?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Hepatocellular carcinomas will emerge as a major form of malignancy in the coming decades. When these tumors are in advanced stages, few therapeutic options are available. Therefore, it is essential to search for new treatment modalities to fight this disease.
Aim
Evaluate the possible protective and therapeutic effects of Cannabis extract on dimethylnitrosamine (DMNA)-induced hepatocarcinogenicity in mice.
Methods
Seventy-five male mice were divided into five groups of 15 each: group I mice received corn oil only as the control group; group II mice were injected intraperitoneally with DMNA (10 μg/kg body weight) weekly for 12 weeks; group III mice were pretreated orally with cannabis extract (0.5 ml/kg body weight) every other day for two weeks before the injection of DMNA, and continued until the end of the experiment (12 weeks); group IV mice were treated orally with cannabis extract every other day simultaneously with DMNA injection and continued until the end of the experiment; group V mice were treated orally with cannabis extract every other day after receiving the last intraperitoneal injection of DMNA. A real time PCR was used to quantify telomerase reverse transcriptase and caspase-8 m-RNA expression level.
Results
As compared to the control group, mTERT mRNA expression level was significantly increased in group II. The gene in groups (III, IV, and V) was insignificantly higher than the control group but it was significantly decreased as compared to group II. The caspase-8 mRNA expression level was significantly decreased in all groups as compared to the control group. As compared to group II, caspase-8 mRNA level was significantly increased in group III.
Conclusion
The protective effect of cannabis extract is more pronounced in group taking cannabis before DMNA. Cannabinoids might exert their anti-tumor effects by the direct induction of apoptosis and can decrease telomerase activity by inhibiting the expression of the TERT gene. Coordination between inhibition of telomerase activity and induction of apoptosis might be a potential therapeutic agent for cancer treatment.
1 Introduction
Hepatocellular carcinoma (HCC) is one of the most common solid tumors and the third leading cause of cancer-related death worldwide.Citation1 Its prognosis remains reserved, with a 5-year survival rate of <5%.Citation2 Hepatocarcinogenesis is a multi-step process involving different genetic alterations that ultimately lead to malignant transformation of the hepatocytes.Citation3,Citation4 One of the molecular events that underlie the multigenetic process of hepatocarcinogenesis is the activation of human telomerase reverse transcriptase (hTERT) which is normally suppressed in most human somatic tissues after birth.Citation5,Citation6
The replicative potential of eukaryotic cells is regulated through specialized DNA structures called telomeres, which cap the ends of the chromosomes. Telomerase is a ribonucleoprotein complex composed of two essential components: a catalytic subunit with reverse transcriptase activity (hTERT) and RNA subunit with human telomerase RNA (hTR). In the adult organism, telomerase expression is restricted to a few cell types, most notably germ cells and stem/progenitor cells. Telomerase has been a target of increasing interest because high telomerase activity is one of the mechanisms that sustain the unlimited growth of cancer cells.Citation7,Citation8 The hTERT gene encodes the catalytic subunit of telomerase, which mediates pleiotropic effects, including the regulation of senescence and proliferation and plays an important role in carcinogenesis.Citation9
Programed cell death (apoptosis) is a potent mechanism that limits the expansion of tumor cells by triggering their suicide, while defects in apoptosis underpin both tumorigenesis and metastasis.Citation10 Caspase-8 belongs to the caspase family of proteases and plays a key role in the regulation of apoptosis during normal development as well as in adult life. Since signaling via the death receptor (extrinsic) pathway critically depends on caspase-8, the distribution of caspase-8 expression or function may contribute to human diseases.Citation11
Cannabis (bhang, ganja, charas, hashish, kif, marijuana etc.) is one of the popular plants among common people since time immemorial due to its various uses and abuses. The hemp plant Cannabis sativa produces unique compounds known as cannabinoids (CND).Citation12 The most important cannabinoids found in the cannabis plant are tetrahydrocannabinol (Δ9THC) and cannabidiol (CBD). Cannabinoids have been shown to be effective in the treatment of nausea and vomiting associated with cancer chemotherapy, anorexia and cachexia seen in HIV/AIDS patients, as well as neuropathic pain, and spasticity in multiple sclerosis.Citation13,Citation14
Therefore, the aim of the present study was to evaluate the possible protective and therapeutic effects of Cannabis extract on dimethylnitrosamine-induced hepato-carcinogenicity in mice through studying TERT m-RNA, caspase-8 m-RNA gene expression levels, alanine transaminase (ALT), aspartate transaminase (AST) and gamma-glutamyl transpeptidase (γ-GT) activities.
2 Materials and methods
2.1 Preparation of plant extract
Cannabis sativa plant (the flowering tops of plants) was obtained by permission from the Public Prosecutor. One hundred gms of dry cannabis plant were minced into very small pieces and boiled with water for 5 min. The boiling water was discarded and the minced plant was left to dry at room temperature. Extraction was carried out by boiling the dried minced plant with 100 ml of ethyl alcohol for 10 min followed by filtration. The filtrate was transported into a specific apparatus used to evaporate cannabis residue by heating at specific temperatures (180–220 °C) to obtain cannabinoids free of some carcinogenic compounds (such as benzopyrene, benzene, toluene and naphthalene). The residue was heated up to 180 °C, the vapor was collected in petroleum ether (for trapping hydrocarbons) and then discarded. On raising the temperature from 180 °C to 220 °C the resulting vapor (contains cannabinoids) was received in methyl alcohol (trapping agent).Citation15
Mass spectroscopy coupled with gas chromatography (GC/MS)Citation16 was used as a method for detection of the received cannabinoids using column hp 5, capillary 30 m, GC type is Agilent 6890N, mass is Agilent 5973 N. Temperature program: start of 50 °C for 3 min, then temperature was increased at a rate of 50 °C/min up to 280 °C for 25 min. Injection temperature = 250 °C, detector temperature = 280 °C, injection volume = 3 μl and wavelength λ = 50–550 M/Z (M = molecular weight, Z = charge). Methyl alcohol was evaporated and the cannabinoids’ residue was dissolved in 100 ml of corn oil until time of use.
2.2 Animals and drug administration schedule
The experiments were conducted on seventy-five male mice weighing 20 ± 2 g and were housed in plastic cages at room temperature of 22 ± 1 °C under a 12 h light-dark cycle. The studies were carried out in accordance with the current ethical guidelines for investigation approved by the Ethics Committee of Medical Research Institute.
Animals were divided into five groups of 15 each. The groups were as follows: group I mice received corn oil only and served as the control group; group II mice were injected intraperitoneally with dimethylnitrosamine (DMNA 10 μg/kg body weight) weekly for 12 weeks;Citation17 group III mice were pretreated orally with cannabis extract (0.5 ml/kg body weight)Citation18 every other day for two weeks before the intraperitoneal injection of DMNA, and continued until the end of the experiment (12 weeks); group IV mice were treated orally with cannabis extract (0.5 ml/kg body weight) every other day simultaneously with an intraperitoneal injection of DMNA and continued until the end of the experiment (12 weeks); group V mice were treated orally with cannabis extract (0.5 ml/kg body weight) every other day after receiving the last intraperitoneal injection of DMNA (12 weeks).
At the end of experiment, the mice were fasted overnight and then sacrificed under light ether anesthesia and the liver was divided into three parts:
| - | First part was used for total RNA extraction. | ||||
| - | Second part was used for liver tissue homogenate preparation. | ||||
| - | Third part was fixed in 10% formalin for histopathological examination. | ||||
2.3 Relative quantification of TERT mRNA and caspase-8 mRNA gene by real time PCR using SYBR green.Citation19,Citation20
2.3.1 RNA extraction
Total RNA was extracted using Gene JET™ RNA Purification Kit (Fermentas) following the manufacturer’s instructions and the standard protocol. RNA was detected by electrophoresis using a 1.0% agarose gel containing ethidium bromide (0.5 μg/ml) for 40 min at 100 V and the gel was observed under UV light. The eluted RNA was collected immediately, placed in ice or stored at −20 °C for further processing.
2.3.2 cDNA preparation
cDNAs were synthesized from the mRNA by high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA, USA) using a PCR thermocycler (Applied Biosystem). cDNA prepared for real time PCR was stored at −15 to −25 °C.
2.3.3 Real time PCR
For each sample, 5 μl cDNA was used for RT-PCR using the Power SYBR® Green RT-PCR Master Mix kit (Applied Biosystems, Foster City, CA, USA) and the real time machine (Applied Biosystems one step, Foster City, CA, USA) to detect relative expression levels of mTERT mRNACitation19 and caspase-8 mRNA.Citation20 The reaction conditions used to detect TERT and caspase-8 levels were: initial DNA denaturation at 95 °C for 10 min and then 40 cycles of denaturation at 95 °C for 15 s, annealing and elongation at 60 °C for 1 min. GAPDH (glyceraldehyde 3-phosphate dehydrogenase; housekeeping gene was used as an endogenous control) was included in each experiment.
2.3.4 Primers sequence
(Sigma-Aldrich Co., St. Louis, MO, USA) mTERT was (5′-ATGGCGTTCCTGAGTATGGGTGC-3′) mTERT-forward and (5′-ACTTCAACCGCAAGACCGACAGG-3′) mTERT-reverse.Citation21
Caspase-8 was (5′-TCGCCCGAGCTGGAGTTGTGA-3′) caspase-8-forward and (5′-CTCGGTTGCAGTCTAGGAAGTTGA-3′) Caspase-8-reverse.Citation20
GAPDH was (5′-ACCACAGTCCATGCCATCAC-3′) GAPDH-forward and (5′-TCCACCACCCTGTTGCTGTA-3′) GAPDH-reverse.Citation21
The amount of target, normalized to an endogenous reference and relative to a calibrator, is given by 2−ΔΔCT where CT = Threshold cycle. ΔCT = CTt − CTr where ΔCT = the difference in threshold cycles for target and reference.
2.4 Biochemical analysis of hepatic liver enzymes
The liver was immediately removed, weighed, washed using chilled saline solution and homogenized (10% w/v) in 0.01 M sodium phosphate buffer (pH 7.4) in a Potter-elvehjem type homogenizer. The homogenate was centrifuged at 10,000 × g for 20 min at 4 °C, and the resultant supernatant was used for ALT, AST and γ-GT levels determination using a kit (spectrum, Germany), according to the manufacturer’s instructions. Enzyme activities were expressed in terms of U/L.
2.5 Histopathological examination
Liver tissues were fixed in 10% formalin, sectioned, and stained with hematoxylin and eosin (H and E).
2.6 Statistical analysis
Data were fed to the computer using IBM SPSS software package version 20.0. Quantitative data were described using mean and standard deviation. For normally distributed data, comparisons between different groups were analyzed using F-test (ANOVA) and Post Hoc test (Scheffe) for pair wise comparison. Significance test results are quoted as two-tailed probabilities. Significance of the obtained results was judged at the 5% level. A difference was considered significant at p ⩽ 0.05. Spearman’s correlation coefficient was used to measure the closeness of a linear relationship between all parameters in all studied groups.
3 Results
3.1 Chromatography results
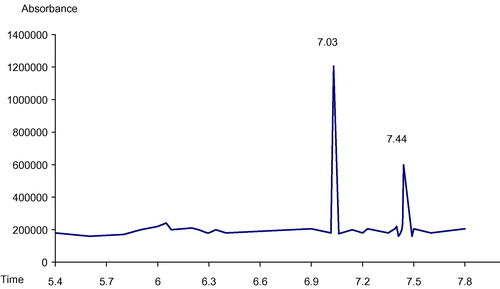
In , the GC chromatogram showed that the retention times of cannabis vapor extract constituents are 7.03 and 7.44 min, identical to the retention times of Δ9THC and CBD standards, respectively. The relative amounts are 67.9% for Δ9THC and 32.1% for CBD. The phenotype ratio of cannabis extract = THC%/CBD% = 67.9%/32.1% > 1.
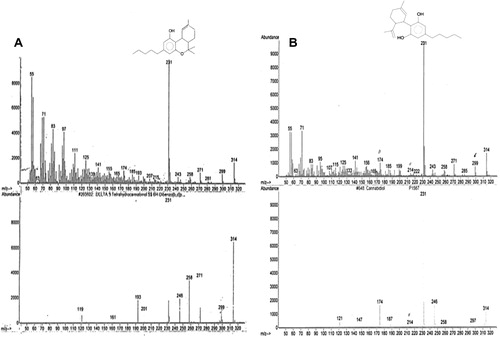
GC/MS allows positive identification of Δ9THC and CBD in cannabis vapor extract which is shown in (A and B) with base peaks at 231 for each constituent.
3.2 Molecular and biochemical results
The amplification plots of fluorescence intensity against the PCR cycle from tissue samples and melting curves of both TERT mRNA and caspase-8 mRNA are shown in and respectively.
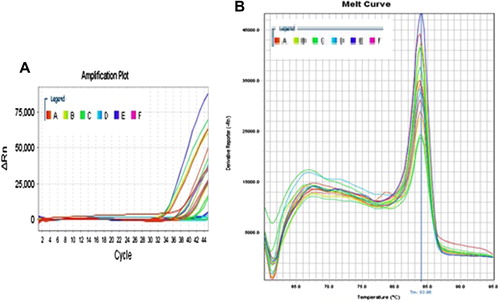
The mTERT expression level was significantly increased in group II as compared to the control group (p1 < 0.001). The gene level in groups (III, IV, and V) was insignificantly higher than the control group (p1 = 1.000, 1.000, and 0.952 respectively) but it was significantly decreased as compared to group II (p2 < 0.001). The mTERT expression in groups (IV, V) did not show any significant difference when compared with group III (p3 = 1.000, 0.971 respectively) or with each other (p4 = 0.981) ().
Table 1 Hepatic TERT mRNA, caspase-8 m-RNA expression level (copies), ALT, AST and γ-GT activities (U/L) for all studied groups.
The caspase-8 expression level was significantly decreased in all groups as compared to the control group (p1 < 0.001). As compared to group II, caspase-8 expression level was significantly increased in group III (p2 = 0.031), while in groups IV and V it did not show any significant difference (p2 = 0.992, 1.000 respectively). As compared to group III, caspase-8 expression level in group IV did not show any significant difference (p3 = 0.098), while in group V it was significantly decreased (p3 = 0.038). Group V did not show any significant difference when compared with group IV (p4 = 0.996) ().
ALT and AST activities were significantly decreased in all groups as compared to the control group (p1 < 0.001) and significantly increased in groups (III, IV and V) as compared to group II (p2 < 0.001). As compared to group III, ALT activity was significantly decreased in group IV (p3 = 0.001), while in group V it did not show any significant difference (p3 = 0.998). In group V, the ALT activity was significantly higher than in group IV (p4 = 0.002). AST activity in groups (IV and V) did not show any significant difference when compared with group Ш (p3 = 0.131, 0.752 respectively) or with each other (p4 = 0.752) ().
The γ-GT activity was significantly increased in all groups as compared to the control group (p1 < 0.001) while it was significantly decreased in groups (III, IV and V) as compared to group II (p2 < 0.001). As compared to group III, γ-GT activity was significantly increased in group IV (p3 = 0.008), while in group V it did not show any significant difference (p3 = 0.721). γ-GT activity was significantly decreased in group V as compared to group IV (p4 = 0.013) ().
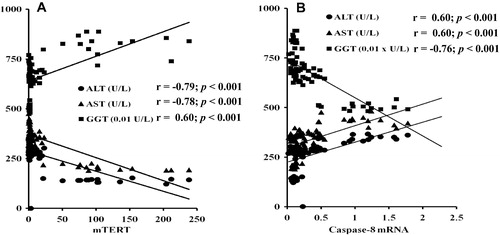
There was a significant positive correlation between ALT, AST (r = 0.951, p ⩽ 0.001) and caspase-8 gene expression and also between γ-GT activity and TERT gene expression. The significant negative correlation between γ-GT and each of ALT (r = −0.886, p < 0.001), AST activities (r = −0.883, p < 0.001), and caspase-8 gene expression was observed and also between TERT gene expression and the activities of both ALT and AST ().
3.3 Histopathological results
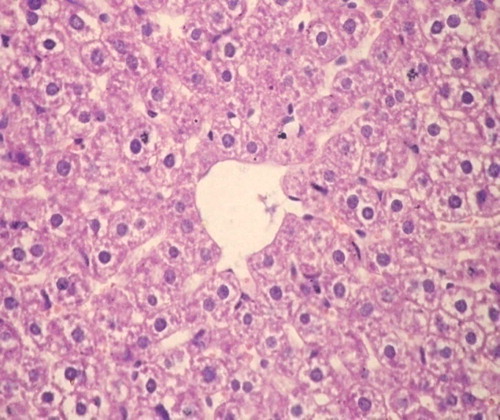
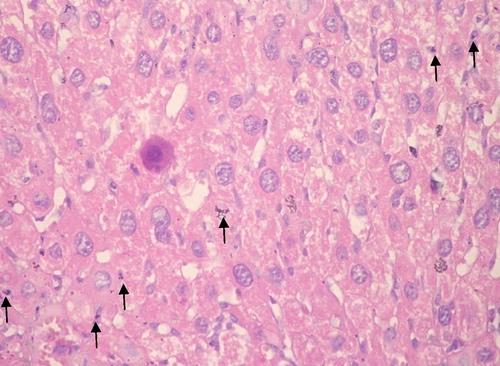
Group I: showed normal hepatic architecture with no evidence of inflammation, fibrosis, necrosis, neoplasia or dysplasia ().
Group II: Receiving DMNA: All hepatic sections showed well to moderately differentiated HCC. Trabeculae several cells thick are seen lined by pleomorphic hepatocytes with hyperchromatic nuclei and abnormal mitotic figures. The stroma was scantly formed of sinusoidal vessels. Necrosis when present was always focal and there was no fibrosis ().
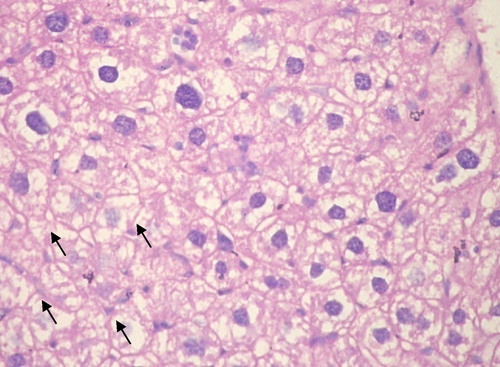
Group III: Cannabis before DMNA: All cases developed well to moderately differentiated HCC. Signs of tumor regression were present in the form of frequent apoptotic hepatocytes with condensed pyknotic nuclei surrounded by perinuclear halo. Wide areas of necrosis were seen with secondary infiltration by acute and chronic non-specific inflammatory cells ().
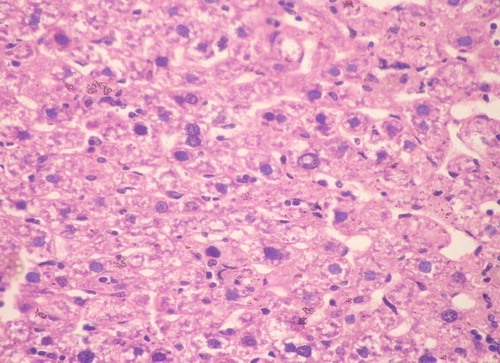
Group IV: Cannabis plus DMNA: All cases developed HCC of well differentiated morphology. Coagulative necrosis and scattered apoptotic hepatocytes and bodies characterized this group ().
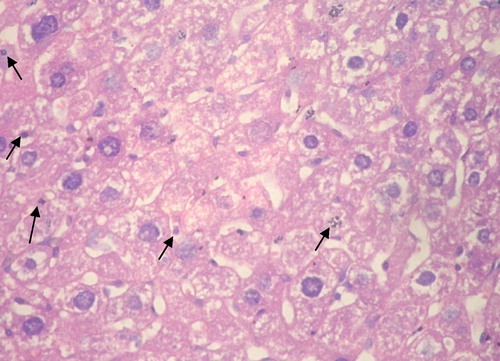
Group V: Cannabis after DMNA: All cases showed well to moderately differentiated HCC. The majority of cases showed viable tumor with occasional spotty foci of coagulative necrosis and moderate number of apoptotic cells and bodies ().
4 Discussion
The most important mechanism of liver cancer progression is cell proliferation. Although in recent years several clinical trials have tested the efficacy of agents that selectively target important signaling pathways involved in the control of this process, no relevant improvement in the prognostic/survival of patients with HCC has been achieved so far, and, therefore, it is necessary to identify novel therapeutic strategies for the management of HCC.Citation22
Cannabis vaporization is a relatively new technology aimed at suppressing respiratory toxins (e.g. benzopyrene, naphthalene, benzene and toluene) by heating cannabis to temperatures below the point of combustion when smoke and associated toxins are produced (near 230 °C). The electric vaporizer can successfully generate THC at 185 °C while completely suppressing toxins.Citation23,Citation24
The results obtained from the chromatograms indicate that the cannabis vapor extract consists of 67.9% of Δ9THC and 32.1% of CBD free from hazardous compounds. The phenotype ratio of cannabis plant, where cannabis preparation with ratio greater than 1.0, is classified as the drug phenotype or biologically active or more precisely as Δ9THC phenotype. However, that is with a ratio smaller than 1.0 classified as fiber phenotype or non-biologically active or cannabidiol phenotype.Citation25
The relative amounts in cannabis vapor extract give a drug phenotype or more precisely Δ9THC phenotype. Where the phenotype ratio of cannabis extract = THC% content/CBD% = 67.9%/32.1% > 1.
The investigation of the therapeutic effects of cannabinoids on some cancer-related disorders has provided evidence of their effectiveness in the alleviation of several symptoms observed in cancer patients, such as appetite loss and neuropathic pain.Citation26 Beyond the palliative effects induced by these compounds, previous advances in the knowledge of the biological role of endocannabinoids and novel insights into the molecular signaling of cannabinoid receptors, support the participation of the endocannabinoid system in the regulation of key processes involved in the development of cancer.Citation27,Citation28 So this study aimed to evaluate the possible protective and therapeutic effects of cannabis extract on DMNA-induced hepatocarcinogenicity in mice.
In this study, there was a significant increase in the expression of TERT mRNA in the DMNA group (group II) as compared to the control group. The higher increase of TERT mRNA level in the DMNA group was in parallel to that reported by Braicu et al.Citation29 and Miura et al.Citation30 Although telomerase is not carcinogenic, it plays a direct role in oncogenesis by allowing the precancerous cells to proliferate continuously and become immortal. These results were confirmed by the histopathological findings in the control group, and DMNA group.
Telomerase has been found to be expressed in most immortal cell lines, but it was undetectable in adult normal tissues, so in malignant tumors telomerase is thought to be activated to maintain their immortality. It has been suggested that telomerase is one of the critical steps in malignant transformation and its strong enhancement considered an important part of hepatocarcinogenesis.Citation31
On the other hand, there was a significant decrease in the expression of TERT mRNA in groups III, IV, and V as compared to group II. These results may be due to the protective effect of the pretreatment and therapeutic effect of (simultaneous and post-treatment) cannabis extract. Vara et al.Citation32 study of cannabinoids on HCC showed that Δ9-THC and JWH-015 (synthetic cannabinoids) efficiently reduced ascites development and alpha fetoprotein (AFP) expression in HCC, which also paralleled mammalian target of rapamycin C1 (mTORC1) inhibition, adenosine monophosphate-activated kinase (AMPK) activation and autophagy stimulation in those tumors. Their data represent the first evidence for the anti-proliferative action of cannabinoids in HCC cells in vivo and support that the ability of cannabinoids to inhibit mTORC1, stimulate AMPK and enhance autophagy could be therapeutically exploited for the management of HCC. In addition, the ability of THC to reduce inflammation (a pro-tumorigenic function of the immune system), and this effect may seem to be beneficial for preventing certain types of cancer.Citation33
Our findings suggested that the treatment with cannabis extract inhibits TERT mRNA expression level which leads to down regulation of telomerase activity.
In the current work, TERT mRNA expression level in groups III, VI and V showed insignificant change when compared with each other. This insignificant difference may be attributed to cannabinoid treatment, where cannabinoids impair tumor progression at different levels through the inhibition of cancer cell proliferation. These results are in line with in vivo experiments which indicated that cannabinoids impair tumor angiogenesis and block invasion and metastasis.Citation34
These results are confirmed by the histopathological study which revealed that the liver shows frequent apoptotic hepatocytes with condensed nuclei and wide areas of necrosis in group III, spotty coagulative necrosis in group IV and coagulative necrosis in group V.
In the present study, there was a significant decrease in the expression of caspase-8 mRNA in groups II, III, IV and V as compared to the control group. The lower level of caspase-8 mRNA expression in the DMNA group was in agreement with previous studies which found that caspase-8 is frequently silenced by promoter hypermethylation indicating that tumorigenesis is associated with inactivation of caspase-8.Citation35,Citation36
Caspase-8 is a key signaling molecule of apoptosis, loss of caspase-8 expression or function has a profound impact on the cancer cell’s ability to undergo apoptosis. Various mechanisms including genetic, epigenetic and posttranslational alterations have been identified that cause inactivation of this central apoptosis regulator in human cancers.Citation11 Loss or dysfunction of caspase-8 results in increased cellular transformation, enhanced tumor progression, poor response to chemotherapy, and impaired prognosis of patients of different cancer entities.Citation37,Citation38
On the other hand, there was a significant increase in the expression of caspase-8 mRNA in group III as compared to the corresponding values in group II. Our results may be due to the administration of cannabis plant extract as a protective treatment. These results were in agreement with a previous study of Lombard and his associatesCitation39 which showed that THC treatment of the cells led to the activation of caspases 2, 8, 9, and 10 in that order, and cleavage of Bid occurred 2 h after treatment. Overall, these studies demonstrated that THC-induced apoptosis was occurring through cross-talk between the extrinsic and intrinsic pathways, with the intrinsic pathway playing the primary role. Also, Giuliano et al.Citation40 found that modulation of both cannabinoid receptors by some synthetic cannabinoids induces apoptosis in a hepatocellular carcinoma cell line.
The most prevalent effect of cannabinoids is the induction of cancer cell death by apoptosis and the inhibition of cancer cell proliferation. At least one of these actions has been demonstrated in almost all the cancer cell types tested.Citation34
This study revealed that, there was no significant change in caspase-8 mRNA expression level in groups IV and V as compared to group II as well as in group IV as compared to group III, while it was significantly decreased in group V as compared to group III. These results may be due to that, the percentage of apoptotic cells after treatment was increased both in a time-dependent manner and in a dose-dependent manner.Citation41
Our study demonstrated that there was a significant decrease in the hepatic activities of both ALT and AST in groups II, III, IV, and V as compared to the control group. DMNA induces liver fibrosis in a highly reproducible manner, first inducing a central hemorrhagic necrosis followed by the formation of septa and establishing micronodular cirrhosis after 3 weeks of treatment.Citation42 Lower hepatic AST and ALT activities might be attributed to the damage of the liver tissue due to the alteration of the membrane components in the tissue, resulting in the release of these enzymes into the blood.Citation43 We suggested that the decrease in the hepatic activities of AST and ALT in our results has been positively correlated with the increased liver damage.
The hepatic activities of ALT and AST were significantly increased in groups III, IV, and V as compared to group II, but still significantly decreased as compared to the control group. This partial improvement in the enzyme activities may be due to the protective effect of the pretreatment and therapeutic effect of (simultaneous and post-treatment) cannabis extract, in addition to non toxicity of the cannabis extracts in the liver tissue.Citation13 Also, cannabidiol pretreatment significantly attenuated the elevations of serum aminotransferases, decreased oxidative and nitrative stress, and reduced the inflammatory response in the liver tissue. The antioxidant, anti-inflammatory and antiapoptotic activities can be considered the main factors responsible for the hepatoprotective effect of cannabidiol. Therefore, cannabidiol may be a feasible therapeutic candidate to prevent liver tissue injury.Citation44
In the present study there was a significant increase in the hepatic activity of γ-GT in groups II, III, IV, and V as compared to the control group. These results are in agreement with a previous study.Citation45 The elevated values of γ-GT activity in their study are an indication of parenchymal cell damage and induction of hepatic necrosis and premalignant hepatocellular damage induced by DMNA administration. γ-GT is the most sensitive indicator of hepatobiliary disease.Citation46
The activities of γ-GT are rather low in normal liver tissues, expressed mainly on the border of epithelial cell membrane of biliary duct and intrahepatic cholangioles with strong secretory and absorptive functions. With the feature of carcinoembryonic protein, hepatoma-related γ-GT is produced and secreted when the genes controlling γ-GT synthesis were expressed abnormally. Hence γ-GT is usually considered as the early enzyme marker of hepatocarcinogenesis.Citation47 These results were confirmed by the histopathological study which revealed that the liver showed well differentiated HCC with foci of necrosis in group II.
On the other hand, there was a significant decrease in γ-GT activity in groups III, IV, and V as compared to group II. These results may be due to the protective and therapeutic effects of cannabis extract. Numerous studies show that THC and other cannabinoids exhibit anti-tumor effects on a wide range of animal models of cancer.Citation13,Citation48 Administration of cannabinoids to tumor-bearing mice decreased the activity and expression of matrix metalloproteinase-2 (a proteolytic enzyme that allows tissue breakdown and remodeling during angiogenesis and metastasis).Citation49
The obtained results were confirmed by the correlation study, which indicated that there was a significant positive correlation between ALT, AST activities and caspase-8 gene expression and also between γ-GT activity and TERT gene expression. In addition the significant negative correlation between γ-GT activity and each of ALT, AST activities, caspase-8 gene expression and also between TERT gene expression and the activities of both ALT and AST. These results may be due to that hepatic injury, cellular leakage and loss of the functional integrity of the liver cell membrane induced by DMNA lead to decreasing levels of ALT, AST activities, and caspase-8 gene and increasing levels of both TERT gene expression and γ-GT activity.
In conclusion, exposure to DMNA plays a role in pathogenesis of liver disease leading to carcinogenicity and causes disturbances in the activities of mice liver enzymes while cannabis causes a partial improvement in these enzymes. The protective effect of cannabis extract is more pronounced than other groups and this is demonstrated in group III. Cannabinoids might exert their anti-tumor effects by direct induction of apoptosis and can decrease telomerase activity by inhibiting the expression of hTERT gene. Coordination between inhibition of telomerase activity and induction of apoptosis might be a potential therapeutic agent for cancer treatment.
It is widely believed that strategies that aim to reduce mortality from cancer should consist of targeted therapies that are capable of providing the most efficacious and selective treatment for each individual tumor and patient.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 23 May 2014
References
- J.D.YangL.R.RobertsHepatocellular carcinoma: a global viewNat Rev Gastroenterol Hepatol72010448458
- M.I.ShariffI.J.CoxA.I.GomaaS.A.KhanW.GedroycS.D.Taylor-RobinsonHepatocellular carcinoma: current trends in worldwide epidemiology, risk factors, diagnosis and therapeuticsExpert Rev Gastroenterol Hepatol32009353367
- S.M.CaldwellD.M.CrespoH.S.KangA.M.Al-OsaimiObesity and hepatocellular carcinomaGastroenterology127200497103
- D.MoradpourH.E.BlumPathogenesis of hepatocellular carcinomaEur J Gastroenterol Hepatol172005477483
- J.NakayamaH.TaharaE.TaharaM.SaitoK.ItoH.NakamuraTelomerase activation by hTERT in human normal fibroblasts and hepatocellular carcinomaNat Genet1819986568
- M.ShimojimaF.KomineH.HisatomiT.ShimiruM.MoriyamaY.ArakawaDetection of telomerase activity, telomerase RNA component, and telomerase reverse transcriptase in human hepatocellular carcinomaHepatol Res2920043138
- M.W.ChangJ.GrillariC.MayrhoferK.FortscheggerG.AllmaierG.MarzbanComparison of early passage, senescent and hTERT immortalized endothelial cellsExp Cell Res3092005121136
- N.W.KimM.A.PiatyszekK.R.ProwseC.B.HarleyM.D.WestP.L.HoSpecific association of human telomerase activity with immortal cells and cancerScience266199420112015
- C.ZhangY.P.TianY.WangF.H.GuoJ.F.QinH.NiHTERT rs2736098 genetic variants and susceptibility of hepatocellular carcinoma in the Chinese population: a case-control studyHepatobiliary Pancreat Dis Int1220137479
- P.MehlenA.PuisieuxMetastasis: a question of life or deathNat Rev Cancer62006449458
- sFuldaCaspase-8 in cancer biology and therapyCancer letters2812009128133
- M.Ben AmarCannabinoids in medicine: a review of their therapeutic potentialJ Ethnopharmacol1052006125
- M.GuzmanCannabinoids: potential anticancer agentsNat Rev Cancer32003745755
- W.PollmannW.FenenbergCurrent management associated with multiple sclerosisCNS Drugs222008291324
- E.RichardCannabis, “Vaporization”: a promising strategy for smokeJ Cannabis Therapeutic1200134
- D.J.HarveyComparison of fourteen substituted silyl derivatives for the characterization of alcohols, steroids and cannabinoids by combined gas–liquid chromatography and mass spectrometryJ Chromatogr1471978291298
- V.L.SouliotisJ.R.HennemanC.D.ReedS.K.ChhabraB.A.DiwanL.M.AndersonDNA adducts and liver DNA replication in rats during chronic exposure to N-nitrosodimethylamine (NDMA) and their relationships to the dose-dependence of NDMA hepatocarcinogenesisMutat Res50020027587
- S.SheweitaNarcotic drugs changes the expression of cytochrome p450 2E1, 2C6 and other activities of carcinogen metabolizing in the liver of male miceToxicology1912003133142
- A.A.El-FadleN.F.Al HusseiniA.F.El-kholyO.Al-SaidN.Al-ToukhyM.M.AttaTelomerase reverse transcriptase gene expression as a tumor marker for hepatocellular carcinomaAm J Biochem Biotech720115562
- X.ZhangX.ZhengH.SunB.FengG.ChenC.VladauPrevention of renal ischemic injury by silencing the expression of renal caspase-3 and caspase-8Transplantation82200617281732
- J.M.RitzO.KuhleS.RiethdorfB.SiposW.DeppertC.EnglertA novel transgenic mouse model reveals humanlike regulation of an 8-kbp human TERT gene promoter fragment in normal and tumor tissuesCancer Res65200511871196
- A.DuffyT.GretenDeveloping better treatments in hepatocellular carcinomaExpert Rev Gastroenterol Hepatol42010551560
- M.PolenHealth care use by frequent marijuana smokers who do not smoke tobaccoWest J Med1581993596601
- D.TashkinIs frequent marijuana smoking hazardous to health?West J Med1581993635637
- C.W.WallerJ.A.SciglianoThe national marihuana program. Report to commission on problems of drug dependenceNat Acad Sci N R C419702832
- W.HallM.ChristieD.CurrowCannabinoids and cancer: causation, remediation, palliationLancet Oncol620053542
- T.W.KleinCannabinoid-based drugs as anti-inflammatory therapeuticsNat Rev Immunol52005400411
- S.PisantiC.BorselliO.OlivieroC.LaezzaP.GazzerroM.BifulcoAntiangiogenic activity of the endocannabinoid anandamide: correlation to its tumor-suppressor efficacyJ Cell Physiol2112007495503
- C.BraicuC.BurzI.Berindan-NeagoeO.BalacescuF.GraurHepatocellular carcinoma. Tumorigenesis prediction markersGastroenterol Res22009191199
- N.MiuraY.OsakiM.NagashimaM.KohnoK.YorozuK.ShomoriA novel biomarker TERT mRNA is applicable for early detection of hepatomaBMC Gastroenterol10201046
- E.TakumayK.NousoY.KobayashiS.NakamuraH.TanakaE.MatsumotoTelomerase reverse transcriptase gene amplification in hepatocellular carcinomaJ Gastroenterol Hepatol19200413001304
- D.VaraM.SalazarN.Olea-HerreroM.GuzmánG.VelascoI.Dı́az-LaviadaAnti-tumoral action of cannabinoids on hepatocellular carcinoma: role of AMPK-dependent activation of autophagyCell Death Differ18201110991111
- W.M.LiuD.W.FowlerA.G.DalgleishCannabis-derived substances in cancer therapy – an emerging anti-inflammatory role for the cannabinoidsCurr Clin Pharmacol52010281287
- G.VelascoC.SanchezM.GuzmanTowards the use of cannabinoids as antitumour agentsNat Rev Cancer122012436444
- Y.H.SoungJ.W.LeeS.Y.KimY.J.SungW.S.ParkS.W.NamCaspase-8 gene is frequently inactivated by the frame shift somatic mutation 1225 1226delTG in hepatocellular carcinomasOncogene242005141147
- C.LiedtkeN.H.ZschemischA.CohrsT.RoskamsJ.BorlakM.P.MannsSilencing of caspase-8 in murine hepatocellular carcinomas is mediated via methylation of an essential promoter elementGastroenterology129200516021615
- C.FlothoE.Coustan-SmithD.PeiS.IwamotoG.SongC.ChengGenes contributing to minimal residual disease in childhood acute lymphoblastic leukemia: prognostic significance of CASP8AP2Blood108200610501057
- J.F.MataV.S.SilveiraE.C.MateoM.A.CortezR.G.QueirozJ.A.YunesLow mRNA expression of the apoptosis-related genes CASP3, CASP8, and FAS is associated with low induction treatment response in childhood acute lymphoblastic leukemia (ALL)Pediatr Blood Cancer552010100107
- C.LombardM.NagarkattiP.S.NagarkattiTargeting cannabinoid receptors to treat leukemia: role of cross-talk between extrinsic and intrinsic pathways in delta9-tetrahydrocannabinol (thc)-induced apoptosis of jurkat cellsLeuk Res292005915922
- M.GiulianoO.PelleritoP.PortanovaG.CalvarusoA.SantulliA.De BlasioApoptosis induced in hepg2 cells by the synthetic cannabinoid win: Involvement of the transcription factor PPAR gammaBiochimie912009457465
- DuQing-YouXiao-BoWangXue-JunChenWeiZhengSheng-QiWangAnti-tumor mechanism of antisense cantide targeting human telomerase reverse transcriptaseW J Gastroenterol9200320302035
- Y.L.JinH.EnzanN.KurodaY.HayashiM.ToiE.MiyazakiVascularization in tissue remodeling after rat hepatic necrosis induced by dimethylnitrosamineMed Mol Morphol320063343
- A.O.OluduroB.I.AderiyeEffect of Moringa oleifera seed extract on vital organs and tissue enzymes activities of male albino ratsAfrican J Microbiol Res32009537540
- P.MukhopadhyayM.RajeshB.HorváthS.BátkaiO.ParkG.TanashiaCannabidiol protects against hepatic ischemia/reperfusion injury by attenuating inflammatory signaling and response, oxidative/nitrative stress, and cell deathFree Radic Biol Med50201113681381
- S.PriyaP.VijayalakshmiP.VivekanandanS.KarthikeyanInfluence of N-acetylcysteine against dimethylnitrosamine induced hepatotoxicity in ratsToxicol Ind Health272011914922
- D.M.VasudevanS.SreekumariKannanVaidyanathanText Book of Biochemistry for Medical Studentsthird ed.2001Japee Brothers Medical Publishers (P) Ltd.New Delhi
- T.Qi-YunY.Deng-FuL.Jian-XinW.Xin-HuaM.Xian-YongExpression and alterations of different molecular form γ-glutamyl transferase and total RNA concentration during the carcinogenesis of rat hepatomaW J Gastroenterol51999356358
- S.SarfarazV.M.AdhamiD.N.SyedF.AfaqH.MukhtarCannabinoids for cancer treatment: progress and promiseCancer Res682008339342
- C.BlázquezM.L.CasanovaA.PlanasT.Gómez Del PulgarC.VillanuevaM.J.Fernández-AceñeroInhibition of tumor angiogenesis by cannabinoidsFASEB J172003529531