Abstract
Foxp3 has been studied as a biomarker of Treg cells in many solid malignant diseases, although its role as an immunomodulator in B-NHL remain poorly understood and the effect of traditional chemotherapy on its expression remains unclear. In this study the role of circulating and intra-tumoral Treg and TGF-β in patients with B-NHL before and after chemotherapy was evaluated. Enumeration of Treg cells was carried out by flow cytometric staining of their cell surface markers CD4 and CD25 as well as by molecular analysis of its signature transcription factor FoxP3. Expression of FoxP3 was done using quantitative real-time PCR while TGF-β mRNA expression was semi-quantitatively assayed by the conventional reverse transcription-PCR. In addition, spontaneous versus mitogen-induced release of TGF-β by PBMCs was assessed by a short term cell culture followed by ELISA. This was done before and after six cycles of CHOP chemotherapy. The results were evaluated in relation to the clinicopathological data.
A significant increase in mRNA transcripts of both Fox P3 and TGF-β as well as the percentage of CD4+/CD25+ in B-NHL patients before receiving the chemotherapy were recorded, when compared either to healthy controls or to patients after completion the treatment regimen. Interestingly 6 cycles of CHOP treatment caused significant reduction in all parameters under study, relative to the situation before treatment. A significant enhancement in spontaneous TGF-β release in B-NHL patient either before or after chemotherapy was obtained. These results strongly confirm the possible involvement of Treg cells and TGF-β in orienting the clinical course of the disease as well as the ability of targeting them in immunotherapeutic approaches.
KEYWORDS:
1 Introduction
NHL is a heterogeneous group of malignancies involving uncontrolled clonal expansion of transformed B or T cells in the periphery. In Egypt, the incidence of NHLs is very high. According to Middle East Cancer consortium (MECC), the NHLs age standardized incidence rates (ASRs) are (16.3/100.000 person). This very high incidence makes NHLs the third most common cancer in Egyptian males and the second most common cancer in females as reported by National Cancer Institute (NCI) accounting for 10.9% of all cancers in Egypt diagnosed every year.Citation1,Citation2 The incidence of NHL has increased during the past several decades at an average rate of 3–4% per year. Most of this increase can be attributed to a rising incidence of mature B-NHL, which represent about 90% of all NHL cases.Citation3 The clinical course of this disease is variable and the molecular and cellular mechanisms responsible for the clinical heterogeneity of B cell NHL are largely unknown. Although many therapeutic regimens have been developed for B-NHL, many of them remain incurable with frequent relapse/remission cyclesCitation4 De Jong et al., (2005)Citation5 showed that the complex intractions between the neoplastic B cells and the surrounding microenvironment plays an important role in disease severity and clinical outcome as well as response to therapy. More recently, analysis of gene expression using microships in follicular and diffuse B cell lymphomas have indicated that the nature of non malignant lymphocytes within the affected lymphomas is of considerable prognostic importance.Citation6 Peripherally induced Treg cells play an important role in suppressing the antitumor immune response particularly within the tumor microenvironments where increased Treg cell frequencies and function might favour tumor development and growth interfering with CD8+ T cell-mediated immunity and subsequently, influencing the course of the disease.Citation7 Meanwhile, the mechanisms and the signal pathways by which TGF-β generates and expands these diverse Treg subsets remain largely unknown.Citation8,Citation9 It is now largely accepted that manipulation of Treg cells either by chemo- or immunotherapeutic regimens might represent a promising approach in the field of cancer management.Citation10
2 Aim and Methods
The present study aimed at assessment of peripheral and intratumoral Treg populations in Egyptian patients diagnosed as B-NHL either before or after defined cycles of chemotherapy in order to elucidate their possible role in orienting the clinical course of the disease as well as the possibility of targeting them in future prospects of antitumor approaches.MethodologySubjects: The study was carried out on 25 patients with B-NHL recruited from the Clinical Oncology Department at Medical Research Institute, Alexandria University (at year 2009 and follow up till 2011) in addition to 10 normal healthy age- and sex-matched individuals as controls. Following surgical excision and histopathological diagnosis, B-NHL patients received 4–6 cycles of CHOP as a selected therapeutic regimen, at 21 days intervals.Criteria for positive selection
| • | Histologically proven B-NHL.Citation11 | ||||
| • | Intermediate grade based on working formulation system. | ||||
| • | Performance status 0 and 1 based on Eastern Cooperative Oncology Group (ECOG) scale.Citation12 | ||||
| • | Adequate kidney, liver and cardiac functions. | ||||
All patients in the study were subjected to the following
| (1) | Full history taking with special reference to personal history of solid or hematological malignancies. | ||||
| (2) | Thorough clinical examination with special reference to pan or localized lymph node enlargement. | ||||
| (3) | Laboratory investigations including CBC, LDH, liver and kidney function tests. | ||||
| (4) | Radiological investigations including CT chest, CT or U/S abdomen and pelvis. | ||||
| (5) | Cardiac assessment by echocardiography. | ||||
| (6) | Follow up of the patients was carried out by radiological and laboratory investigations as well as clinical examination every 3 months in the first year after the end of treatment and every 6 months in the second year. | ||||
Methodology
| • | Excisional lymph node biopsy | ||||
Following surgical excision of suspected lymph nodes, they were immediately preserved in a solution of 10% buffered formaline for routine histopathological assessment and for molecular verification of both FoxP3 and TGF-β.
| • | Blood sampling | ||||
Approximately 5 ml of heparinised peripheral blood were obtained from all the subjects under study by vein puncture and used for total RNA extraction.
From all B-NHL patients (either before or after chemotherapy) as well as from normal healthy controls, PBMCs were isolated by density gradient centrifugation over Ficoll-Histopaque and were subjected to a short-term culture either in the presence or absence of PHA mitogenic stimulation. Culture supernatents were harvested and assayed for TGF-β release by ELISA. PBMCs were also used as a starting material for phenotypic analysis of CD4/CD25 double positive cells by flow cytometry. Furthermore, RNA extracted out of PBMCs was also used to study the expression of both TGF-β and Foxp3 (by real time-PCR). Finally, Foxp3 and TGF-β expression was also assayed in the tumor microenvironment in RNA extracts of excised lymph nodes by PCR.
2.1 Immunological studies
Separation of peripheral blood mononuclear cells by density gradient centrifugation over Ficoll-Histopaque, according to the method adopted by Boyum (1986).Citation13
| (A) | Assessment of TGF-β release by PBMC Details of the tissue culture assay were adopted from the original method described by Lederman et al., (1998).Citation14 TGF-β levels in the culture suppernatents of all samples, without and with PHA supplementation were determined using a quantitative enzyme- linked immunosorbant assay “ELISA kit.” Bender MedSystems GmbHH, Campus Vienna.Citation15 | ||||
| (B) | Enumeration of Treg (CD4 + CD25+) by flow cytometry This was done by a flow cytometric enumeration of both of them using relevant fluorescen and phycoerythrin-labelled monoclonal antibodies according to the method of Vaickus et al., (1991).Citation16 | ||||
2.2 Molecular studies
The present study involves molecular detection of mRNA of both Foxp3 and TGF-β in PBMCs and L.N extracts by PCR technique using quantative (real-time) PCR and conventional PCR respectively before and after chemotherapy.
| (a) | RNA extraction from PBMCs Total RNA was prepared from fresh PBMCs isolated from all subjects under study. This was done adopting the method described by n Boom et al., (1990)Citation17 and using specific columns and kits commercially available from Qiagen, USA. All extraction steps were carried out at room temperature under strictly aseptic conditions. | ||||
| (b) | RNA extraction from paraffin-blocked LN Total cellular RNA was extracted from paraffin blocks of B-NHL tissues with Trizol reagents according to the guidelines provided by Rupp et al., (1988)Citation18 under strictly sterile conditions in a biosafety cabinet, under RNAse-free conditions. | ||||
| (c) | Assessment of RNA integrity and Purity The most common method used to assess the integrity of total RNA is to run a sample of 5 μl of the extracted RNA on 1.5% agarose gel electrophoresis with 0.5× TBE buffer followed by staining with ethidium bromide.Citation19 The purity of extracted RNA was tested by 260/280 spectrophotometric analysis.Citation20 | ||||
| (d) | Reverse transcription of RNA All RNA extracts prepared either from PBMCs or LNs were immediately used as templates for preparation of respective complementary DNA sequences. According to Berchtold (1989)Citation21 using Gene Amp RT-PCR System (Gene). The obtained cDNA was used for the second amplification of the Foxp3 gene using the specific forward and reverse primers as well as specific probes.Citation22,Citation23 | ||||
| (e) | Foxp3 amplification in PBMCs and LN by real time PCR Foxp3 and GADPH specific primers and probes employed in real time PCR. | ||||
RQ = Ct target gene/Ct reference (housekeeping) gene.
Copy number = 2ΔCt where ΔCt is the calculated normalized Ct.
| (a) | Qualitative PCR detection of TGF-β gene The TGF-β gene was qualitatively monitored by standard PCR As described by Kufer et al., (2002).Citation24 | ||||
2.3 Statistical analysis
The statistical and graphical analysis of the results were carried out using statistical package for the social sciences (SPSS) version 10.0 Student’s t test was used to analyze the obtained data, the differences between groups and the correlation between all the measured parameters. P < 0.05 was considered statistically significant. The actuarial survival analysis was performed according to the method described by Kaplan and Meier.
3 Results
3.1 Study population
The present study was conducted on a total of 35 individuals; including 25 B-NHL patients diagnosed according to the results of histopathological and immunohistochemical assessment of surgically excised lymph nodes. They received 4–6 cycles of CHOP as a selected chemotherapy regimen. In addition, 10 healthy age- and sex-matched individuals were included in the study as negative controls.
illustrates the clinicopathological characteristics of our patients. They included 15 males and 10 females with male/female ratio of 3:2 having age range of 45–60 years with a mean ± S.D of 48.38 ± 5.65. The frequency of major pathological types encountered as well as the performance status of the patients and clinical stage. According to this table, 15/25 (60%) of the involved patients had B-NHL of small follicular type, while 10/25 (40%) were diagnosed as B-NHL of the large diffuse type. Concerning tumor grade, the study was done only on those diagnosed as grade II B-NHL while other grades were omitted from the study.
Table 1 Clinico-pathological characteristics of B-NHL patients.
3.2 Routine laboratory findings
Investigations were performed for all patients before starting the recommended CHOP treatment, regularly before each cycle and at the end of treatment cycles. This was done in order to monitor the effects of chemotherapy on liver and kidney functions as well as haemoglobin level. Only results obtained before starting treatment and those recorded at the end of chemotherapy are concerned and summarized in (). Statistical analysis of these data showed that significant variations were observed.
Table 2 Statistical comparison of hemoglobin, liver and kidney function tests in B-NHL patients before and after CHOP chemotherapy.
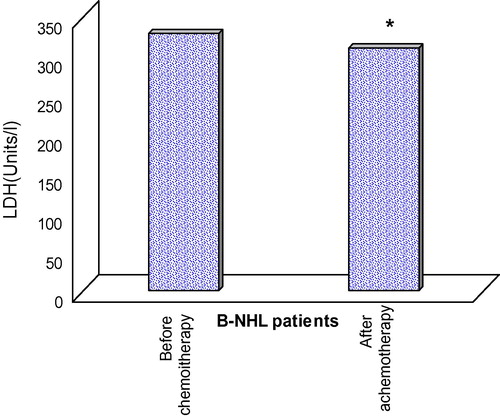
Lactate dehydrogenase (LDH)
As an important conventional prognostic marker for NHL, all patients were further assayed for serum LDH before starting and after completion of therapeutic CHOP regimen. Statistical analysis of the obtained data showed that a significant reduction was recorded in serum LDH level of B-NHL patients at the end of CHOP treatment as compared to that registered before chemotherapy ().
3.3 Immunological findings
3.3.1 Spontaneous and activation-induced release of TGF-β (pg/ml)
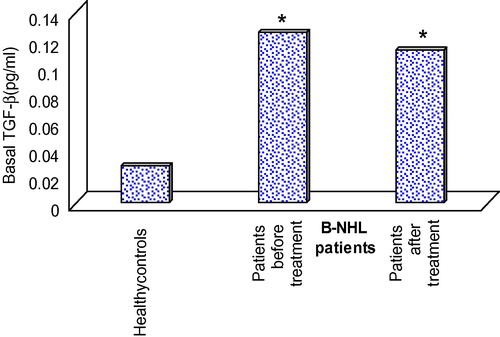
Spontaneous TGF-β release from PBMCs in healthy controls had a mean ± S.D of 0.027 ± 0.011 compared to 0.125 ± 0.102 and 0.182 ± 0.137 in B-NHL patients either before or after CHOP treatment respectively. Statistical analysis of these results revealed a significant enhancement in spontaneous TGF-β release by PBMCs of B-NHL patients when compared to healthy controls (p = 0.0136 and 0.032 respectively). However, no significant difference was recorded upon comparing basal TGF-β release by PBMCs of B-NHL patients before and after chemotherapy (p = 0.147) (see ).
Table 3 TGF-β spontaneous release (pg/ml) by PBMCs.
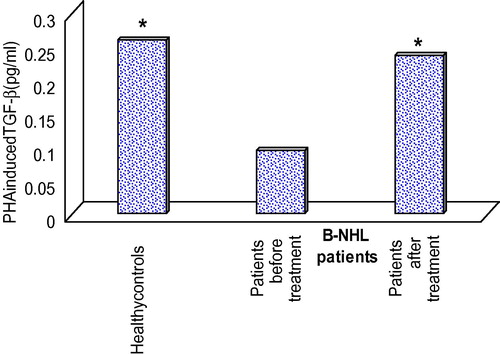
Concerning PHA- stimulated TGF-β release, a mean of 0.259 ± 0.282 pg/ml was recorded in healthy controls compared to 0.094 ± 0.113 pg/ml and 0.236 ± 0.186 in B-NHL patients before and after chemotherapy respectively (). Statistical analysis of these data showed a remarkable impairment in PHA-induced TGF-β release by PBMCs from B-NHL as shown in .
Table 4 Statistical analysis of the results of PHA induced TGF-β release (pg/ml) by PBMCs of healthy controls as compared to B-NHL patient either before or after CHOP treatment.
3.3.2 TGF-beta expression in PBMCs
The RT-PCR results of PBMCs mRNA expression of TGF-β are semi-quantitatively expressed as a relative index denoting the ratio between TGF-β expression to that of a housekeeping gene (β-actin) (see ). Results are summarized in and are represented in . According to the obtained data, statistical analysis revealed that there was a significant elevation in TGF-β mRNA expression in PBMCs of B-NHL patients either before or after treatment as compared to healthy controls (p < 0.001). In addition, there was a significant reduction in spontaneous mRNA expression of TGF-β in B-NHL patients by the end of the CHOP therapy in comparison to records obtained before treatment (P < 0.001).
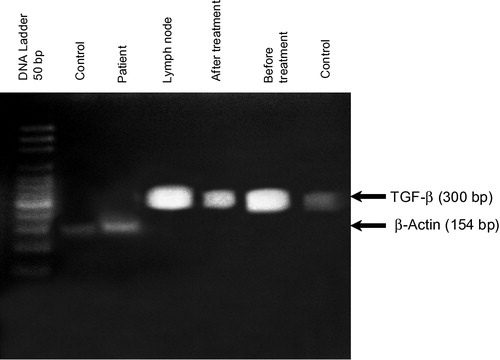
Table 5 mRNA expressionof TGF-β (relative index) in PBMCs from B-NHL patients.
3.3.3 Enumeration of peripheral blood CD4 and CD25
Peripheral blood detection of CD4 and CD25 was done for all subjects under study by flow cytometry using relevant monoclonal antibodies and results are expressed as percentage of double positive cells and are summarized in , the mean ± S.D of CD4+ cells in healthy controls was 36.04 ± 12.84 compared to 31.21 ± 7.33 and 34.9 ± 4.31 in B-NHL patients before and after CHOP treatment respectively. CD25+ cells of healthy individuals had a mean ± S.D of 14.9 ± 2.28 relative to 24.8 ± 5.07 and 24.4 ± 7.07 in B-NHL patients before and after chemotherapy respectively. Concerning CD4/CD25 double positive cells, the mean ± S.D of healthy controls was 10.9 ± 2.38 in comparison with 25.2 ± 6.27 and 16.3 ± 2.06 in B-NHL patients before and after treatment respectively. and .
Table 6 Statistical comparison of CD4+, CD25+ and CD4+/CD25+ double positive cells amonghealthy donors as compared to B-NHL patients before and after CHOP treatment.
3.3.4 Foxp3 expression in PBMCs
The real-time PCR results of Foxp3 expression in RNA extracts of PBMCs are expressed as relative index. Statistical analysis of these results point out to a significant increase in mRNA transcripts of Foxp3 in B-NHL patients before receiving CHOP treatment when compared either to healthy controls (p = 0.03) or to patients after completion of treatment (p = 0.05, and ).
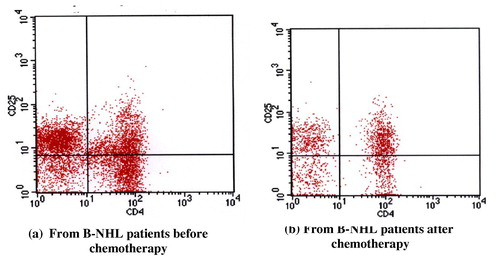
Table 7 Statistical comparison between the expression of Foxp3 (relative index) in RNA extracts of PBMCs from B-NHL patients (either before or after treatment) as compared to their corresponding healthy controls.
3.3.5 TGF-beta and Foxp3 expression in lymph nodes
Results are summarized in . Statistical analysis of the results shows that neither of the clinico-pathological parameters had significant effect on the intratumoral mRNA expression of TGF-β while that of Foxp3 was positively influenced by higher age (P < 0.001), male gender (p = 0.004), high serum LDH level (p = 0.003) and follicular type of affected lymph nodes (p = 0.039).
Table 8 Statistical analysis of intratumoral mRNA expression of Foxp3 and TGF-β (relative index) distributed according to patient‘s age, sex, gender and serum LDH level.
Correlation analysis and the outcome of the patients
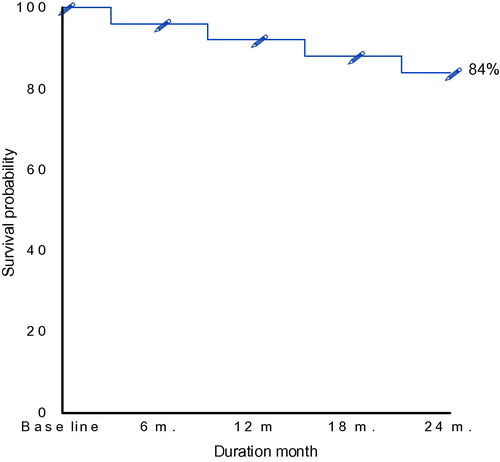
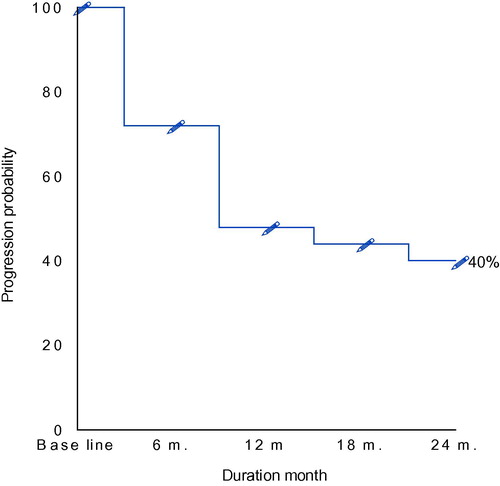
The median follow up of our patients was 24 months, progression, relapse, or death were observed in the studied patients. The overall survival (OS) was defined as the interval from date of diagnosis until death from any cause. Progression-free survival (PFS) was defined as the interval between diagnosis and progression of lymphoma or death due to lymphoma or toxicity of treatment. ( and and and ) represents the overall survival and the progression free survival of our patients which were 84% and 40% respectively at the event end point.
Table 9 Distribution of the studied patients regarding the outcome.
Table 10 Overall survival of B-NHL patients all over the period of follow up.
The response to treatment was assessed according to conventional criteria, Citation25 among the 25 patients 24% achieved complete response (CR)and 28% partial response, whereas 24% failed to respond to treatment.().
Table 11 Progression free survival of the studied patients all over the period of follow up.
Pearson correlation analysis (2-tailed) were performed between different parameters in all patients under study. Results are summarized in revealed the following:
| (1) | Significant positive correlation between serum LDH and of peripheral blood CD4/CD25 double positive phenotype (r = 0.494, p < 0.001). | ||||
| (2) | Significant positive correlation between peripheral blood Foxp3 expression and percentage of peripheral blood CD4/CD25 double positive phenotype (r = 0.319, p = 0.013). | ||||
| (3) | Significant positive correlation between peripheral blood Foxp3 expression and the performance status of the patients as well as the stage of the disease. | ||||
| (4) | Significant positive correlation between peripheral blood mRNA expression of TGF-β and CD4/CD25 double positive phenotype (r = 0.509, p = <0.001). | ||||
| (5) | Significant positive correlation between peripheral blood TGF-β expression and the performance status of the patients as well as the stage of the disease. | ||||
Table 12 Relation between response and over all survive in relation to both Foxp3 and TGF-β.
4 Discussion
NHL is a heterogeneous group of malignancies involving uncontrolled clonal expansion of transformed B or T cells in the periphery. The incidence of NHL has increased during the past several decades in the United States at an average rate of 3–4% per year. Most of this increase can be attributed to arising incidence of mature B-NHL, which represent about 90% of all NHL cases.Citation3
The most common B-cell lymphoma subtypes include follicular lymphoma, diffuse large B-cell lymphoma, marginal zone lymphoma and mantle cell lymphoma.Citation26 The clinical course of this disease is variable and the molecular and cellular mechanisms responsible for the clinical heterogeneity of B cell NHL are largely unknown. Although many therapeutic regimens have been developed for B-NHL, many of them remain incurable with frequent relapse/remission cycles.Citation4 Malignant B cells develop in specialized tissue microenvironments that are characterized by different populations of stromal cells and various subsets of T cells, including CTL and regulatory T-cells. These cells interact with malignant blood cells with variable degrees of dependency on signals from the microenvironment that involves number of immunologic molecules as chemokines, adhesion molecules and cytokines.Citation27
De Jong et al., (2005)Citation5 showed that the complex interactions between the neoplastic B cells and the surrounding microenvironment plays an important role in disease severity and clinical outcome as well as response to therapy. More recently, analysis of gene expression using microchips in follicular and diffuse B-cell lymphomas have indicated that the nature of non malignant lymphocytes within the affected lymphomas is of considerable prognostic importance.Citation6 In the present study, serum levels of LDH were monitored as a pivotal serological marker of all haematological malignancies including lymphomas. According to our results, 14 out of 25 (56%) of B-NHL patients enrolled in the study at the time of diagnosis had serum LDH levels exceeding 460 U/L. At the end of CHOP treatment on the other hand, neither of the examined cases had serum LDH levels above the reference range. Statistical analysis of these results showed a mildly significant reduction in serum LDH at the end of CHOP chemotherapy relative to that recorded before treatment. This finding is enforced by a recent observation by Mazhar et al., (2010)Citation28 who showed that LDH level was significantly raised in NHL patients and is positively influenced in patients with bone marrow infiltration than those without. They concluded that this non-invasive parameter is a useful indicator of the extent of the disease (see ).
Table 13 Correlation study of different parameters.
Moreover, Yang et al., (2009) Citation29 studied the clinical significance of serum LDH in NHL patients and showed that it was correlated positively, together with β2 microglobulin, to the advancement of the disease since both parameters were higher in patients with late stage NHL having B symptoms and bone marrow involvement. They found also that this marker was obviously lower in responders than non-responders to chemotherapy. Interestingly, the same authors proved that neither age nor gender of patients influenced serum level of LDH.
In the current work, we investigated the gene expression of Foxp3 and TGF-β in nodal RNA extracts of B-NHL patients. The results of such an intratumoral expression of both markers were sorted out according to some of the available clinicopathological parameters of NHL including patient‘s age, gender, serum LDH and tumor type. The results revealed that expression of Foxp3, but not TGF-β, was significantly higher in males, older age, elevated serum LDH and follicular lymphomas than their corresponding partners. This finding was in accordance with Zhi-Zhang et al., (2006)Citation30 who compared Foxp3 expression in nodal versus extra-nodal RNA extracts and showed that lymph node-derived Foxp3 expression was higher than that of peripheral blood, non-malignant tonsils, spleens and others.
These results were even reproducible when repeatedly investigated more recently by Lin et al., (2009).Citation31 They stated that NHL is generally a disease with higher male than female and older than younger age prevalence. However, the mechanism underlying this higher male incidence of the disease remains speculative and needs further investigation. The same authors claimed also that peripheral blood Treg level was not significantly correlated to other clinical features of NHL and attributed this finding to the fact that immune suppression by Treg had certain role in the occurrence of lymphomas but might not be related to subsequent tumor advancement once the disease is established.
In addition, Laurenti et al., (2011)Citation32 found that T reg population was correlated to the LDH level where increased T reg percentage was associated with elevated serum LDH. It would be convincing that Foxp3 expression in LDH-high group of B-NHL patients was higher than that of LDH-normal group since elevated serum levels of LDH might reflect more aggressive forms of the disease and it constitutes a putative component of the complex international prognostic index of all haematological malignancies including, in particular, B-NHL.Citation33
In the present study, peripheral blood genetic expression of Foxp3 and TGF-β was assessed by PCR in parallel to flow cytometric enumeration of peripheral CD4/CD25 double positive cells. These parameters were taken as characteristic markers for assessment of the proportion of peripheral Treg populations. Our study showed that the peripheral blood mRNA expression of TGF-β and Foxp3 was significantly higher in B-NHL patients relative to healthy controls. This was accompanied by increased percentage of CD4/CD25 double positive cells in peripheral blood of B-NHL patients (25%) as compared to healthy controls (10.9%). The above mentioned results point out to a significant enhancement of Treg cell frequencies in B-NHL patients relative to their corresponding healthy subjects.
These findings are in agreement with Mittal (2008)Citation33 who demonstrated that Treg populations in PBMCs of NHL patients were significantly higher than controls and were significantly correlated with both disease stage and serum LDH level. In addition, Liu et al., (2006)Citation34 studied the peripheral blood expression of Treg in B-NHL and proved that the percentage of this suppressive subset of T cells was significantly higher than controls. They concluded that the relative increase of CD4+/CD25high Treg cells in peripheral blood of B-NHL patients may be independently related to both the state of immune suppression and tumor progression.
In haematological malignancies other than NHL, Laurenti et al., (2011)Citation32 found that Treg population was also expanded in CLL patients compared to controls and was also positively correlated to specific markers of disease status. Also, Schmitt et al., (2008)Citation35 found that B-CLL patients have peripheral blood Treg percentage higher than healthy volunteers and further increased with progression of the disease. In the present study, the role of TGF-β in modulating peripheral Treg population and influencing the outcome of B-NHL was further assessed by comparison of the spontaneous versus activation-induced release of this cytokine following a short term culture of PBMCs. The results revealed that, similar to mRNA expression, the spontaneous release of TGF-β by PBMCs from B-NHL patients was significantly higher than that of healthy controls. Surprisingly, upon polyclonal T cell activation by PHA mitogen, cells of B-NHL patients were rendered non-responders when compared to cells of healthy donors. In fact, it remains unclear if the non-responsiveness of PBMCs from B-NHL patients represents a manifestation for the generalized state of immune suppression accompanying late stages of malignancy or simply due to accelerated rates of programmed cell death following in vitro mitogenic activation of B-NHL cells relative to those from healthy controls.
It has been suggested that the increased spontaneous release of TGF-β in B-NHL patients might be attributed to the enhanced mRNA expression for the same cytokine together with Foxp3 and CD4/CD25 double positive cells denoting enhanced peripheral blood Treg population. In accordance with this explanation, Fu et al., (2004)Citation36,Citation37 found that TGF-β is a key regulator of the signaling pathways that initiate and maintain Foxp3 expression and suppressive function in CD4 + CD25− precursors.
CHOP treatment has been selected as the regimen of choice on the basis that it is one of the most commonly adopted regimens for the majority of different subtypes of B-NHL. In addition, it is one of the first generation of combination treatments that may be given alone or together with monoclonal antibodies.Citation38,Citation39 After a median of 2 years of follow up, the overall survival of the studied group was 84% while the progression free survival was 40% and only 24% of our patients showed complete response. in accordance with our study, Sonneveld et al. (1995)Citation40, demonstrated that inspite that CHOP regimen is the standard treatment for younger and elderly patients with diffuse large –B- cell lymphoma, but it induces complete responses in only 40–50% of elderly patients, with three-year event free and overall survival rates of 30% and 35–40%, respectively.
According to the plan of the present work, all B-NHL patients enrolled in the study received CHOP chemotherapy in the form of 4–6 cycles at 21 days intervals and were assessed at the end of chemotherapy for the characteristic surface markers of Treg cells Foxp3 and CD4/CD25 positivity.
According to the obtained results, the peripheral blood percentage of CD4/CD25 double positive cells showed significant reduction as compared to the situation before chemotherapy. This was coincident with a similar reduction in mRNA expression of Foxp3 and TGF-β by PBMCs. Concerning the results of basal TGF-β release, it was shown that although a slight and insignificant decrease was recorded upon comparison with patients before treatment, it was still significantly higher than that of healthy subjects. Regarding mitogen-induced release of TGF-β, our results revealed that PBMCs of B-NHL patients after treatment almost retained the normal synthetic rate of this cytokine recorded before the start of chemotherapy.
This package of findings denotes a significant depletion of the elevated frequency of Treg cells that was recorded in B-NHL patients at the time of diagnosis. In fact, most of the immunomodulatory properties of CHOP combination of chemotherapy might be attributed to cyclophosphamide since Ghiringhelli et al., (2007)Citation41 observed that small repeated doses of metronomic cyclophosphamide in patients with different types of malignancies caused a reduction in the peripheral blood proportion of Treg cells (assessed by the percentage positivity of CD4/CD25high cells. This was associated with improved clinical manifestations and ameliorated overall survival rate of patients. In addition, Ladoire et al., (2009)Citation42 showed that a combination neoadjuvant chemotherapy containing both adriamycin and cyclophosphamide was effective in depleting Foxp3+ Treg cell infiltrates in tumor tissue in patients with breast cancer and this was associated with better improvement in patients with bad prognostic markers such as nodal affection and ER-/PR-phenotyping.
Zhang et al., (2008)Citation43 hypothesized that the use of chemotherapy, although known for its cytotoxic effect, may also benefit immunotherapy by preferential impairment of Treg cells. As a clinical evidence for this hypothesis, Lissoni et al., (2009)Citation44 demonstrated that cancer patients receiving a combination of chemo and immunotherapy manifested a significant decrease in Treg cells, enhanced anti-tumor immunity and improved therapeutic outcome compared to those receiving chemotherapy or radiotherapy but not immunotherapy. This finding seems of special interest since the traditional thought in this context is that chemotherapy adversely affects anti-tumor immunity by depleting immune cells and subsequently antagonizing tumor immunotherapy. However, the mechanisms by which the use of chemotherapy paradoxically benefits immunotherapy await profound elucidation.
More recently, Reeder and Ansell (2010)Citation45 investigated the possibility of using a fusion protein composed of IL-2 sequence and active fragments of diphtheria toxin (Dd) in treatment of aggressive B-NHL subtypes. They claimed that since CD25+ naturally occurring Treg cells are highly expressed in B cell lymphoma and suppress other intratumoral immune cells, the use of Dd to deplete Treg cells may be of clinical benefit. Interestingly, they found good response in both CD25 positive and negative B-NHL tumors suggesting an off-target effect for the drug.
Wu et al., (2011)Citation46 showed also that targeting Treg by an alternative drug between cycles of chemotherapy in a murine mesothelioma model almost completely depleted T reg cells and could improve the outcome of the disease.
On the contrary, Shi et al., (2004)Citation47 and Liu et al., (2006)Citation34 showed that peripheral blood CD4CD25 Treg cells were elevated following chemotherapy in B-NHL patients. Although contradictory to our findings, these conflicting results might be explained by ethnic variations between study populations in addition to variations in the stage of the disease, pathological type and schedule of chemotherapy as well as time of follow up after treatment. In addition, the state of Foxp3 expression was not addressed by both articles rendering their conclusion about Treg population incomplete and, subsequently, lack further experimental and clinical evidence.
In addition to possible induction and expansion of Treg phenotyping by malignant B-NHL cells, recruitment of these cell populations by lymphoma cells is another mechanism for increased intratumoral frequency of T reg. This mechanism was supported by Yang et al., (2006)Citation29, who investigated the expression of Treg cells in B-NHL and found that intratumoral Treg cells expressed CCR4 while lymphoma B cells expressed CCL22. They concluded that CCR4+ intratumoral Treg cells were attracted in vitro by supernatants from cultured lymphoma B cells secreting CCL22 and are involved in chemotaxis and migration of intratumoral Treg cells to tumor sites.
In summary, the magnitude of the present study is a confirmation for the role played by both intratumoral and peripheral Treg cell populations in suppressing antitumor immunity.
Our results indicated that selected chemotherapeutic agents in a mixture (the commonly used CHOP regimen) resulted in a remarkable impairment of peripheral Treg population manifested by reduced mRNA expression of Foxp3, decreased percentage of CD4CD25 double positive cells and impaired spontaneous molecular release of TGF-β. It remains unknown however, if chemotherapy selectively deplete peripheral blood and intratumoral Treg populations or this depletion is a spontaneous consequence of the pancytotoxic effect of CHOP components on tumor cell populations including Treg cells. Clarification of how chemotherapy exerts its immunomodulatory effects will aid in the development of better combination strategies of chemo-immunotherapies in enriching the field of cancer management.
Conflict of interest
None declared.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 24 September 2013
References
- Nadia M, Iman G, Iman A: cancer pathology registry and time trend analysis, NCI Egypt 2007.
- Elattar IA, Hassan NM, Lamee MM, Elbasmy AA. Cancer Profile at the National Cancer Institute, Egypt, 2002–2003. J Clin Oncol 2005 (ASCO Annual Meeting Proceedings.); 23: 9653–614.
- C.A.ClarkeS.L.GlaserChanging incidence of non-Hodgkin lymphomas in the United StatesCancer94200220152023
- J.DrachS.SeidlH.KaufmannTreatment of mantle cell lymphoma: targeting the microenvironmentExpert Rev Anticancer Ther52005477485
- D.De JongMolecular pathogenesis of follicular lymphoma: a cross talk of genetic and immunologic factorsJ Clin Oncol23200563586363
- S.MontiK.J.SavageJ.L.KutokMolecular profiling of diffuse large B-cell lymphoma identifies robust subtypes including one characterized by host inflammatory responseBlood105200518511861
- S.G.ZhengJ.H.WangJ.D.GrayH.SoucierD.A.HorwitzNatural and induced CD4+CD25+ cells educate CD4+CD25-cells to develop suppressive activity: The role of IL-2, TGF-beta and IL-10J Immunol172200452135221
- W.J.ChenJ.E.KonkelTGF-β and ‘Adaptive’ Foxp3 regulatory T cellsJ Mol Cell Biol220103036
- D.A.HorwitzS.G.ZhengJ.D.GrayThe role of the combination of IL-2 and TGF-beta or IL-10 in the generation and function of CD4+ CD25+ and CD8+ regulatory T cell subsetsJ Leukoc Biol742003471478
- R.H.SchabowskyS.MadireddiR.SharmaE.S.YolcuH.ShirwanTargeting CD4+CD25+FoxP3+ regulatory T-cells for the augmentation of cancer immunotherapyCurr Opin Investig Drugs8200710021008
- A.Lopez-GuillermoE.MontserratF.BoschApplicability of the International index for aggressive lymphomas to patients with low-grade lymphomaJ Clin Oncol12199413431348
- P.Solal-CelignyP.RoyP.ColombatFollicular Lymphoma International Prognostic IndexBlood104200412581265
- A.BoyumIsolation of leukocytes from human bloodScand J Clin Invest211986929
- M.M.LedermanE.ConnickA.LandayD.R.KuritzkesJ.SpritzlerSt. Clair M, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315J Infect Dis17819987079
- H.ZhangP.YangH.ZhouQ.MengX.HuangInvolvement of Foxp3-expressing CD4+ CD25+ regulatory T cells in the development of tolerance induced by transforming growth factor-β2-treated antigen-presenting cellsImmunology1242008304314
- L.VaickusE.D.BallK.A.FoonImmune markers in hematologic malignanciesCritical Rev Oncol/Hematol111991267297
- R.BoomC.J.SolM.M.SalimansC.L.JansenP.M.Wertheim-van DillenJ.van der NoordaaRapid and simple method for purification of nucleic acidsJ Clin Microbiol281990495503
- G.M.RuppJ.LockerPurification and analysis of RNA from paraffin embedded tissuesBiotechniques619885660
- B.A.PayerT.ReibergerK.RutterS.BeinhardtA.F.StaettermayerM.Peck-RadosavljevicSuccessful HCV eradication and inhibition of HIV replication by intravenous silibinin in an HIV-HCV coinfected patientJ Clin Virol492010131133
- B.GoergenS.JacobsP.SymmonsE.HomesK.-H.Meyer zum BuschenfeldeG.GerkenQuantification of HCV replication using one step competitive RT-PCR and a solid phase colorimetric detection methodJ Hepatol211994678682
- M.W.BerchtoldA simple method for direct cloning and sequencing of cDNA by the use of a single oligonucleotide and oligo (dT) in a polymerase chain reactionNucleic Acids Res171989453459
- A.OverberghD.GiuliettiB.ValckxR.DecallonneV.BouillonC.MathieuThe use of real-time reverse transcriptase PCR for the quantification of cytokine gene expressionJ Biomol Tech1420033343
- N.A.SaundersQuantitative Real- Time PCR. In: Real- time PCR: An Essential Guide. Edwards K, Logan J, Saunders N, London HorizonBioscience62004303333
- J.R.BrodyS.E.KernHistory and principles of conductive media for standard DNA electrophoresisAnal Biochem3332004113
- B.D.ChesonS.J.HorningB.CoiffierEt al. Report of an international workshop to standerize response criteria for non-hodjkin lymphoma: NCI Sponsored international Working groupJ Clin Oncol17199912441253
- Morton LM, Turner JJ, Cerhan JR, et al. Proposed classification of lymphoid neoplasms for epidemiologic research from the Pathology Working Group of the International Lymphoma Epidemiology Consortium (InterLymph). Blood 2007; 11.
- Burger JA, Ghia P, Rosenwald A, Cappio FC. The microenvironment in mature B-cell malignancies: a target for new treatment strategies. Blood 2009; 114: 152–3367-75.
- N.MazherZ.IqbalN.AslamN.MazherInt J Pathol81201019
- L.YangX.XuC.PengJ.WeiZ.SongZ.CongPrognostic values of serum LDH and β2-MG in patients with non-Hodgkin’s lymphoma Chinese-GermanJ Clin Oncol82009353355
- Yang ZZ, Anne J. Novak, Mary J. Stenson, Thomas E. Witzig and Stephen M. Ansell. Intratumoral CD4+CD25+ regulatory T-cell-mediated suppression of infiltrating CD4 + T cells in B-cell non-Hodgkin lymphoma. 2006 107: 3639–3646.
- Lin H, Sun XF, Zhen Z, Xia Y, Yu J, Lin TY, et al. Correlation between peripheral blood CD4+ CD25high CD127low regulatory T cells and clinical characteristics of patients with non-Hodgkin lymphoma. Chinese J of Cancer 2009;28:59–64.
- L.LaurentiM.M.MinerviniS.DeaglioL.BonelloL.De MartinoL.De PaduaRegulatory T-cell number is increased in chronic lymphocytic leukemia patients and correlates with progressive diseaseLeuk Res352011363368
- Mittal VS, Marshall NA, Duncan L, Culligan DJ, Barker RN, Mark A. Local and systemic induction of CD4+CD25+ regulatory T-cell population by non-Hodgkin lymphoma. 2008; 111: 5359–70.
- L iu L, Yao JX, Ding Q, Huang SA. CD4+ CD25 high regulatory T cells in peripheral blood of patients with B cell non-Hodgkin’s lymphoma. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 2006; 14:119–22.
- SchmittM, Giannopoulos K, http://www.ncbi.nlm.nih.gov/pubmed?term=%22 Schmitt%20 M%22%5BAuthor%5D, Kowal M, Wlasiuk P, Bojarska-Junak A, Chen J, et al. Characterization of regulatory T cells in patients with B-cell chronic lymphocytic leukemia. Oncol Rep. 2008 Sep; 20(3):677-82.
- M.L.ChenM.J.PittetL.GorelikRegulatory T cells suppress tumor-specific CD8 T mcell cytotoxicity through TGF-{beta} signals in vivoProc Natl Acad Sci20051022005419424
- S.B.JakowlewTransforming growth factor-beta in cancer and metastasisCancer Metastasis Rev252006435457
- J.O.ArmitageM.A.FyfeJ.LewisLong-term remission durability and functional status of patients treated for diffuse histiocytic lymphoma with the CHOP regimenJ Clin Oncol21984898902
- M.P.LinkJ.J.ShusterS.S.DonaldsonTreatment of children and young adults with early-stage non-Hodgkin’s lymphomaN Engl J Med337199712591266
- P.SonneveldM.de RidderH.van der LelieComparison of doxorubcin and mitoxantrone in the treatment of elderly patients with advanced diffuse non-hodjkin lymphoma using CHOP versus CNOP chemotherapyJ Clin Oncol13199525302539
- F.GhiringhelliC.MenardP.E.PuigMetronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatoryT cells and restoresTand NK effector functions in end stage cancer patientsCancer Immunol Immunother562007641648
- S.LadoireL.ArnouldL.ApetohPathologic complete response to neoadjuvant chemotherapy of breast carcinoma Is associated with the disappearance of tumor-infiltrating Foxp3+ regulatory T CellsClin Cancer Res14200824132420
- Menard C, Puig PE, et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatoryT cells and restoresTand NK effector functions in end stage cancer patients. Cancer Immunol Immunother 2007;56:641–8.
- L.ZhangM.JinK.DermawanS.XiongY.ChuDoes chemotherapy augment anti-tumor immunotherapy by preferential impairment of regulatory T cellsMed Hypothesis712008802804
- P.LissoniF.BrivioL.FunagalliG.MessinaS.MagalliG.PorroEffects of the conventional antitumor therapies surgery, chemotherapy, radiotherapy and immunotherapy on regulatory t lymphocytes in cancer patientsAnticancer Res29200918471852
- C.B.ReederS.M.AnsellNovel therapeutic agents for B cell Lymphoma: developing rational combinationsBlood117201114531462
- Y.X.ShiX.S.ZhangD.G.LiuY.Q.LiZ.Z.GuanW.Q.JiangCD4+CD25+T regulatory cells in peripheral blood of B-NHL patients with or without chemotherapyAi Zheng2352004 May597601