Abstract
Background
ICSI/ET in endometriosis patients has poor outcome by traditional protocols. The key enzyme in the biosynthesis of estradiol, aromatase, has been demonstrated within endometriosis. Combined administration of aromatase inhibitor and GnRH-agonist may efficiently suppress estrogen biosynthesis through a combined pituitary, ovarian and local factors in the implants.
Objective
Evaluate the effect of using letrozole in improvement of the results of ICSI/ET in endometriosis women with long agonist protocol.
Patients
Sixty infertile women with minimal and mild endometriosis according to the revised American Fertility Society classification were scheduled for ICSI/ET.
Methods
Women were randomized into two groups. Group 1: using the traditional luteal long agonist protocol using triptorelin 0.1 and Group 2: using letrozole 5 mg/day started 5 days after the start of GnRH agonist for 5 days. All patients were monitored with day 6 serum estradiol level and estradiol at day of HCG. The number of days of stimulation, number of retrieved oocytes, number of MII oocytes, cleavage rate, and pregnancy rate were studied in both groups.
Results
Days of stimulation were significantly higher in the treated group (p = 0.019). Oocytes number was not affected (10.57 ± 6.14) and (11.21 ± 6.41) (p = 0.516) in groups 1 and 2 respectively also, the number of embryos was not affected (p = 0.955). Nine (32.1%) pregnant cases of 28 were in the first group while 8 from 27 in the second group (29.6%).
Conclusion
Letrozole significantly affected days of stimulation of ICSI cycle in endometriosis patients without affecting pregnancy rate.
Keywords:
1 Introduction
Endometriosis is an estrogen-dependent disorder defined as the presence of endometrial tissue outside of the uterine cavity.Citation1
The increased incidence of infertility with endometriosis may reflect a higher incidence of abnormal oocytes, defective embryos or failed implantation. Patients with moderate or severe endometriosis may have anatomic distortion of the fallopian tubes and ovaries.Citation2,Citation3
Several studies have compared the success rates of IVF in women with endometriosis with women who have other indications for IVF. Results suggested that fertilization rates were reduced in women with endometriosis compared with those with tubal or unexplained infertility.Citation4 Others indicate lower pregnancy and implantation rates in women with endometriosis.Citation5,Citation6
Prospective randomized trials have suggested that laparoscopic surgery improves fertility in mild/moderate endometriosis.Citation7 Based on these studies surgical treatment of minimal to mild endometriosis seems to offer a small but significant benefit with regard to fertility outcome. Furthermore, the surgical removal of peritoneal endometriosis may also be important to prevent progression of endometriosis in some patients. However, care is needed to prevent adhesion formation that could result as a consequence of over-enthusiastic excision of minimal to mild endometriosis. After laparoscopic resection and ablation of stages I–II endometriosis, monthly fecundity rate in a randomized control trial was 4.7%, as compared with 2.4% for women who underwent only a diagnostic procedure.Citation8 However, in some cases of minimal and mild endometriosis non-visualized endometriotic spots either on the peritoneal surface or deep implantation were identified on visually normal peritoneum.Citation9
In a meta-analysis of three large studies it was found that prolonged pretreatment with gonadotropin-releasing hormone analogue before IVF has been reported to improve clinical pregnancy rates in infertile women with endometriosis. The administration of GnRH agonists for a period of 3–6 months prior to IVF or ICSI in women with endometriosis increases the odds of clinical pregnancy by fourfold.Citation10
Another study, confirmed that GnRH-a does not generally improve results of ART. In mild endometriosis after 4 months of GnRH-a therapy, the pregnancy rates per embryo transfer were not higher than those obtained with conventional GnRH-a therapy in the long protocol before IVF–ET.Citation11 Also, in a randomized trial it was found that in patients with stage I or II endometriosis, there was no significant difference between long and ultra long protocol with respect to clinical pregnancy rate per cycle, also it is not suitable for poor responders plus the cost and the duration of therapy.Citation12 The recent evidence showed that suppression of ovarian function to improve fertility in minimal and mild endometriosis is not effective and should not be offered for this indication alone.Citation13
Studies have shown an increase in aromatase enzyme expression and high levels of the enzyme itself in endometriotic tissue. Small studies using the aromatase inhibitor anastrozole have demonstrated a marked reduction in size of endometriotic implants as well as a reduction or disappearance in pelvic pain. Furthermore, the aromatase inhibitors not only block aromatase activity within the ovary but also act directly on the aromatase enzyme found in the endometriotic tissue, lowering both estrogen levels and PG E production. These two actions may potentially give it an advantage over the medical treatments available.Citation14,Citation15
2 Aim of the work
The aim of this study is to evaluate the effect of using letrozole (aromatase inhibitor) to improve the success of ICSI/ET in women with minimal to mild endometriosis using the long protocol.
2.1 Patients
All the women included in the study were recruited from infertility clinic at the Elshatby Maternity University Hospital.
Sixty cases of minimal to mild endometriosis infertile women were scheduled for ICSI.
2.2 Inclusion criteria includes
| I. | Age less than 35 years. | ||||
| II. | Basal serum FSH less than 10 IU. | ||||
| III. | Minimal to mild endometriosis as diagnosed by laparoscopy and lesions classified according to the revised American Society for Reproductive Medicine scoring. | ||||
2.3 Exclusion criteria include
| I. | Moderate or severe endometriosis according to the revised American Society for Reproductive Medicine scoring. | ||||
| II. | Potentially poor responders. | ||||
| III. | Previous ovarian surgery. | ||||
3 Methods
All the women in the study started the luteal long agonist protocol at day 21 (midluteal) and the informed consent was taken from the patients before the beginning of the study.
All the women were subjected to a peritoneal biopsy from the suspected lesions during diagnostic laparoscopy. Histological examination of the excised tissue was systematically carried out after hematoxylin–eosin staining, and the nature of the lesions was histologically confirmed in all cases (presence of glands and stroma). Then the patients were randomized into two groups.
3.1 Group A 30 patients
All the women continued the long agonist protocol starting GnRH agonist triptorelin (Decapeptyl®) at midluteal phase and starting stimulation using combined human menopausal gonadotrophin and purified FSH after complete suppression (300 IU).
3.2 Group B 30 patients
The patients continued the long protocol and started the aromatase inhibitor (letrozole) 5 days after the start of the agonist for 10 days of 5 mg/day as was done in a pilot study.
In both groups suppression was verified by: serum estradiol level less than 50 pg/ml, thin endometrium and no ovarian activity. Both groups were followed up by follicular scanning and serial serum estradiol level till the criteria of HCG fulfilled.
3.3 Criteria of HCG administration
| • | Most of the follicles were 18–20 mm. | ||||
| • | Serum estradiol level was 150–200 pg/ml for each follicle >15 mm in diameter. | ||||
| • | Endometrial thickness was >9 mm. | ||||
Women were given 10,000 IU of HCG (Pregnyl, Organon, Egypt).
Transvaginal guided oocyte retrieval was done 36 h after HCG administration. Semen was prepared using the double wash and swim up technique.
After oocyte preparation metaphase II oocytes were injected. Fertilization was checked after 16–18 h and embryo transfer after 48 h.
3.4 Main outcomes
Day 6 serum estradiol level, final serum estradiol, endometrial thickness and pattern, number of retrieved oocytes, number of MII oocytes, fertilization rate, cleavage rate, and number of class A embryos.
3.5 Secondary outcome
Pregnancy rate per embryo transfer was calculated.
4 Statistical analysis
Data were fed to the computer using the Predictive Analytics Software (PASW Statistics 18).
Quantitative data were described using median, minimum and maximum as well as mean and standard deviation.
The distributions of quantitative variables were tested for normality using Kolmogorov–Smirnov test, and Shapiro–Wilk test. D’Agstino test was used if there was a conflict between the two previous tests. If it reveals normal data distribution, parametric tests were applied. If the data were abnormally distributed, non-parametric tests were used.
For abnormally distributed data, Mann–Whitney Test (for data distribution that was significantly deviated from normal) was used to analyze two independent populations. Correlations between two quantitative variables were assessed using Spearman coefficient.
Significance test results are quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level.
5 Results
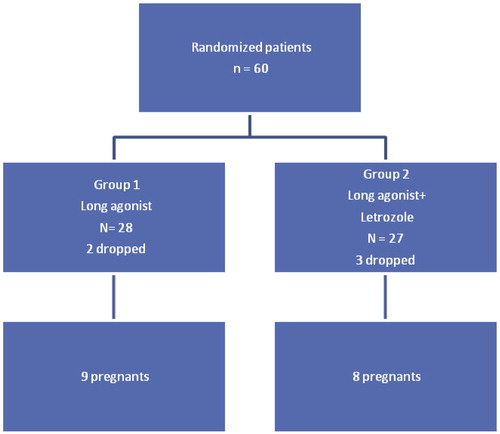
Sixty women were included in the study population. They were randomized into two groups. As shown in .
comparing the two studied groups, as regards the demographic data, it was found that there was no difference between both groups as regards age and years of infertility.
It was found that the mean age in the treated group was 29.07 ± 5.09 and 28.43 ± 5.08 in the control group (p = 0.646).
Comparing both groups as regards days of stimulation it was found that there was a significant difference between the days of stimulation between both groups (p, 0.019). In the study group it was 13.04 ± 1.53 and 12.07 ± 1.60 in the control group ().
Table 1 Comparison between the two studied groups according to days of stimulation.
As regards the number of oocytes after ovum pick up, it was found that there was no significant difference between the numbers of oocytes in both groups (11.21 ± 6.41) and (10.57 ± 6.14) in study and control group respectively. Also there was no significant difference in the percentage of mature oocytes in both groups (78.05 ± 16.93) and (78.98 ± 23.14) (p, 0.516).
As regards the number of embryos, there was no significant difference between both groups (4.85 ± 3.29) and (4.0 ± 2.52) in study and control group respectively.
Pregnancy rate was a secondary outcome measure in this study and it did not show any significant difference between the two studied groups. It was 29.6% and 32.1% in the letrozole treated group versus control group (p, 0.631).
6 Discussion
Endometriosis is associated with reduced response after ICSI/ET.Citation16 In a meta-analysis of 22 published studies the conclusion was that women with endometriosis have a reduced pregnancy rate (21% for stages I–II and 14% for stages III–IV) compared with that in women with tubal infertility. In addition, other indicators, such as circulating estradiol levels, numbers of retrieved oocytes, and decreased fertilization and implantation rates, have shown similar results.Citation6
In the two meta-analysis of Sallam et al.Citation10 it was concluded that the administration of GnRH agonists for a period of 3–6 months prior to IVF or ICSI in women with endometriosis increases the odds of clinical pregnancy. But prolonged suppression has high expenses and adverse effects of prolonged pituitary down regulation.Citation17
Till now there is no satisfactory stimulation protocol for cases of endometriosis as regards cost and for improving results of IVF.
This study used an aromatase inhibitor during the phase of down regulation before ICSI. This hypothesis is justified by the intracrine production of estrogen by the implants which is dependent not only on FSH but also locally regulated by PGE2 which at the same time is dependent on estrogen produced by the implants. So, the pituitary down regulation by GnRH agonist does not lead to complete suppression of the implants.
As regards days of stimulation, compared to the classical long agonist protocol, letrozole increased stimulation days significantly (p, 0.019). This may be due to high expression of P450 aromatase in endometriosis that is suppressed by letrozole.
As regards the serum level of day 6 stimulation and final E2 there was no significant difference between the studied groups. The percent of class A embryos was the same in both the study and the control group. This is due to the short term effect of letrozole so, less harmful effects on endometrium and embryos. These results are not different from what was found by Shahine et al. 2009 that the surgical treatment of endometriosis did not alter embryo quality.Citation18
As regards pregnancy rate, there was no significant difference between the studied groups. This may be due to the small number of each group. A big number may be needed to show the effect. The dose of letrozole may be small for the expected effect. Also, the effect of letrozole on endometrial receptivity and implantation is a big question still not answered. But still our results as regards pregnancy rate were comparable to pregnancy rate in the literature.Citation6,Citation19
Only one pilot study in 2009 had shown a new a protocol for the preparation of endometriosis patients for ICSI similar to ours but in cases of endometriomas. In the IVF/ICSI cycle, five (25%) had a clinical pregnancy, and three (15%) delivered healthy children (two singletons and one twin).Citation19 This pregnancy rate is comparable to our study which was 29.5–32.1%.
7 Conclusion
Letrozole has an effect on the duration of stimulation of ICSI cycle in endometriosis patients without affecting pregnancy rate.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 15 May 2012
References
- S.OzkanW.MurkA.AriciEndometriosis and infertility: epidemiology and evidence-based treatmentsAnn N Y Acad Sci1127200892100
- C.SimónA.GutiérrezA.VidalOutcome of patients with endometriosis in assisted reproduction: results from in-vitro fertilization and oocyte donationHum Reprod941994725729
- A.PellicerC.AlbertN.GarridoJ.NavarroJ.RemohiC.SimonThe pathophysiology of endometriosis – associated infertility. Follicular environment and embryo qualityJ Reprod Fertil Suppl552000109119
- T.SuzukiS.LzumiH.MetsubayashiH.AwajiK.YoshikataT.MakinoImpact of ovarian endometrioma on oocyte and pregnancy outcome in in-vitro fertilizationFertil Steril8342005908913
- E.TavmergenM.UlukusE.N.GokerLong-term use of gonadotropin-releasing hormone analogues before IVF in women with endometriosisCurr Opin Obstet Gynecol1932007284288
- F.AzemJ.B.LessingE.GevaA.ShaharL.Lerner-GevaI.YovelPatients with stages III and IV endometriosis have a poorer outcome of in vitro fertilization–embryo transfer than patients with tubal infertilityFertil Steril72199911071109
- T.Z.JacobsonD.H.BarlowP.R.KoninckxD.OliveC.FarquharLaparoscopic surgery for subfertility associated with endometriosisCochrane Database Syst Rev42002CD001398
- S.MarcouxR.MaheuxS.BerubeThe Canadian collaborative group on endometriosis. Laparoscopic surgery in infertile women with minimal or mild endometriosisN Engl J Med3371997217222
- F.NezhatC.J.AllanC.NezhatNonvisualized endometriosis at laparoscopyInt J Fertil3661991340343
- H.N.SallamJ.A.Garcia-VelascoS.DiasA.AriciLong-term pituitary down-regulation before in vitro fertilization (IVF) for women with endometriosisCochrane Database Syst Rev2520061
- F.FàbroguesJ.BalaschM.CreusS.CivicoF.CarmonaB.PuertoLong term down-regulation does not improve pregnancy rates in an invitro fertilization programFertil Steril7019984651
- C.H.KimY.K.ChoJ.E.MokSimplified ultralong protocol of gonadotrophin-releasing hormone agonist for ovulation induction with intrauterine insemination in patients with endometriosisHum Reprod1121996398402
- E.HughesJ.BrownJ.J.CollinsC.FarquharOvulation suppression for endometriosisCochrane Database Syst Rev1820073
- S.E.BulunK.M.ZeitounK.TakayamaH.SasanoMolecular basis for treating endometriosis with aromatase inhibitorsHum Reprod Update652000413418
- H.ShigetaH.MinaguchiK.NoguchiM.IkedaFundamental and phase 1 clinical study of YM511: a new aromatase inhibitorH.MinaguchiO.SugimotoEndometriosis today1996The Parthenon Publishing, GroupLondon334339
- A.BergendalS.NaffahC.NagyA.BergqvistP.SjoblomT.HillensjoOutcome of IVF in patients with endometriosis in comparison with tubal-factor infertilityJ Assist Reprod Genet151998530534
- H.FernandezC.LucasB.HédonOne year comparison between two add-back therapies in patients treated with a GnRH agonist for symptomatic endometriosis: a randomized double-blind trialHum Reprod196200414651471
- L.K.ShahineR.O.BurneyB.BehrEmbryo quality before and after surgical treatment of endometriosis in infertile patientsJ Assist Reprod Genet262–320096973
- K.LosslA.LoftN.L.FreieslebenCombined down-regulation by aromatase inhibitor and GnRH-agonist in IVF patients with endometriomasEur J Obstet Gynecol Reprod Biol144120094853