ed.2001Appleton & LangeNew York, Chicago, San Francisco, Lisbon, London, Madrid, Mexico City, Milan, New Delhi, San Juan, Seoul, Singapore, Sidney, Toronto617635
- G.M.FrankA.J.LepistoM.L.FreemanB.S.SheridanT.L.CherpesEarly CD4+ cell help prevents partial CD8+ T cell exhaustion and promotes maintenance of herpes simplex virus 1 latencyJ Immunol1842010277286
- Y.HoshinoL.PesnicakJ.I.CohanS.E.StrausRates of reactivation of latent herpes simplex virus from mouse trigeminal ganglia ex vivo correlate directly with viral load and inversely with number of infiltrating CD8+ T cellsJ Virol8115200781578164
- T.LiuK.M.KhannaB.N.CarriereR.L.HendricksGamma interferon can prevent herpes simplex virus type 1 (HSV-1) reactivation from latency in sensory neuronsJ Virol7520011117811184
- K.M.KhannaR.H.BonneauP.R.KinchingtonR.L.HendricksHerpes simplex virus-specific memory CD8+ T cells are selectively activated and retained in latently infected sensory gangliaImmunity162200328952905
- H.KisakiN.HatoM.MizobuchiN.HondaH.TakahashiH.WakisakaY.HitsumotoN.YanagiharaRole of T-lymphocyte subsets in facial nerve paralysis owing to the reactivation of herpes simplex virus type 1Acta Otolaryngol12532005316321
- L.T.FeldmanA.R.EllisonC.C.VoytekL.YangP.KrauseT.P.MargolisSpontaneous molecular reactivation of herpes simplex virus type 1 latency in micePNAS9922002978983
- M.L.FreemanB.S.SheridanR.H.BonneauR.L.HendricksPsychological stress compromises CD8+ T cell control of latent herpes simplex virus type 1 infectionsJ Immunol1792007322328
- B.SerafiniB.RosicarelliD.FranciottaR.MagliozziR.ReynoldsP.CinqueDysregulated Epstein-Barr virus infection in the multiple sclerosis brainJ Exp Med204200728992912
- P.KlenermanA.HillT cells and viral persistence. lessons from diverse infectionsNat Immunol62005873879
- H.TakahashiY.HitsumotoN.HondaN.HatoM.MizobuchiS.MurakamiH.KisakiH.WakisakaK.GyoMouse model of Bell's palsy induced by reactivation of herpes simplex virus type 1JNEN6062001621627
- T.JiangH.B.WangZ.M.FanY.G.HanL.XuPrevention of facial paralysis induced by herpes simplex virus type 1 (HSV-1) in mouse and establishment of a relapse model induced by reactivation of latent HSV-1Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi4292007683686
- K.E.WeckS.S.KimH.I.VirginIVS.H.SpeckB cells regulate murine gamma herpes virus 68 latencyJ Virol73199946514661
- Z.MikloskaP.P.SannaA.L.CunninghamNeutralizing antibodies inhibit the axonal spread of herpes simplex virus type 1 to epidermal cells in vitroJ Virol73199959345944
- P.P.SannaD.R.BurtonRole of Antibodies in Controlling Viral Disease: Lessons from Experiments of Nature and Gene KnockoutsJ virol7421200098139817
- L.JonssonO.SjobergL.ThomanderDepression of T cells in Bell's palsyAnn Oto Rhinol Laryngol972 pt 11988138141
- C.A.VedelerR.MaireH.NylandP.MollerImmunoglobulins, complement components and lymphocyte subpopulations in Bell's palsyEur Neurol2531986177182
- R.GussenPathogenesis of Bell's palsyRetrograde epineurial edema and postedematous fibrous compression neuropathy of the facial nerve. Ann Otol Rhinol laryngol864 pt 11977549558
- N.HondaN.HatoH.TakahashiH.WakisakaH.kisakiS.MurakamiK.Gyopathophysiology of facial nerve paralysis induced by herpes simplex virus type 1 infectionAnn Otol Rhinol Laryngol1117 pt 102002616622
- Y.MatsumotoJ.L.pulecM.J.PattersonN.YanagiharaFacial nerve biopsy for etiologic clarification of Bell's palsyAnn Oto Rhinol Laryngol13719882227
- H.JurokawaI.NakagawaM.KubotaElectrophysiological examination in Bell's palsy using ENoG and strength duration curveActa Neurochir4571996842
- D.H.LeeS.Y.ChaeY.S.ParkS.W.YeoPrognostic value of electroneuronography in Bell's palsy and Ramasy- Hunt syndromeClin Otolaryngol3122006144148
- J.MontaltR.BaronaM.ArmengotJ.Lopez TrigoJ.BasterraThe blink reflex in the electrophysiologic exploration of Bell's palsy and its prognostic valueAn Otorrinolaryngol Ibero Am41999401412
- A.A.NashaT cells and regulation of herpes simplex virus latency and reactivationJEM199200014551458