Abstract
Background
The objective of the present work is to determine the prevalence of Cyclospora cayetanensis in symptomatic and asymptomatic immune-competent children less than five years in Alexandria, Egypt.
Subjects and methods
This study was conducted on two groups: Group I: 100 children suffering from acute diarrhea for less than 14 days. Group II: 100 apparently healthy children without diarrhea. All patients were subjected to history taking, physical examination and stool examination by: direct smear examination, concentration using formol ether sedimentation and Sheather's sugar floatation technique and staining using modified Ziehl–Neelsen and modified trichrome stains.
Results
There was a significant difference between Cyclospora infected children in symptomatic (17%) and asymptomatic (6%) groups. Cryptosporidium was detected in 10 diarrheic children (10%), five cases were combined with Cyclospora infection and not detected in any of the asymptomatic group. Microsporidia, Giardia lamblia and Hymenolepis nana were also detected in the symptomatic group. There was no significant difference as regards age and residency of Cyclospora positive and negative cases in both groups. In asymptomatic group, Cyclospora infected cases were males while in negative cases, 50% were males. This was statistically significant. There was no significant difference between the type of feeding and the Cyclospora infected cases in the two groups. As regards weight for height standard deviation (SD), there was no significant difference between the number of cases below normal in infected and noninfected diarrheic children. All asymptomatic cases were within the normal range without a significant difference. There was no significant difference between symptomatic Cyclospora infected and noninfected cases as regards the duration of diarrhea and clinical presentations.
Conclusion
Cyclospora infection in immune-competent symptomatic and asymptomatic children in Alexandria is common. Physicians should request a routine fecal examination for this parasite in any case with diarrhea or gastrointestinal troubles.
Abbreviation:
1 Introduction
Diarrhea is defined as the passage of three or more loose or liquid stools per day or more frequent passage than is normal for the individual.Citation1 For infants and children, this would result in stool output more than 10 g/kg/24 h, or more than the adult limit of 200 g/24 h.Citation2
Diarrheal disease is the second leading cause of death in children under five years of age.Citation1 It accounts for a large proportion (18%) of childhood deaths, with an estimated 1.8 million deaths per year globally. The World Health Organization (WHO) suspects that there are more than 700 million episodes of diarrhea annually in children less than five years of age in developing countries. While global mortality may be declining, the overall incidence of diarrhea remains unchanged at about 3.2 episodes per child per year.Citation3
Egypt Demographic and Health Survey (EDHS) 2008 noted that the prevalence of diarrhea among children under five years of age was nine percent. Children under age 24 months, particularly those age 6–11 months, were more likely to suffer from diarrhea than older children. Looking at the residential differentials, diarrheal episodes were more common among children living in Upper Egypt and the Urban Governorates than in Lower Egypt and the Frontier Governorates.Citation4
Diarrhea is caused by a variety of bacterial, viral, and parasitic pathogens. In developed countries, the vast majority of episodes of diarrhea are caused by viral pathogens.Citation5–Citation7 In developing countries with poor hygiene and sanitation, enteric bacteria and parasites are more prevalent.Citation8–Citation10
In Egypt, parasitic agents that most commonly cause acute diarrheal illness in children are Cryptosporidium parvum, Giardia lamblia and Entamoeba histolytica.Citation11
Coccidian protozoa of genus Cyclospora are obligate intracellular apicomplexan parasites that infect the mucosal epithelium of the small intestine or bile duct of a variety of hosts, mostly vertebrates.Citation12 Cyclospora was first identified as a human pathogen in three patients from Papua, New Guinea but it was thought to be a coccidium, probably a new species of Isospora.Citation13 The parasite was described from human fecal material in Peru and was identified as a coccidian of the genus Cyclospora because when the oocysts were induced to sporulate, they yielded two sporocysts, each containing two sporozoites. The human species was named Cyclospora cayetanensis. It differs significantly from all other Cyclospora species not only in its host but also in its oocyst stage, which is much smaller and spherical rather than oblong.Citation14,Citation15
Cyclospora is an important emerging cause of diarrhea worldwide that leads to significant morbidity and mortality. In immune-competent hosts, mild-to-moderate, self-limiting diarrhea is common while in immune-compromised hosts, severe intestinal injury and prolonged diarrhea is observed.Citation16 The clinical presentation of C. cayetanensis also includes gastrointestinal (GI) symptoms such as loose or watery diarrhea, nausea, vomiting, abdominal cramps and loss of appetite; or constitutional symptoms such as unintentional weight loss, fever, chills, muscle aches, joint aches, generalized body aches, headache, or fatigue.Citation17 In developed countries, the disease has been associated with cases of travelers. The parasite is commonly isolated from travelers to Latin America, the Indian subcontinent and South East Asia.Citation18–Citation20
Direct person-to-person transmission of Cyclospora is highly unlikely because of the period needed by the oocysts outside the host to sporulate and become infectious.Citation19 Thus, a transmission vehicle must be involved. Cyclospora oocysts can be transmitted in humans through exposure to fecally contaminated environmental water, food or soil. In areas where environmental sanitation may be compromised, such as disadvantaged community settings, the frequency of transmission may be high.Citation21 Waterborne oocysts are a common source of infection, but definitive documentation is lacking.Citation19 A study designed to address the prevalence and risk factors for infection in El-Sharkia showed that water was an important source of infection. Cyclospora oocysts were detected in several water sources suggesting water as the main vehicle of transmission. The densities of water contamination by the oocysts indicated sewage contamination.Citation22 In Alexandria, the parasite was identified in different water sources, including swimming pools.Citation23 In the developing world, cyclosporiasis has been associated with eating vegetables in NepalCitation24 and Jordan.Citation25 In Egypt, the coccidium was isolated from lettuceCitation26 and bivalves (shell fish) collected in markets from Alexandria.Citation27 Contact with soil has been a risk factor for cyclosporiasis. Studies from Peru,Citation28 GuatemalaCitation29 and EgyptCitation22 showed this factor as an important source of infection among children.
Variations in prevalence of Cyclospora infections in endemic countries may be influenced by study design, geographic area, age, immunologic status of the population studied, seasonal variability of the parasite, methods of detection used and expertise of the microscopist.Citation21 From a review of 47,642 apparently immune-competent individuals attending health care centers in Peru, most of them with diarrhea, infection rates ranged from 0% to 13% (average 1.7%), whereas the isolation rates from matched asymptomatic controls varied from 0% to 4.2% (average 0.4%). Among 3340 immune-compromised persons, mostly HIV/AIDS patients with diarrhea, the percentages of Cyclospora infections ranged from 0% to 36% (average 4.5%). It appears that in endemic areas in Indonesia, the situation at the general population level is quite different than that observed in health center populations in whom a strong association of the parasite with diarrhea has been recognized.Citation30–Citation33
The diagnosis of Cyclospora infection is based on microscopic detection of oocysts in fecal specimens. Examination of wet mounts of fresh, unpreserved stool by means of bright-field microscopy reveals nonrefractive spheres that are 8–10 μm in diameter and contain numerous refractive globules enclosed within membrane.Citation14 Because of potential low oocyst numbers, we routinely use a concentration procedure before examination. The standard formalin-ethyl acetate (FE) sedimentation (centrifugation) concentration procedure has been routinely used and found to be efficient.Citation34 Floatation procedures for the concentration of Cyclospora oocysts also can be used. A variety of solutions have been used to float parasite oocysts.Citation34 Sheather's sugar floatation procedure, as recommended for detection of Cryptosporidium oocysts, is also the preferred procedure for Cyclospora. The use of acid-fast-stained smears serves as the standard for detecting Cyclospora oocysts. Other stains include routine trichrome modified trichrome, Giemsa, chromotrope, Gram-chromotrope, Kinyoun acid-fast, auramine–rhodamine and safranin stains.Citation35 Polymerase chain methods (PCR) have also been developed for diagnosis and detection in the environment, but the primers appear to cross react with Eimeria spp.Citation36–Citation38
The objective of the present work is to determine the prevalence of C. cayetanensis in symptomatic and asymptomatic immune-competent children less than five years of age attending Alexandria University Children's Hospital.
2 Subjects and methods
The present study was conducted on 200 children less than five years of age presented to the outpatient clinic of Alexandria University Children's Hospital in the period from July through December 2010. Any case suffering from a chronic disease or receiving treatment leading to immune-suppression was excluded. They were classified into two groups: Group I: 100 children aged from one to 59 months old and suffering from acute diarrhea persistent for less than 14 days. Group II: 100 apparently healthy children attending the outpatient clinic due to other causes than diarrhea. All children were subjected to the following:
| (1) | Thorough history taking, stressing upon: demographic data (age, sex, residency), feeding history (breast, formula or cow's milk feeding), duration of illness, presence of blood in stool, associated symptoms as nausea, vomiting, or fever. | ||||
| (2) | Complete physical examination, including: anthropometric measures (weight, height, weight for height) temperature and degree of dehydration according to WHO guidelines (no signs of dehydration, some and severe dehydration). Systemic examination was done to detect any associated illness. | ||||
2.1 Investigations
2.1.1 Collection of stool samples
Stool was collected in a clean container: (1) for the potty-trained child, the child was instructed to have a bowel movement in the container without urination. (2) Child in diapers, an urine bag was placed on the child to prevent urine from coming into contact with the stool specimen. Specimens were collected in a disposable diaper by turning the diaper inside out with the plastic side facing the skin. Specimens collected on the absorbent side were not acceptable. Specimens were placed in the appropriate container. Patient name, date, & time of collection were written on the container. The specimens were transported to the Lab as soon as possible.Citation39
2.2 Stool examination
| (a) | Direct smear examination (saline and iodine smear).Citation40 | ||||
| (b) | Concentration of each sample using Sheather's sugar floatation technique and formol ether sedimentation technique.Citation40 | ||||
| (c) | Staining using modified Ziehl–NeelsenCitation41 and modified trichromeCitation42 stains | ||||
Cyclospora oocysts were identified by their size measured by ocular micrometer and morphological criteria of different stains.Citation41–Citation43 Other pathogenic parasites found in the samples were also detected.
2.2.1 Data analysis
Statistical analysis was performed with SPSS (Version12). It was presented as mean (X) ± standard deviation (SD) for each subgroup and compared using Fisher Exact test (FEp) to assess the difference of means among the two groups and Mann–Whitney nonparametric test (MWp) and Monte Carlo test (MCp) to assess differences in two independent groups. Probability level of ⩽ 0.05 was considered significant.
2.2.2 Ethical considerations
Ethical approval was obtained from the committee of research, Publications and Ethics of Alexandria University. All procedures were explained to parents or guardians of the participating children and written informed consent was obtained. Infected patients were informed of their diagnosis. Concerned physicians were also informed in order to prescribe suitable treatment and follow-up the patients.
3 Results
3.1 Analysis of symptomatic and asymptomatic groups
The age of the diarrheic cases ranged between 1 month and 59 months, with a mean age of 13.01 ± 15.23 months. Whereas the age of the asymptomatic group ranged between 1 month and 42 months with mean age of 16.47 ± 12.80 months. This difference was statistically significant (p = 0.020). Regarding sex, the percentage of males among symptomatic cases was 65%, whereas in asymptomatic cases, it was 56%; however, this was not statistically significant (p = 0.193). As regards residency, 68% of symptomatic and 62% of asymptomatic cases were from rural areas; this also was not statistically significant (p = 0.374). 52% of symptomatic cases were breast fed, 47% were formula fed, and 1% was cow milk fed, whereas 64% of asymptomatic cases were breast fed and 36% were formula fed, with no statistical significance between the two groups (p = 0.120). As regards the nutritional state of the two studied groups (weight percentile, height percentile and weight for height standard deviation) one third of diarrheic cases were below 3rd percentile. However in the nondiarrheic cases, all children were in the normal range (between 3rd and 97th percentile). This difference was statistically significant (p ⩽ 0.001). At the time of presentation, 88% of cases presented with vomiting, 73% with fever, 3% with dysentery and 61% came with moderate or severe dehydration.
3.2 Analysis of Cyclospora infected cases
3.2.1 Results of stool analysis
Cyclospora was detected in stool of 17 children among symptomatic (17%), and of 6 children among asymptomatic cases (6%) (). This was statistically significant (p = 0.015). The pure Cyclospora infected cases were 12 only (12%), but this was not statistically significant (p = 0.387). Cryptosporidium was detected in 10 diarrheic children (10%), five cases were combined with Cyclospora infection (these cases were excluded from the total number of Cyclospora infected cases as shown above) and the other five cases were pure Cryptosporidium infection while it was not detected in any of control group, which was statistically significant when compared with the control group (p = 0.031). Microsporidia was detected in five diarrheic children (5%) and not in the nondiarrheic group. G. lamblia was also detected in two cases of diarrheic children (2%) and one case of control group (1%). Hymenolepis nana eggs were detected in one case of diarrheic children (1%) and not in any of the control group ().
Figure. 1 Cyclospora cayetanensis oocyst (arrows) in stool samples stained with Modified Ziehl–Neelsen stain ×1000.
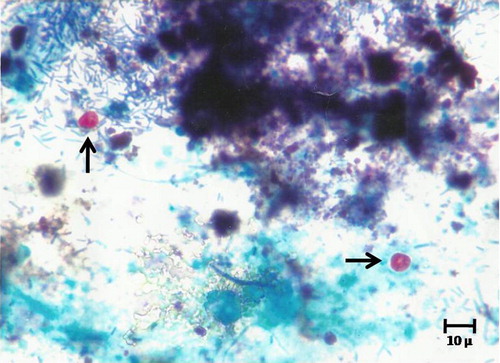
Table 1 Different parasites detected in stool samples from diarrheic and nondiarrheic children included in this study.
3.2.2 Demographic data
The results were summarized in . There was no significant difference as regards age and residency of Cyclospora positive and negative cases in the symptomatic and asymptomatic groups. Ten out of twelve (83.3%) symptomatic Cyclospora infected cases were less than two years of age. Two out of six (33.3%) patients of asymptomatic infected cases were less than two years of age.
Table 2 Demographic data of Cyclospora positive and negative cases among symptomatic and asymptomatic groups.
As regards the gender, Cyclospora positive cases in the symptomatic group are not statistically significant when compared with negative cases (p = 0.606). However in the asymptomatic group, all Cyclospora infected cases were male while in negative cases, 50% were males. This was statistically significant.
3.2.3 Feeding
There was no significant difference between the type of feeding and the Cyclospora infected cases in the two groups as shown in .
Table 3 Relation between Cyclospora positive and negative cases and type of feeding in symptomatic and asymptomatic groups.
3.2.4 Anthropometric measures
In the symptomatic group, Weight percentile of 25% of Cyclospora cases was below third percentile. Among Cyclospora negative cases, 28.4% were below third percentile in growth curves. There was no significant difference between the two groups (p = 0.175). As regards height percentile, 33.3% of Cyclospora cases in symptomatic group were below third percentile. While in Cyclospora negative cases, 21.6% were below third percentile. Also there was no significant difference (p = 0.107). Weight for height standard deviation (SD) for 33.3% of Cyclospora cases with diarrhea was below −2 SD. Whereas in Cyclospora negative cases in the same group, 17% of them were below −2 SD which is the normal value for this age group. There was no significant difference between the two groups (p = 0.192). As regards the asymptomatic cases, all of them were between 3rd and 97th percentiles of weight and height. Also, weight for height standard deviation (SD) of all these cases was within the normal range. There was a significant difference between nondiarrheic and diarrheic cases ().
Table 4 Relation between Cyclospora and anthropometric measures in the two studied groups.
3.2.5 Duration of diarrhea
The duration was selected to be less than 14 days to fulfill the criteria of acute infection. The difference between the duration of diarrhea in Cyclospora infected and noninfected cases of symptomatic group was not statistically significant (p = 0.141), as shown in .
Table 5 Relation between Cyclospora positive and negative cases and duration of diarrhea in the symptomatic group.
3.2.6 Clinical presentation
As shown in , there was no significant difference between symptomatic Cyclospora infected and noninfected cases as regards vomiting, fever, dysentery and dehydration.
Table 6 Relation between Cyclospora positive and negative cases and clinical presentations in the symptomatic group.
4 Discussion
Diarrheal disease is the second leading cause of death in children under five years old, and is responsible for killing 1.5 million children every year. Globally, there are about two billion cases of diarrheal disease every year, and mostly results from contaminated food and water sources.Citation1 In Egypt, enteric pathogens were identified in 46% of children less than five years of age.Citation44
C. cayetanensis is a coccidian protozoan that has emerged as an enteric pathogen.Citation14 Numerous recent reports implicating Cyclospora in diarrheal disease have suggested that the organism has a wide geographic distribution, however, most of these reports have been among adults with a history of travel to developing countries or among human immune-deficiency virus-infected individuals.Citation45–Citation47
In the present work, there was a significant difference between age of diarrheic, and nondiarrheic groups. Also, there was a significant difference between the two groups as regards the weight for age, the height for age and weight for height standard deviation. This means that diarrhea affect the nutritional state of children in this age group significantly. These findings may have important implications for the effects on growth at age less than 24 months. These results were supported by previous studies that have shown deleterious effects on growth and development after symptomatic infections in children who acquired the infection at less than one year of age. Also boys were affected more significantly rather than girls.Citation48
At the time of presentation, 88% of cases presented with vomiting, 73% with fever, 3% with dysentery and 61% came with moderate or severe dehydration. In accordance with several studies in the same age group, in Fayoum in 2006, 84% had fever, 54% had vomiting, 9% had dysentery, and 48% came with dehydration.Citation44 In a study in Nepal in 2004, 67% had abdominal pain, 38% had vomiting, and 9% were reported to have had blood in their stools.Citation49
The current study findings revealed that Cyclospora infection was common among immune-competent children with 23% prevalence. Prevalence of cyclosporiasis in diarrheic children was 17% while of asymptomatic nondiarrheic children it was 6%. Because of the absence of follow-up information it is not known if the six infections in controls represented true asymptomatic carriage, as has been documented in Peru,Citation28 or infection during the incubation period prior to developing symptoms. In comparison with previous studies, it was detected in 12.1% of cases and 5.7% of controls in Nepal,Citation50 11% of cases and 4.2% of controls in Saudi Arabia,Citation31 in 9% of cases in El-MenoufiaCitation51 and Jordan,Citation25 with no available data about the asymptomatic control in the last two studies. Higher prevalence rates were reported in Ismailia, Egypt (19.6% of immune-competent and 34.4% of immune-compromised children with diarrhea)Citation52 and outside Egypt in Peru (14.3% of cases and 13.7% of controls).Citation53 Lower prevalence rates were reported in Cuba (4.4% in cases with no infection in controls),Citation32 and in another study in Nepal in 1995 (5% of cases and 2% in controls).Citation49
Detection of Cryptosporidium in 10% of cases but not in any control, in the present study, was in accordance with previous studies in Ismailia (11.6% of immune-competent diarrheic children and 0% in the controls)Citation52 and Cuba (11.5% of cases and 0% in the controls),Citation32 and it was less prevalent in Nepal (5%),Citation49 and Peru (3.4%)Citation53 of cases and also 0% in the controls. Five cases out of ten in diarrheic children infected with Cryptosporidia were co-infected with Cyclospora. These five co-infected cases were excluded from the 17 cases infected with Cyclospora because the cause of diarrhea may be due to one or both parasites. Cryptosporidium was the only associated parasite with Cyclospora among the symptomatic group. The high rate of co-infection in cyclosporiasis patients was also found in Peruvian children with a prevalence rate of 13%, and frequent multiple parasitism in 45.6%.Citation54 The high rate of co-infection between cyclosporiasis and cryptosporidiosis may cause nonspecific symptoms, including abdominal pain, loose or watery stool, which could easily be confused with other common intestinal diseases. In the present study, this finding was expected as cryptosporidiosis in most surveys was among the four major pathogens causing diarrheal diseases in children.Citation55
The age of Cyclospora positive cases ranged from 2 to 36 months. Ten of twelve cases (83.3%) of Cyclospora diarrhea were less than two years of age; a finding similar to that of Ortega et al.Citation14 Abdel-Wahab et al.Citation52 revealed that the infection rate was significantly higher in the age group 1–4 years and that infection rate decreased with age.Citation52 On the other hand, it was suggested that the development of partial immunity in older age groups protects them from the pathogenic effects of the coccidium, but not from re-infection.Citation56 Studies from Peruvian towns suggest that immunity becomes complete by adolescence.Citation57 Bern et al.Citation54 stated that after an initial episode of cyclosporiasis, the likelihood of diarrhea decreased significantly with each subsequent infection.Citation54 There was no significant difference between the number of males and females among positive Cyclospora cases as many previous studies reported.Citation49,Citation52,Citation58
In agreement with other studies,Citation52,Citation56 most of the infected children (75%) were living in rural areas. The authors related their data to personal hygiene and living environmental condition. In rural areas, plenty of simple toilets, deficiency of sanitary facilities and diffusing feces contamination were commonly seen, and most people were unaware of health knowledge and good hygiene habits.
In the present study, 66.7% of Cyclospora cases were formula fed, whereas 33.3% were breast fed, so breast-feeding showed a trend toward being protective against Cyclospora infection. Similar finding was also observed by Hoge et al.Citation49
Regarding nutritional state, 33.3% of Cyclospora cases were malnourished (they had Z-scores of less than −2 SD for weight-for-height). The same percentage was detected by Nimri et al.Citation25while it was 20% in Abdel-Wahab et al. study.Citation52 Rizk et al. detected Cyclospora among 5.6% of malnourished children.Citation59 Moreover, severe malnutrition was associated with C. cayetanensis and Cryptosporidium spp. causing persistent diarrhea in Nepal.Citation60
At the time of presentation, duration of diarrhea among Cyclopspora cases ranged from 1 to 7 days, with mean duration of 3.33 ± 1.67 days. Also it was ranged from 3 to 18 days with a mean of 7.2 ± 6.14 days in Nepal.Citation49 Abdel-Wahab et al.Citation52 reported that 83% of Cyclospora diarrhea had duration of 3 days or less.Citation52 However, there were reports of a longer duration of diarrhea in children with Cyclospora in El-Menoufia (mean duration of illness was 28 ± 8 days).Citation51
Among Cyclospora diarrhea, 100% of cases presented with vomiting, 58.3% with fever, no case presented with dysentery. One third of cases came with no dehydration, 58.3% with mild dehydration, and 8.4% with severe dehydration. In Ismailia, 96% of cases were associated with abdominal pain, 80% with fever, 76% with nausea, 35% with flatulence, 17% with vomiting and 1.6% with dysentery.Citation52 In Cuba, clinical characteristics associated with Cyclospora were abdominal pain (80%), vomiting (60%), fever (40%) and anorexia (20%).Citation32 In Peru, two types of clinical manifestations were found; an acute type that can cause dehydration, and a chronic condition with several digestive signs and/or symptoms, particularly abdominal pain.Citation61 Puente et al.Citation20 detected heart burn as a frequent symptom, a finding not often previously described. On the other hand, dehydration was reported as the only significant manifestation in infected patients from Colombia.Citation62
5 Conclusion
In conclusion, Cyclospora infection in immune-competent symptomatic and asymptomatic children in Alexandria is common. Further studies in indigenous populations are needed to determine the relative rates of symptomatic and asymptomatic infection among persons of all ages (including older children and adults), assess natural sources of the parasite and routes of transmission as well as seasonality as risk factor for infection. Physicians should request a routine fecal examination of this parasite in any case with diarrhea or gastrointestinal troubles.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 24 April 2012
References
- World Health Organization. Diarrheal disease. Fact sheet No. 330, August 2009.
- K.G.FayezChronic DiarrheaR.M.KliegmanR.E.BehrmanH.B.JensonB.F.StantonNelson Textbook of Pediatrics18th ed.2007Saunders ElsevierPhiladelphia, Pa19131914
- A.B.ZulfiqarAcute Gastroenteritis In ChildrenR.M.KliegmanR.E.BehrmanH.B.JensonB.F.StantonNelson Textbook of Pediatrics18th ed.2007Saunders ElsevierPhiladelphia, Pa19101912
- El-Zanaty F, Way A. Egypt Demographic and Health Survey 2008. Cairo, Egypt: Ministry of Health, El-Zanaty and Associates 2009;157–8.
- D.BassH.GreenbergGroup A Rota virusesM.BlaserP.SmithJ.RavdinInfections of the Gastrointestinal TractVol 11995Raven PressNew York967982
- X.L.PangJ.JoensuuT.VesikariHuman Calicivirus-associated sporadic gastroenteritis in Finnish children less than two years of age followed prospectively during a rotavirus vaccine trialPediatr Infect Dis J181999420426
- H.MustafaE.A.PalomboR.F.BishopEpidemiology of Astrovirus infection in young children hospitalized with acute gastroenteritis in Melbourne, Australia, over a period of four consecutive years, 1995 to 1998J Clin Microbiol38200010581062
- K.OnoS.K.RaiM.ChikahiraT.FujimotoH.ShibataSeasonal distribution of enteropathogens detected from diarrheal stool and water samples collected in Kathmandu, NepalSoutheast Asian J Trop Med Public Health322001520526
- M.AlamY.N.AkhtarS.S.AliM.AhmedM.AtiqA.AnsariF.A.ChaudhryH.BashirM.A.BangashA.AwaisA.SafdarS.F.HasnainA.ZafarSeasonal variation in bacterial pathogens isolated from stool samples in KarachiPakistan. J Pak Med Assoc532003125129
- M.J.FarthingGiardia lambliaM.BlaserP.SmithJ.RavdinInfections of the Gastrointestinal Tract1995Raven Press LtdNew York10811105
- A.M.El ShazlyS.E.AwadD.M.SultanG.S.SadekH.H.KhalilT.A.MorsyIntestinal parasites in Dakahlia Governorate, with different techniques in diagnosing protozoaJ Egypt Soc Parasitol363200610231034
- R.LainsonThe genus Cyclospora: (Apicomplexa), with a description of Cyclospora schneideri n.sp. in the snake from Amazonian BrazilMem Inst Oswaldo Cruz1002005103115
- R.W.AshfordOccurrence of an undescribed coccidian in man in Papua New GuineaAnn Trop Med Parasitol731979497500
- Y.R.OrtegaC.R.SterlingR.H.GilmanV.A.CamaF.DíazCyclospora species new protozoan pathogen of humansN Engl J Med328199313081312
- Y.R.OrtegaR.H.GilmanC.R.SterlingA new coccidian parasite Apicomplexa: Eimeriidae from humansJ Parasitol801994625629
- C.R.SterlingY.R.OrtegaCyclospora: an enigma worth unravelingEmerg Infect Dis519994853
- A.CristC.MorningstarR.ChambersT.FitzgeraldD.StoopsM.DeffleyOutbreak of cyclosporiasis associated with snow peasMorb Mortal Wkly Rep53372004876878
- D.R.ShlimM.T.CohenM.EatonR.RajahE.G.LongB.L.UngarAn alga-like organism associated with an outbreak of prolonged diarrhea among foreigners in NepalAm J Trop Med Hyg451991383389
- L.S.MansfieldA.A.GajadharCyclospora cayetanensis a food-and waterborne coccidian parasiteVet Parasitol12620047390
- S.PuenteA.MorenteT.Garcia-BenayasM.SubiratsJ.GasconJ.M.Gonzalez-LahozCyclosporiasis: a point source outbreak acquired in GuatemalaJ Travel Med132006334337
- L.Chacín-BonillaEpidemiology of Cyclospora cayetanensis: a review focusing in endemic areasActa Tropica1152010181193
- E.M.El-KaramanyT.I.ZaherM.M.El-BahnasawyRole of water in the transmission of cyclosporiarsis in Sharkia Governorate, EgyptJ Egypt Soc Parasitol3532005953962
- M.Y.YouseffA.M.KhalifaM.Z.El-AzzouniDetection of Cryptosporidia in different water sources in Alexandria by monoclonal antibody test and modified Ziehl–Neelsen stainJ Egypt Soc Parasitol281998487496
- J.B.SherchandJ.H.CrossEmerging pathogen Cyclospora cayetanensis infection in NepalSoutheast Asian J Trop Med Public Health2001143150
- L.F.NimriCyclospora cayetanensis and other intestinal parasites associated with diarrhea in rural area of JordanInt Microbiol62003131135
- I.F.Abou el NagaStudies on a newly emerging protozoal pathogen: Cyclospora cayetanensisJ Egypt Soc Parasitol291999575586
- A.Y.NegmHuman pathogenic protozoa in bivalves collected from local markets in AlexandriaJ Egypt Soc Parasitol332003991998
- G.MadicoJ.McDonaldR.H.GilmanL.CabreraC.R.SterlingEpidemiology and treatment of Cyclospora cayetanensis infection in Peruvian childrenClin Infect Dis241997977981
- C.BernB.HernandezM.B.LopezM.J.ArrowoodM.A.de MejiaA.M.de MeridaEpidemiologic studies of Cyclospora cayetanensis in GuatemalaEmerg Infect Dis561999766774
- R.ZerpaN.UchimaL.HuichoCyclospora cayetanensis associated with watery diarrhea in Peruvian patientsJ Trop Med Hyg981995325329
- F.A.Al-BraikenA.AminN.J.BeechingM.HommelC.A.HartDetection of Cryptoporidium amongst diarrheic and asymptomatic children in Jeddah, Saudi ArabiaAnn Trop Med Parasitol972003505510
- F.A.Nu˜nezO.M.GonzalezI.GonzalezA.A.EscobedoR.A.CordoviIntestinal coccidia in Cuban pediatric patients with diarrheaMem Inst Oswaldo Cruz982003539542
- D.J.FryauffR.KrippnerP.ProdjodipuroC.EwaldS.KawengianK.PagelowT.YunC.Von Heydwolff-WehnertB.OyofoR.GrossCyclospora cayetanensis among expatriate and indigenous populations of West Java IndonesiaEmerg Infect Dis51999585588
- M.L.EberhardN.J.PieniazekLaboratory diagnosis of Cyclospora infectionsArch Pathol Lab Med12181997792797
- T.R.GhimireJ.B.SherchanHuman Infection of Cyclospora cayetanensis: a review on its medico-biological and epidemiological pattern in global scenarioJ Nepal Health Res Counc420063132
- J.M.ShieldsB.H.OlsonPCR-Restricted Fragment Length Polymorphism method for detection of Cyclospora cayetanensis in environmental waters without microscopic confirmationAppl Environ Microbiol698200346624669
- D.A.RelmanT.M.SchmidtA.GajadharM.SoginJ.CrossK.YoderMolecular phylogenetic analysis of Cyclospora, the human intestinal pathogen, suggests that it is closely related to Eimeria speciesJ Infect Dis1731996440445
- N.J.PieniazekS.B.SlemendaA.J.da SilvaE.M.AlfanoM.J.ArrowoodPCR confirmation of infection with Cyclospora cayetanensisEmerg Infect Dis21996357359
- Cincinnati Children's [Internet]. Ohio: Cincinnati Children's Hospital Medical Center; 1999–2011. Stool Collection: Giardia/Cryptospordium and/or Ovum and Parasitic Infection; [updated 2009 Sep; cited 2011 Mar 9]; [about 2 screens]. Available from: <http://www.cincinnatichildrens.org/health/alpha/s/giardia-crypto.htm81>.
- D.T.JohnExamination of stool specimensE.K.MarkellM.VogeD.T.JohnMedical parasitology7th ed.1992W.B. Saunders CoPhiladelphia, London, Toronto, Montreal, Sydney, Tokyo406428
- M.A.BrondsonRapid dimethyl modified acid fast stain of Cryptosporidium oocyst in stool specimensJ Clin Microbiol191984952955
- E.KokoskinT.W.GyorkosA.CamasL.CandiottiT.PurtillB.WardModified technique for efficient detection of MicrosporidiaJ Clin Microbiol32199410741075
- L.TuliD.K.SinghA.K.GulatiS.SundarT.M.MohapatraA multiattribute utility evaluation of different methods for the detection of enteric protozoa causing diarrhea in AIDS patientsBMC Microbiol1520101011
- H.El-MohamadyI.A.Abdel-MessihaF.G.YoussefM.SaidcH.FarageH.I.ShaheenEnteric pathogens associated with diarrhea in children in Fayoum, EgyptDiagn Microbiol Infect Dis56200615
- E.G.LongA.EbrahimzadehE.H.WhiteB.SwisherC.S.CallawayAlga associated with diarrhea in patients with acquired immunedeficiency syndrome and in travelersClin Microbiol28199011011104
- C.W.HogeD.R.ShlimR.RajahJ.TriplettM.ShearJ.G.RaboldP.EcheverriaEpidemiology of diarrheal illness associated with coccidian-like organism among travelers and foreign residents in NepalLancet341199311751179
- J.W.PapeR.I.VerdierM.BoncyJ.BoncyW.D.JohnsonCyclospora infection in adults infected with HIVAnn Intern Med1211994654657
- D.FraserR.DaganL.NagganV.GreeneJ.El-OnY.Abu-RbiahR.J.DeckelbaumNatural history of Giardia lamblia and Cryptosporidium infections in a cohort of Israeli Bedouin infants: a study of a population in transitionAm J Trop Med Hyg.571997544549
- C.W.HogeP.EcheverriaR.RajahJ.JacobsS.MalthouseE.ChapmanL.M.JimenezD.R.ShlimPrevalence of Cyclospora species and other enteric pathogens among children less than 5 years of age in NepalJ Clin Microbiol33199530583060
- J.B.SherchandJ.H.CrossCyclospora cayetanensis in Nepal: a study of microbiological and epidemiological aspectsNHRC3200418
- N.E.NassefS.A.El-AhlO.K.El-ShafeeM.NawarCyclospora: a newly identified protozoan pathogen of manJ Egypt Soc Parasitol281998213219
- Amina M.Abdel-WahabSonia G.El-SharkawyHanan Z.E.RayanEman M.HusseinDetection of Cyclospora cayetanensis Infections among Diarrheal Children Attending Suez Canal University HospitalPUJ1120083746
- S.O.Cordova PazV.F.VargasV.A.GonzalezIntestinal parasitism in Peruvian children and molecular characterization of Cryptosporidium speciesParasitol Res9862006576581
- C.BernY.R.OrtegaW.CheckleyJ.M.RobertsA.G.LescanoL.CabreraM.VerasteguiR.E.BlackC.SterlingR.H.GilmanEpidemiologic differences between cyclosporiasis and cryptosporidiosis in Peruvian childrenEmerg Infect Dis82002581585
- A.S.HartM.T.RidingerR.SoundarajanC.S.PetersA.L.SwiatloF.E.KockaNovel organism associated with chronic diarrhea in AIDSLancet3351990169170
- Y.R.OrtegaC.R.SterlingR.H.GilmanCyclospora cayetanensisAdv Parasitol401998339418
- K.KimuraS.K.RaiG.RaiS.InsisiengmayM.KawabataP.KaranisS.UgaStudy on Cyclospora cayetanensis, associated with diarrheal disease in Nepal and Lao PDRSoutheast Asian J Trop Med Public Health36200513711376
- K.WangC.LiJ.WangY.TianCyclospora cayetanensis in Anhui, ChinaWorld J Gastroenterol86200211441148
- H.RizkM.SolimanCoccidiosis among malnourished children in Mansoura, Dakahlia Govemorate, EgyptJ Egypt Soc Parasitol312001877886
- C.MukhopadhyayG.WilsonD.PradhanP.G.ShivanandaIntestinal protozoal infestation profile in persistent diarrhea in children below age 5 years in western NepalSoutheast Asian J Trop Med Public Health38120071319
- S.Burstein AlvaCyclosporosis: an emergent parasitosis: I: clinical and epidemiological aspectsRev Gastroenterol Peru2542005328335
- J.Botero-GarcésM.N.Montoya-PalacioJ.I.BarguilA.Castaño-GonzálezAn outbreak of Cyclospora cayetanensis in MedellínColombia Rev Salud Publica832006258268