Abstract
Background and purpose
Oestrogen receptor (ER) and HER (human epidermal growth factor receptor) family signaling pathways are fundamental to guide treatment and determine prognosis. Categorizing breast cancer tumors as ER and Her-2 positive or negative is usually performed by immunohistochemistry (IHC), however the technique lacks standardization in handling tissues, staining techniques, and scoring systems.
The current study aimed to compare the conventional IHC and the RT-PCR techniques in assessing the ER-alpha status in breast cancer. It also validates the application of RT-PCR technique in detecting the Her-2/neu status.
Subjects and methods
The study included 40 patients with IDC (NOS) (invasive ductal carcinoma; not otherwise specified). Breast tissue specimens were collected at the time of the elective surgery. Specimens were subjected to routine pathological examinations. ER alpha and PR receptor status; assessed by immunohistochemical staining. RNA was extracted, reverse transcribed, and amplified by PCR using ER alpha and HER-2 specific primers. Relative expression was detected by comparing the expression in normal and tumor samples relative to the housekeeping (β-actin) gene expression.
Results
IHC and RT-PCR techniques showed great discrepancies in detecting ER alpha status which might be due to the presence of ER alpha variants. RT-PCR showed higher sensitivity (72.7%) and accuracy (57.6%) than IHC in detecting ER alpha status. Gene expression of HER-2 was detected (by RT-PCR) in 67.5% (27/40) with overexpression in 25% (10/40) of the cases.
Conclusion
IHC and RT-PCR must be combined for more informative results regarding the ER alpha and HER-2 status. Further studies are mandatory to evaluate the efficacy of anti-estrogens on ER alpha variant expression.
1 Introduction
Breast cancer is the most common form of cancer among women and the second cause of death in childbearing period. The average age of presentation is 10 years lesser than in the west.Citation1 In Egypt, breast cancer represents 35.1% of all female cancers with 80–90% of cases of advanced stages (III or IV) at presentation.Citation2
Breast cancer is characterized by genetic heterogeneity which makes its diagnosis and treatment challenging.Citation3 Despite having similar histological appearance, individual breast tumors can exhibit tremendous variations in clinical presentation, disease aggressiveness, and treatment response in different patient and ethnic populations.Citation4 Therefore, breast cancer is currently regarded as a heterogeneous disease that has been classified into various molecular subtypes according to the gene expression profile of ER, PR, and Her-2/neu including: basal cell-like or triple negative (ER-, PR-, and HER2-), Her-2/neu (ER-, PR-, and HER2+), luminal A (ER + and/or PR+, HER2-), luminal B (ER + and/or PR+, HER2+), and normal breast like.Citation5–Citation6Citation7Citation8 It is evident now that the breast cancer heterogeneity is more complicated than thought before. Recently, Mosoyan et al.Citation9 isolated five cell lines (Her2, ER, CK8/18, CD44, and CD24) from a single patient’s primary breast cancer tissue. The isolated cells showed variable tumorigenic and metastatic potential when tested in immunocompromised nude mice.
ER is a member of a family of nuclear receptors. It functions as transcriptional regulator that mediates the biological responses to the sex hormone estrogen which is essential for reproduction, cardiovascular, skeletal and nervous systems.Citation10 Currently there are two different forms of estrogen receptors; α and β receptors that are co-expressed in many cell types. Each encoded by separate genes (ESR1 and ESR2) that are present on different chromosomes. ESR1gene is located on q arm of chromosome 6, while ESR2 gene is located on q arm of chromosome 14.Citation11–Citation12Citation13 ERα and ERβ show significant overall sequence homology with the greatest homology (close to 100%) in their DNA binding domains, and both are composed of seven domains. Hormone activated estrogen receptors form homo-[ERα (αα) or ERβ (ββ)] or heterodimers [ERαβ (αβ)] ERα.Citation10 ER-α gene (ESR1) comprises eight exons of more than 140 Kb separated by seven introns of ∼ 40 kb. Each exon encodes a certain region of the protein.Citation14,Citation15
Researchers have identified 20 ER alpha splice variants in human breast cancers.Citation16,Citation17 The most abundant variants are created by splicing deletions in one or more exons.Citation18–Citation19Citation20Citation21Citation22 Patterns of splice variant expression differ between tumors and are quite heterogeneous. Splice variants could potentially affect the evaluation of ER status, but this has not been proven yet.Citation23 The generation of human ER alpha mRNA transcripts is a complex process that involves at least seven different promoters and exhibits cell line-dependent promoter usage.Citation24 Most ER alpha variants differ in the 5′ untranslated region and result in the expression of the full-length 66-kDa form of ER alpha.Citation24 Among others, 46-kDa isoforms of ER alpha (ER alpha 46) generated from an internal ATG start codon, lacks exon 1 and consequently the N-terminal AF-1 region.Citation25–Citation26Citation27 Wang and co-workers identified and cloned a 36-kDa isoform of ER alpha (named ER alpha 36). ER alpha 36 is generated from a promoter located in the first intron of the ER alpha gene and it lacks both of the two transcriptional activation domains AF-1 and AF-2.Citation28,Citation29
In some estrogen-dependent cells and tissues, ER might directly activate G-protein-coupled receptors and/or heterodimeric Her (human epidermal growth factor) receptors, resulting in downstream activation of MAPK and PI3K pathways.Citation30 In turn, p38 (protein 38), a member of the MAPK family, is capable of phosphorylating and activating ER in a ligand-independent manner. This cross-talk activation of ER results in the loss of inhibitory effects of tamoxifen.Citation31,Citation32
Her-2 (human epidermal growth factor receptor-2; also known as c-erb-B2, HER-2/neu, p185, CD340) proto-oncogene is located at the long arm of human chromosome 17 (17q21–q22).Citation33 It is part of a family of genes that play roles in regulating cell growth. The protein it makes is a tyrosine kinase (TK) growth factor receptor expressed by a number of normal tissues and probably has a role in normal cell function, regulating growth and proliferation.
The full length 4.6 Kb Her-2/neu transcript encodes a 185 kDa transmembrane glycoprotein receptor with intrinsic tyrosine kinase (TK) activity. It contains an N-terminal extracellular domain (ECD), a single transmembrane helix, a TK domain, and an intracellular regulatory domain. The ECD is ∼600 residues long and contains four (I–IV) domains including a site of potential ligand binding between domains I and III. The ECD can undergo proteolytic cleavage in the juxtamembrane region that releases soluble Her-2/neu ECD (90–110 kD).Citation34 The intracellular domain is ∼500 residues long and consists of a TK domain and an intracellular regulatory domain. The TK domain contains the enzymatic sequences necessary for TK activity. The intracellular domain also contains multiple tyrosine residues which serve as substrates for other TK receptors.Citation30
Alternative splicing of Her-2/neu generates a truncated product (100 KDa) consisting of the entire ECD rather than the full-length protein.Citation35 The 5′ region (2.1 Kb) of the truncated transcript (2.3 Kb) is identical to that of the full length. However, the 3′ end diverges revealing an exonic extension with in frame stop codon and a poly (A) addition site. Thus the truncated transcript differs from the full length in 20 amino acids on the C-terminal.
Her-2/neu gene is amplified in 20–25% of primary breast tumors and has been associated with poor prognosis.Citation36 Increases in Her-2/neu at the DNA, RNA, and protein levels have been similarly prognostic for poor outcomes in primary breast cancer.Citation37
The use of molecular classification of breast cancer is limited in many countries including Egypt. IHC is cheap and widely available. However the reproducibility of ER, PR, and HER2 status is a major issue in daily practice and in clinical investigation. Therefore, the current study was designed to evaluate the accuracy and specificity of IHC and RT-PCR in detecting ER alpha status in IDC (NOS). It also validates the use of RT-PCR in detecting Her-2/neu status.
2 Subjects and methods
The study included 40 patients (sample size was based on previously published work) with IDC (NOS) randomly selected from cases presented to the Cancer Management and Research Department of medical research institute (MRI) (from February 2007 to August 2009). Tumor samples were subjected to pathology assessment and only IDC (NOS) cases were included according to simple random sampling without replacement; lottery method. Clinical diagnosis, treatment, and clinical follow up were performed as described by El-Abd et al.Citation38 All subjects were recruited according to the ethical rules approved by the ethics committee of the MRI based on Belmont report.Citation39 Tumor and normal tissue specimens (far from tumor site to serve as self-controls) were collected during the elective surgery and stored at −80 °C until use.
2.1 Pathological investigations
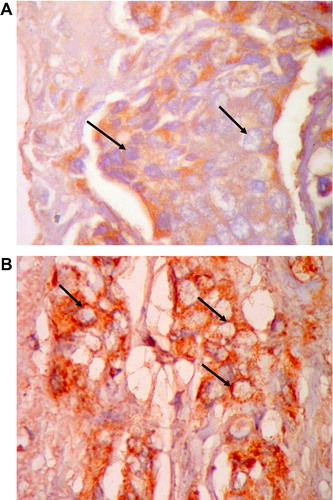
Hematoxylin (Genzyme, England) and Eosin (Chematec, UK) (H&E) stained breast sections (3–4 μm) were examined by the pathologist to determine tumor type, size, grade, stage,Citation40 and number of the involved lymph nodes. ER alpha, total progesterone receptor (PR) and Her’s-2/neu status were detected by IHC using streptavidin–biotin peroxidase labeled specific monoclonal antibodies (Lab Vision, USA)Citation41,Citation42 and scored according to ASCO-CAP test guideline recommendations (http://www.cap.org/apps/docs/committees/immunohistochemistry/summary_of_recommendations.pdf).
2.2 Molecular investigations
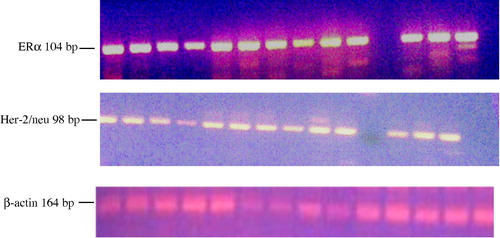
Total RNA was isolated from breast tissue samples using EZ-10 Spin Column Total RNA Minipreps Super Kit (Bio Basic Inc, Canada) according to the manufacturer’s instructions. The quantification and purity of the prepared RNA was examined using the spectrophotometer (UNICAM; Helios Delta, England) at wavelengths of 260 nm and 280 nm and by agarose gel electrophoresis. Full-length cDNA prepared from total RNA (5 μg) using revert aid™ H minus first strand cDNA synthesis kit (MBI Fermentas, Germany) and random hexamer primer according to the manufacturer’s protocol. Fourth of the reaction was then amplified using 2× PCR Master Mix (MBI Fermentas, Germany) and the sequence specific primers for ERα (5′-ATG AGA GCT GCC AAC CTT-3′ and 5′-AAC AAG GCA CTG ACC ATC T -3′) or Her-2 neu (5′-CCT CTG ACG TCC ATC ATC TC-3′ and 5′-ATC TTC TGC TGC CGT CGC TT-3′) (Metabion International AG, Deutschland). To verify the integrity of the extracted total RNA, all samples were additionally assayed for human β-actin (5′-CAC TGT GTT GGC GTA CAG GT-3′ & 5′-TCA TCA CCA TTG GCA ATG AG-3′) (Metabion International AG, Deutschland). Positive and negative controls were included as appropriate. The Amplification protocol has an initial denaturation for 5–8 min at 95 °C, 35 cycles; 30 s at 95 °C, 90 s at 60 °C for ERα or 56 °C for Her-2 neu and β-actin, and 90 s at 72 °C; plus a final elongation for 7–10 min at 72 °C. The amplified products (ERα; 104 bp, Her-2 neu; 98 bp, and β-actin; 164) were separated by agarose gel electrophoresis (3%), stained with ethidium bromide, and visualized by UV. The relative expression level of ER alpha and Her-2/neu (relative to β-actin expression ratio in both normal and tumor samples) was quantified using the Scion image program for Windows.
2.3 Statistical analysis
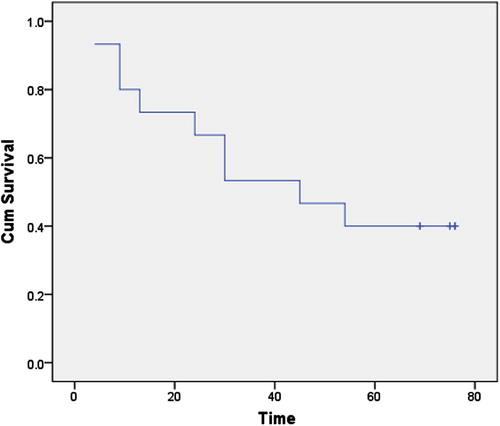
Data analysis was performed using SPSS version 11.5. Normality tests showed abnormally distributed continuous variables (K.S < 0.05) except age. Thus, description of abnormally distributed variables was done using the median and range. For age, description was done using the mean and standard deviation (M ± SD). Comparison of continuous variables was done using the Mann–Whitney U test if it was between two groups while Kruskal–Wallis X2 was used if it was between several groups. Comparison of the age between groups was performed using the student t-test. Regarding qualitative variables, description was done using the percentage and comparison between groups was performed through the Fishers exact test (in case of 2 × 2 tables) or the Monte Carlo test (in case of rxc tables) due to invalid X2. The survival curve was constructed using Kaplan–Meier plots and Wilcoxon–Gehan statistics.
3 Results
3.1 Study population
Age of patients ranged between 20 to 74 years (minimum to maximum; Mean ± SD: 50.7 ± 9.8); menstrual status: 55% postmenopausal, 35% premenopausal, and 10% perimenopausal. Patients were followed up clinically for 76 months. During this period, three (3/40; 7.5%) patients had metastasis and 33 (33/40; 82.5%) patients died ( and ).
Table 1 Clinicopathological characteristics of patients with IDC (NOS).
3.2 ER alpha status
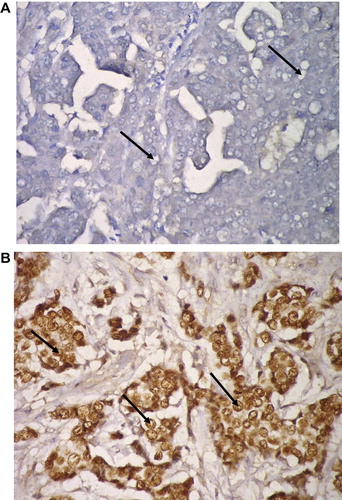
The clinicopathological characteristics of patients with IDC (NOS) are summarized in . Although great discrepancy was noticed in detecting ER alpha status (), 52.5% (21/40) of cases were positive by both techniques (IHC and RT-PCR) (, and ). Similarly 10% (4/40) were negative for ER alpha status by both techniques (, and ) i.e. the complete match in the results of both IHC and RT-PCR techniques was observed in 62.5% (21/40: positive cases; 4/40: negative cases) and the discrepancy between the two techniques was detected in 37.5% (15/40) of cases.
Table 2 ERα as detected by both RT-PCR and IHC techniques.
Fifth of cases (8/40; 20%) were ER alpha positive by IHC only (positive by IHC but negative by RT-PCR) () and 7 cases (7/40; 17.5%) were ER alpha negative by IHC only (negative by IHC but positive by RT-PCR) ().
No significant difference was observed between ER alpha status and any of the tumor grade (p = 0.453), size (p = 0.745), stage (p = 0.268), LNM (p = 0.339), or the number of the involved LNs (p = 0.168).
3.3 PR status
PR was positive in 72.5% of cases as detected by IHC (). PR and ER status differed in one patient only regarding the score of positivity ().
3.4 Her-2/neu status
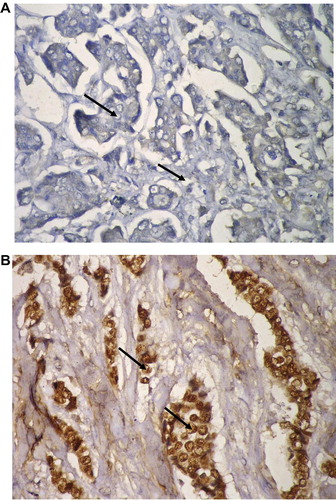
Her-2/neu status is not a routine in the pathological protocols in Egypt. It is only examined in ER alpha negative cases that can afford Herceptin (trastuzumab) and/or other tyrosine kinase inhibitor treatment regimens ( and ). Her-2/neu gene expression was detected (by RT-PCR) in 67.5% (27/40) of tumor specimens (). Overexpression (relative to β-actin) was detected in 25% (10/40) of the cases.
No significant difference was observed between Her-2 status and any of the tumor grade (p = 0.714), size (p = 0.571), stage (p = 0.160), LNM (p = 0.693), the number of the involved LNs (p = 0.889), or ER alpha status (p = 0.354).
4 Discussion
The current study documents the importance of combining IHC and RT-PCR techniques in detecting ER alpha status at the time of diagnosis and treatment decision.
4.1 Study population
The median age of patients with breast carcinoma in this study (50 years) is almost similar to that detected by Omar et al.Citation1 among Egyptian population (30–60 years; median age is 46 years) which is one decade younger than the corresponding age in Europe and North America. Tumor size, grade, histological type, LNM (lymph node metastasis), number of the involved LNs (lymph nodes) and their distribution are also in accordance with disease presentation in Egypt.Citation1
4.2 ER alpha Status; controversies between IHC and RT-PCR results
The discrepancies in ER alpha status might indicate technical limitations and/or presence of ER alpha variants. Although the superiority of the predictive value of ER-immunohistochemistry (ER-IHC) over ligand-binding techniques has been established to everyone’s satisfaction, there remains the controversial issue of immunohistochemical results.Citation43 Several studies reported controversial results between the IHC and RT-PCR techniques.Citation44–Citation43Citation44Citation45Citation46Citation47
Although every step in both techniques can affect the resultsCitation48; and thus would account for such variations; other effectors are not ruled out. Taking into consideration that IHC detects the protein and RT-PCR detects the mRNA, one possible cause for discrepancy may be the differential stability between human ER mRNA and protein; variation in translation efficiency or the rate of mRNA and protein degradation. ERα mRNA usually has a relatively short half-life, which was determined to be approximately 5 h in MCF-7 cell line after actinomycin D treatment.Citation49 Ligand binding, in addition to altering the conformation of the receptor, has been shown to influence the stability of the receptor. In particular, it has been shown that in the absence of ligand, the half-life of ERα is about 4–5 h, whereas estradiol binding accelerates receptor degradation, reducing its half-life to 3–4 h.Citation49–Citation50Citation51Citation52Citation53
4.2.1 RT-PCR negative/ IHC positive cases
Several investigators identified a number of estrogen receptor (ESR) mRNA variants (truncated transcripts, exon deleted transcripts, and larger than wild-type ER mRNA products resulting from nucleotide insertions) in a variety of cancer cell lines and tumors including tissues from human breast cancers.Citation16,Citation54,Citation55 In 35 ESR-positive tumors,Citation56 a common profile of variant ESR transcripts was present, with all tumors containing the exon 2-deleted and exon 7-deleted ESR variants, 94% containing the exon 4-deleted ESR variant, and 83% containing the exon 5-deleted ESR variant. Since the primers in our study were designed against a 104 fragment of exon four, variants that lack this exon will not be detected by RT-PCR technique. Several of the variant transcripts generated by an exon skipping mechanism of the primary ER-α pre-mRNA retain the same reading frame as the full-length transcript, and the corresponding variant proteins have been detected in vivo and in vitro.Citation22,Citation57–Citation58Citation59Citation60 Receptors with ‘outlaw’ function, including both a dominant-positive receptor which was transcriptionally active in the absence of estrogen, and a dominant-negative receptor, which was itself transcriptionally inactive but prevented the action of normal estrogen receptor, were discovered using a yeast transactivation assay and open reading frame analysis.Citation17,Citation21 The monoclonal antibody SP1 used in our study in detecting ER alpha protein using the IHC technique is designed against 18 merit peptide (SLQKYYITGEAEGFPATV) representing the C-terminal of human ERα protein. Thus, the IHC technique can detect variant products that lack exon-4 provided that the resulting protein has the same affinity toward the antibody, same conformational determinants, and the same recognition epitope(s).
4.2.2 RT-PCR positive/IHC negative cases
ERα 36 variant was detected in colorectal cancers and their matched normal colorectal tissues.Citation61 In contrast to full-length ERα 66 functional domains, ERα 36 lacks both transactivation domains AF-1 and AF-2 but retains its DNA-binding, ligand-binding domains, partial dimerization and three potential myristoylation sites. It also possesses an extra, unique 27-amino acids’ domain to replace the last 5 helixes (helix 8–12) of the 12 helixes in the ERα 66. Providing that ERα 36 variant is dominant in some tumor samples, it would not be detectable by SP1 antibody due to the change in helix 8–12 but will be detectable by RT-PCR.
Due to such controversial issues of IHC and RT-PCR results, it is suggested to combine both techniques in detecting ERα status for breast cancer patients. It is also preferred to follow these recommendations:
| 1. | Proper sample transfer, storage, and processing according to the subsequent technique (IHC or RT-PCR or in situ…etc.). | ||||
| 2. | Report should include the sample location, type of antibody or primer, use of standard chemicals and detection kits. | ||||
| 3. | Refer to a reference laboratory as a final evaluator. | ||||
4.3 Her-2/neu status
Her-2/neu mRNA was detected with variable intensities (degree of positivity) in 67.5% (27/40) and over expression was detected in 10/40 (25%) of cases. Only three cases (3/40; 7.5%) had results using the IHC technique since it is not a routine pathological investigation in Egypt. A result of RT-PCR depends only on the presence (positive) or absence (negative) of bands while both negative (0) and positive one (+) scores in IHC are considered negative. If we apply the same scoring system of the IHC (on protein level) on the RT-PCR (on RNA level) relative expression results, the percentage of positive cases (25%) [0: 13 (32.5%); +: 17 (42.5%); ++: 5 (12.5%); +++: 5 (12.5%)] will be within the acceptable levels in the Arab countries (22%). Citation62 It is obvious that results may vary according to the detection technique, molecular detection level, age, race, and tumor stage. Citation63,Citation64 The RNA expression analyses show promise as possible independent methods of Her2 assessmentCitation65 that require small amounts of tumor tissues and deliver both semi- and possible quantitative estimates of the Her2 RNA. Further comparative studies to evaluate the usefulness of these techniques; in both diagnosis and prognosis; are required.
No significant correlation was observed between expression of Her-2/neu and any of breast cancer prognostic variables (tumor size, histological grade, lymph node metastasis) or age or menopausal status. These results are in accordance with the research result of Ellsworth et al.Citation66
5 Conclusion
We can conclude that it would be advantageous to combine the IHC and RT-PCR techniques in detecting ER alpha status to avoid the false negative results especially for triple negative (TN) patients (ER-, PR-, and HER-2) who usually have poor clinical outcome and no specific systemic treatment. Further studies would be mandatory to estimate the influence of antiestrogen drugs on ER alpha variants to improve the clinical response in breast cancer patients.
Her-2/neu was detected by RT-PCR, and found in 70% of our cases. Her-2 represents an ideal therapeutic target because it is accessible; as a cell surface receptor; to treatment with chemotherapy. Recently, herceptin, a monoclonal antibody known as trastzumab, has shown to be effective than chemotherapy alone. Therefore, testing Her-2/neu in breast cancer at the time of primary diagnosis is of a prime importance. Thus, a routine examination of Her-2/neu using both RT-PCR and IHC techniques is highly recommended for ensuring proper classification of the patients’ Her-2/neu status and tailoring therapy.
Conflict of interest
The authors declare that they have no conflict of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 31 March 2014
References
- S.OmarH.KhaledR.GaafarA.R.ZekryS.EissaO.ElkhatibBreast cancer in Egypt: a review of disease presentation and detection strategiesEast Mediterr Health J932003448463
- I.A.El-AttarCancer databases in the Arab WorldEthn Dis151 Suppl 12005 S 1-3-4
- C.U.IhemelanduL.D.LeffallJrR.L.DewittyT.J.NaabH.M.MezghebeK.H.MakambiMolecular breast cancer subtypes in premenopausal and postmenopausal African American women: age-specific prevalence and survivalJ Surg Res14312007109118
- J.K.WienckeImpact of race/ethnicity on molecular pathways in human cancerNat Rev Cancer4120047984
- Cancer Genome Atlas NetworkComprehensive molecular portraits of human breast tumoursNature490741820126170
- T.SørlieC.M.PerouR.TibshiraniT.AasS.GeislerH.JohnsenGene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implicationsProc Natl Acad Sci9820011086910874
- V.AlmendroG.FusterHeterogeneity of breast cancer: etiology and clinical relevanceClin Transl Oncol13112011767773
- J.RheeS.W.HanD.Y.OhJ.H.KimS.A.ImW.HanThe clinicopathologic characteristics and prognostic significance of triple-negativity in node-negative breast cancerBMC Cancer82008307
- G.MosoyanC.NagiS.MarukianA.TeixeiraA.SimonianMultiple breast cancer cell-lines derived from a single tumor differ in their molecular characteristics and tumorigenic potentialPLoS One812013e55145
- X.LiJ.HuangP.YiR.A.BambaraR.HilfM.MuyanSingle-chain estrogen receptors (ERs) reveal that the ER alpha/beta heterodimer emulates functions of the ER alpha dimer in genomic estrogen signaling pathwaysMol Cell Biol2417200476817694
- J.R.GosdenP.G.MiddletonD.RoutLocalization of the human oestrogen receptor gene to chromosome 6q24–q27 by in situ hybridizationCytogenet Cell Genet1433–41986218220
- N.R.CandelariaK.LiuC.Y.LinEstrogen receptor alpha: Molecular mechanisms and emerging insightsJ Cell Biochem201310.1002/jcb.24584 [Epub ahead of print]
- S.GreenP.WalterV.KumarA.KrustJ.M.BornertP.ArgosHuman oestrogen receptor cDNA: sequence, expression and homology to v-erb-ANature32060581986134139
- G.L.GreeneP.GilnaM.WaterfieldA.BakerY.HortJ.ShineSequence and expression of human estrogen receptor complementary DNAScience2314742198611501154
- G.G.KuiperJ.A.GustafssonThe novel estrogen receptor-beta subtype: potential role in the cell- and promoter-specific actions of estrogens and anti-estrogensFEBS Lett410119978790
- W.L.McGuireG.C.ChamnessS.A.FuquaEstrogen receptor variants in clinical breast cancerMol Endocrinol511199115711577
- I.PoolaJ.AbrahamK.BaldwinIdentification of ten exon deleted ERβ mRNAs in human ovary, breast, uterus and bone tissues: alternate splicing pattern of estrogen receptor ß mRNA is distinct from that of estrogen receptor alphaFEBS Lett5161–32002133138
- L.C.MurphyEstrogen receptor variants in human breast cancerMol Cell Endocrinol7421990 C83-C6
- S.A.FuqaD.C.AllredR.J.AuchusExpression of estrogen receptor variantsJ Cell Biochem Suppl17G1993194197
- C.A.EncarnacionS.A.FuquaEstrogen receptor variants in breast cancerCancer Treat Res71199497109
- U.PfefferEstrogen receptor mRNA variants. Do they have a physiological role?Ann N Y Acad Sci7841996304313
- Q.-X.ZhangS.G.HilsenbeckS.A.W.FuquaA.BorgMultiple splicing variants of the estrogen receptor are present in individual human breast tumorsJ Steroid Biochem Mol Biol593–41996251260
- L.K.DiazN.SneigeEstrogen receptor analysis for breast cancer: current issues and keys to increasing testing accuracyAdv Anat Pathol12120051019
- G.ReidS.DengerM.KosF.GannonHuman estrogen receptor-alpha: regulation by synthesis, modification and degradationCell Mol Life Sci5952002821831
- G.FlouriotH.BrandS.DengerR.MetivierM.KosG.ReidIdentification of a new isoform of the human estrogen receptor-alpha (hER-alpha) that is encoded by distinct transcripts and that is able to repress hER-alpha activation function 1EMBO J1917200046884700
- L.LiM.P.HaynesJ.R.BenderPlasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cellsProc Natl Acad Sci USA1008200348074812
- K.H.KimJ.R.BenderRapid estrogen receptor-mediated signaling: why is the endothelium so special?Sci STKE2882005Pe28
- Z.WangX.ZhangP.ShenB.W.LoggieY.ChangT.F.DeuelIdentification, cloning, expression of human estrogen receptor alpha36, a novel variant of human estrogen receptor-alpha66Biochem Biophys Res Commun3364200510231027
- P.AscenziA.BocediM.MarinoStructure–function relationship of estrogen receptor alpha and beta: impact on human healthMol Aspects Med2742006299402
- J.W.ParkR.M.NeveJ.SzollosiC.C.BenzUnraveling the biologic and clinical complexities of HER2Clin Breast Cancer852008392401
- C.C.BenzG.K.ScottJ.C.SarupR.M.JohnsonD.TripathyE.CoronadoEstrogen-dependent tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neuBreast Cancer Res Treat24219928595
- M.C.GutierrezS.DetreS.JohnstonS.K.MohsinJ.ShouD.C.AllredMolecular changes in tamoxifen resistant breast cancer: relationship between estrogen receptor, HER-2, and p38 mitogen-activated protein kinaseJ Clin Oncol2311200524692476
- L.CoussensT.L.Yang-FengY.C.LiaoE.ChenA.GrayJ.McGrathTyrosine kinase receptor with extensive homology to EGF receptor shares chromosomal location with NEU oncogeneScience2304730198511321139
- M.A.MolinaR.SáezE.E.RamseyM.J.Garcia-BarchinoF.RojoA.J.EvansNH (2)-terminal truncated HER-2 protein but not full-length receptor is associated with nodal metastasis in human breast cancerClin Cancer Res822002347353
- G.K.ScottR.RoblesJ.W.ParkP.A.MontgomeryJ.DanielW.E.HolmesA truncated intracellular HER2/neu receptor produced by alternative RNA processing affects growth of human carcinoma cellsMol Cell Biol134199322472257
- M.A.OwensB.C.HortenM.M.Da SilvaHER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6556 breast cancer tissuesClin Breast Cancer5120046369
- D.J.SlamonW.GodolphinL.A.JonesJ.A.HoltS.G.WongD.E.KeithStudies of the HER-2/neu protooncogene in human breast and ovarian cancerScience24449051989707712
- E.El-AbdA.HassanW.FaiedS.ZakiA.SobhiS.El-SwedyClinical relevance of HIF-1 alpha, Cox-2, leptin, and prolactin as hypoxic markers in breast cancerAJC1142012237246
- The Belmont Report. Ethical Principles and Guidelines for the Protection of Human Subjects of Research. The National Commission for the Protection of Human Subjects of Biomedical and Behavioral Research. April 18, 1979. http://www.hhs.gov/ohrp/policy/belmont.html (accessed 28.01.14).
- S.E.SingletaryJ.L.ConnollyBreast cancer staging: working with the sixth edition of the AJCC Cancer Staging ManualCA Cancer J Clin5620063747
- A.SaadM.ShetaA.SobhiE.AlyE.El-AbdClinical and pathological assessment of sentinel lymph node micrometastasis in breast cancer using RT-PCRJ Egypt Soc Path25120058187
- N.Abd EL-MoneimS.EbidA.KazemA.SaadS.MousaE.EL-ABDThe role of angiogenesis assessment in the prognosis of breast carcinoma and in the evaluation of the therapeutic effect of “shark care” drug as an angiogenesis inhibitorTurkish J cancer3832008123134
- M.NadjiQuantitative Immunohistochemistry of estrogen receptor in breast cancer. “much ado about nothing!”Appl Immunohistochem Mol Morphol1622008105107
- D.C.AllredJ.M.HarveyM.BerardoG.M.ClarkPrognostic and predictive factors in breast cancer by immunohistochemical analysisMod Pathol1121998155168
- L.J.LayfieldD.GuptaE.E.MooneyAssessment of tissue estrogen and progesterone receptor levelsBreast J632000189196
- A.RhodesB.JasaniD.M.BarnesL.G.BobrowK.D.MillerReliability of immunohistochemical demonstration of oestrogen receptors in routine practice: interlaboratory variance in the sensitivity of detection and evaluation of scoring systemsJ Clin Pathol5322000125130
- A.M.GownCurrent issues in ER and HER2 testing by IHC in breast cancerMod Pathol21Suppl 22008S8S15
- T.OyamaY.IshikawaM.HayashiK.ArihiroJ.HoriguchiThe effects of fixation, processing and evaluation criteria on immunohistochemical detection of hormone receptors in breast cancerBreast cancer1422007182188
- A.L.WijayaratneD.P.McDonnellThe human estrogen receptor-alpha is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulatorsJ Biol Chem2763820013568435692
- R.L.EckertA.MullickE.A.RorkeB.S.KatzenellenbogenEstrogen receptor synthesis and turnover in MCF-7 breast cancer cells measured by a density shift techniqueEndocrinology11421984629637
- F.J.MonsmaJrB.S.KatzenellenbogenM.A.MillerY.S.ZieglerJ.A.KatzenellenbogenCharacterization of the estrogen receptor and its dynamics in MCF-7 human breast cancer cells using a covalently attaching antiestrogenEndocrinology11511984143153
- S.SchollM.E.LippmanThe estrogen receptor in MCF-7 cells: evidence from dense amino acid labeling for rapid turnover and a dimeric model of activated nuclear receptorEndocrinology1154198412951301
- S.DauvoisP.S.DanielianR.WhiteM.G.ParkerAntiestrogen ICI 164,384 reduces cellular estrogen receptor content by increasing its turnoverProc Natl Acad Sci USA899199240374041
- J.VarayoudJ.G.RamosL.MonjeV.BosquiazzoM.Muñoz-de-ToroE.H.LuqueThe estrogen receptor UTR3 mRNA splicing variant is differentially regulated by estrogen and progesterone in the rat uterusJ Endocrinol186120055160
- T.A.HoppS.A.FuquaEstrogen receptor variantsJ Mammary Gland Biol Neoplasia3119987383
- R.L.BalleineS.M.HuntC.L.ClarkeCoexpression of alternatively spliced estrogen and progesterone receptor transcripts in human breast cancerJ Clin Endocr Metab844199913701377
- E.R.LeygueP.H.WatsonL.C.MurphyEstrogen receptor variants in normal human mammary tissueJ Natl Cancer Inst8851996284290
- P.GallacchiF.SchoumacherS.Eppenberger-CastoriE.M.Von LandenbergW.KuengU.EppenbergerIncreased expression of estrogen receptor exon-5-deletion variant in relapse tissues of human breast cancerInt J Cancer79119984448
- A.A.DaffadaS.R.JohnstonI.E.SmithS.DetreN.KingM.DowsettExon 5 deletion variant estrogen receptor messenger RNA expression in relation to tamoxifen resistance and progesterone receptor/pS2 status in human breast cancerCancer Res5521995288293
- S.G.KoehorstJ.J.CoxG.H.DonkerS.Lopes da SilvaJ.P.BurbachJ.H.ThijssenFunctional analysis of an alternatively spliced estrogen receptor lacking exon 4 isolated from MCF-7 breast cancer cells and meningioma tissueMol Cell Endocrinol1011-21994237245
- H.JiangR.TengQ.WangX.ZhangH.WangZ.WangTranscriptional analysis of estrogen receptor alpha variant mRNAs in colorectal cancers and their matched normal colorectal tissuesJ Steroid Biochem Mol Biol1121–320082024
- M.A.RunnakM.A.HazhaH.A.HeminA.A.WasanR.M.RekawtH.D.MichaelA population-based study of Kurdish breast cancer in northern Iraq: hormone receptor and HER2 status. A comparison with Arabic women and United States SEER dataBMC Womens Health12201216
- Ismail MF, Aly MS, Khaled HM, Mohamed HM. Detection of HER-2/neu, c-myc amplification and p53 inactivation by FISH in Egyptian patients with breast cancer. Ger, Med Sci 2009;7:Doc03.
- K.A.CroninL.C.HarlanK.W.DoddJ.S.AbramsR.Ballard-BarbashPopulation-based estimate of the prevalence of HER-2 positive breast cancer tumors for early stage patients in the USCancer Invest2892010963968
- J.S.RossJ.A.FletcherG.P.LinetteJ.StecE.ClarkM.AyersThe Her-2/neu gene and protein in breast cancer 2003: biomarker and target of therapyOncologist842003307325
- R.E.EllsworthD.L.EllsworthH.L.PatneyB.DeyarminB.LoveJ.A.HookeAmplification of HER2 is a marker for global genomic instabilityBMC Cancer82008297