Abstract
Objective
During the wound healing process, lymphatic regeneration in the injured skin has not been fully investigated. This work was designed to study the regeneration of lymphatic vessels in rat incisional wounds in relation to the duration after the wound infliction.
Material and methods
We studied the regeneration of lymphatic vessels in the rat skin incisional wounds (sutured and unsutured) by immunohistochemistry using an antibody against D2-40, a marker of lymphatic endothelium.
Results
Lymphatic vessels were detectable transiently at the wound edge and depth from day 3 till day 7, and none on day 10 in sutured wounds; and from day 5 till day 10, and none on day 14 in unsutured wounds. On the other hand, the center of the wound area did not show any evidence of lymphatic regeneration up to 60 days after the skin incision, regardless of presence/absence of sutures. Meanwhile, the regenerating blood vessels started to appear in the granulation tissue as early as day 2 in sutured wounds and day 3 in unsutured wounds.
Conclusion
Lymphatic elements appear transiently in the wound edge, concurrent with the appearance of blood vessels but regress earlier. Identification of lymphatic vascular channels in the region of the wound may help to estimate the wound age in the early days after the injury. At later time points in the regeneration process, it may help to recognize the injured area, being the area where the dermis and subcutaneous tissue are devoid of lymphatics.
Abbreviations:
1 Introduction
Wound healing is a dynamic process that proceeds with well-organized interaction between soluble mediators, blood cells, extracellular matrix, and parenchymal cells. Cutaneous wound healing starts immediately after injury and consists of three sequential phases: inflammation, proliferation, and maturation.Citation1 The initial injury causes platelet adhesion and aggregation, and the formation of a clot in the surface of the wound, leading to inflammation. In the proliferative phase, there is formation of granulation tissue, proliferation and migration of connective tissue cells, and re-epithelialization of the wound surface. Maturation involves extracellular matrix deposition, tissue remodeling, and wound contraction.Citation2 In each phase, various kinds of biological substances are closely involved. Examination of the dynamics of such a process would be beneficial to find a clue for wound vitality or wound age.Citation3
Angiogenesis, the development of new blood vessels from pre-existing ones, is a crucial event in the formation of granulation tissue in the proliferative phase of wound healing.Citation4 This process has been studied extensively. Although lymphatic vessels are also present in the subcutaneous tissue, lymphatic regeneration in injured tissues has not been fully investigated.Citation5
Lymphangiogenesis, like angiogenesis, is known to occur through sequential steps that involve production of vascular endothelial growth factor, expression of matrix metalloproteinases; and endothelial cell migration, proliferation and organization into functional vessels.Citation6 Although many similarities exist at the cellular level, the organizational principles of blood and lymph angiogenesis are different, and are probably related to their distinct physiological functions; angiogenesis is a tissue response to hypoxiaCitation4 whereas lymphangiogenesis is a tissue response to interstitial fluid flow.Citation7
Lymphangiogenesis has been reported to occur in adult tissues during inflammation, wound healing, and tumor metastasis.Citation8 Earlier studies have demonstrated at least some lymphatic vessels sprouting in experimental rabbit ear wounds.Citation9The growth of new lymphatic vessels has also been detected in autotransplants of the rat small bowelCitation10 and of the rat hindleg.Citation11 Increasingly, the importance of lymphatic biology is being realized. However, to date, lymphangiogenesis in adult tissues is not adequately understood,Citation6 and studies addressing lymphatic regeneration in the wound healing process remain insufficient, and have yielded contradictory results.
The recent identification of lymphatic endothelial markers has enabled the specific identification of lymphatic vessels.Citation12,Citation13 D2-40 is a commercially available monoclonal antibody that specifically detects a fixation-resistant epitope on podoplanin, which is a mucin-type transmembrane glycoprotein that is specifically expressed by lymphatic endothelial cells,Citation14–Citation16 but not by vascular endothelial cells.Citation17
The aim of the present study was to investigate lymphatic regeneration in a rat incisional wound model along the period of the wound healing process in order to determine the age of the wound to be used in forensic applications.
2 Material and methods
2.1 Experimental design
A total of 66 albino rats (6 week old, 150–200 g) obtained from the animal house of the Medical Research Institute, Alexandria University, were used in the study. The animals were allowed free access to a standard pellet diet and water, and were maintained at 21–23 °C in a 12/12 h light/dark cycle. All procedures complied with the guide lines for the care and handling of animals, and the study protocol was approved by the ethics committee of Alexandria Faculty of Medicine.
2.2 Skin wound model
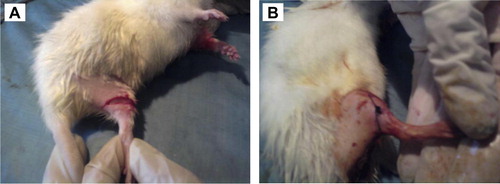
All wounding procedures were carried out under inhalational diethyl ether anesthesia. After shaving the hair of the dorsal thigh on both sides, the skin was wiped once with 70% ethanol. In the shaved area, linear incisions (1.5 cm in length) to the depth of the subcutaneous tissue were performed using sterile scalpels (). Hemostasis was achieved by applying gentle pressure when necessary. The incision was sutured immediately using a 3.0 silk thread to approximate the cut edges of the skin () in one wound in each rat, meanwhile, the other wound was left without sutures.
Figure 1 (A) After shaving the hair of the dorsal thigh, linear incisions to the depth of the subcutaneous tissue were performed in both thighs. (B) One incisional wound was sutured in each rat.
Animals were closely observed for wound closure, and for the appearance of any signs of infection, and those displaying wound swelling or infection were separated, excluded from the study and replaced. The wounds and general health of the animals were monitored daily for up to 60 days post-incision.
After 1 through 60 days, rats were anaesthized by diethyl ether. The wounds with the surrounding tissues were excised after scarification of the rats by cervical dislocation 1, 2, 3, 4, 5, 7, 10, 14, 21, 28, 35, 48, 54 and 60 days after the skin incision.
2.3 The rats were divided into
| A. | Antemortem inflicted skin wound (n = 56): As before four rats were sacrificed at each of the above mentioned 14 time points (i.e. n = 4 at each time point). | ||||
| B. | Postmortem inflicted skin wound (n = 6): The rats in this group were sacrificed and postmortem wounds were inflicted within 30 min after death in the same region as the wounded groups after shaving the hair. The skin samples were collected 1 h and 3 h after the incision. | ||||
| C. | Control group (n = 4): The control specimens were excised from uninjured rats in the same region as the wounded groups after shaving the hair. | ||||
2.4 Histopathological analysis
For histopathological study, the excised wound, together with the surrounding tissue was fixed in formalin and embedded in paraffin. Sections, 5um thick, were prepared and stained with hematoxylin and eosin (H&E). Sections of the excised wound tissue were also subjected to D2-40 immunohistochemistry as follows: sections were deparaffinized in xylene, rehydrated and treated with 0.3% hydrogen peroxide for 10 min to inhibit endogenous peroxidase activity. Antigen retrieval was performed by heating in citrate buffer (0.01 M, pH 6.0) in a microwave oven for 45–60 min. Then the solution was allowed to cool for 10 min. After the wash with phosphate-buffered saline (PBS), sections were incubated with the primary mouse monoclonal antibody D2-40 (CM 266, Biocare medical, CA, USA), dilution 1:100, at 4 °C in a humid chamber overnight. Sections were then incubated with biotinylated goat antipolyvalent (secondary antibody) for 15 min, followed by peroxidase-conjugated streptavidin for another 15 min at room temperature. After the wash with PBS, sections were incubated with 3–3′ Diaminobenzidine tetra hydrochloride (DAB) solution for 5 min at room temperature and then counterstained with hematoxylin. Lymphatics in the adjacent non-wounded skin served as internal positive control. Negative controls, without the primary antibody, were included with each run. D2-40 stained the cytoplasm of lymphatic endothelium.
For quantification, the number of D2-40 positive lymphatic vessels and lymphatic vessel-like structures was counted at ×200 magnification, and the mean value in 4 rats at each time point was expressed as the absolute number of D2-40 positive vessels per 0.74 mm2 (×200 field). Only vessels with a lumen or consisting of more than a single endothelial cell were calculated (18). Lymphatic vessel-like structures were defined as three or more cells associated to form a cord-like structure (19). To assess the relationship between the wound age and the appearance of lymphatics, the mean values at different time points in the regeneration process were compared in the edge of sutured and unsutured wounds using Student’s t-test. A p-value less than 5% was considered statistically significant.
3 Results
3.1 Observation of the wound healing process
3.1.1 A. Sutured versus unsutured wounds
In sutured wounds, on day 1, a blood clot was seen on the wound surface (), with fibrin threads entangling red blood cells and neutrophils (A-inset). On day 2, re-epithelialization of the wound surface was achieved, where a thin, continuous epithelial layer closed the wound (); and early granulation tissue was noted in the dermis, composed of newly-formed blood vessels and fibroblasts (B-inset). On day 5 (), granulation tissue filled the wound area and neovascularization was maximal, with the blood vessels appearing vertical (C-inset). In addition, collagen fibrils started to appear and began to bridge the wound gap. On day 7, the leukocytic infiltrate and edema largely disappeared, associated with accumulation of collagen within the wound area and regression of vascular channels (). On day 10, wound contraction was noted (). On day 14, the blood vessels were less vertical, and gradually became similar to the vessels in non-wounded skin areas thereafter.
Figure 2 H&E-stained sections of rat skin incision wound area. A–F: sutured wound; G&H: unsutured wound. (A) Day 1, a blood clot on the wound surface. Inset: fibrin threads entangling red blood cells and neutrophils. (B) Day 2, re-epithelialization of the wound surface and early granulation tissue beneath (inset). (C) Day 5, numerous blood vessels; collagen fibrils started to appear. Inset: vertical blood vessels. (D) Day 7, accumulation of collagen and regression of vascular channels. (E) Day 10, wound contraction. (F) Day 48, an avascular scar, composed of spindle-shaped fibroblasts, and dense collagen bundles covered by intact epidermis. (G) Day 3, large amount of early granulation tissue. Re-epithelialization has not yet occurred. (H) Day 60: an avascular scar not different from that of sutured wounds. Original magnifications: (A–H) ×100; (insets) ×200.
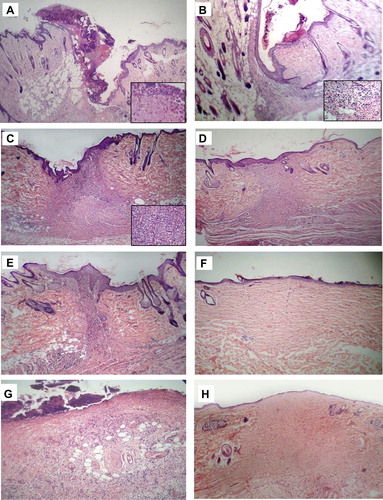
From the 21st day onwards, the wound area was composed of avascular connective tissue, composed of spindle-shaped fibroblasts, and dense collagen bundles covered by intact epidermis (Day 48 is shown in ). The dermal appendages that have been destroyed in the line of the incision have not regenerated.
In unsutured wounds, on day 1, a larger tissue deficit was seen in comparison with sutured wounds, the fibrin clot was larger, and there was more necrotic debris in the wound gap. On day 3, early granulation tissue was seen in the wound gap, the amount was large (). On day 4, re-epithelialization was noted. On day 7, collagen fibrils started to appear and to bridge the incision gap. Wound contraction was noted on day 14. From day 14 onwards, the picture was not different from that of sutured wounds (Day 60 is shown in ).
3.2 Assessment of lymphatic regeneration
Anti-D2-40 immunohistochemistry was used to evaluate the regeneration of lymphatics during the wound healing process ( and ).
Figure 4 Immunohistochemical expression of D2-40 in rat skin incisional wound (sutured): D2-40 positive lymphatic elements were absent in the center of the wound area, and present in the adjacent non-wounded skin throughout the duration of the study. (A) Day 3: D2-40 positive lymphatic vascular structures are noted along the junction between the dermis and subcutaneous tissue at the region of the wound depth. B corresponds to part figure B. (B) Day 3: Few lymphatics are noted at the wound edge and depth (arrows). (C) Day 5: Lymphatics are detectable along the wound margin. D corresponds to part figure D. (D) Day 5: higher magnification demonstrating lymphatics and lymphatic-like structures at the wound edge and depth (arrows). (E) Day 10: lymphatics are no more seen at the wound edge, but are present in the adjacent non-wounded skin (arrows). F corresponds to part figure F. (F) Day 10: higher magnification demonstrating absence of lymphatics in the wound area. Original magnifications: (A, C, E) ×100; (B, D, F) ×200.
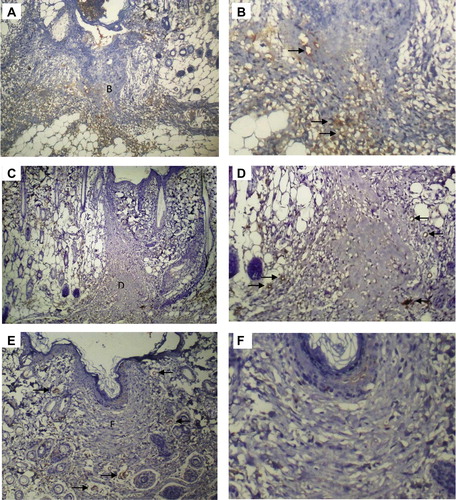
Figure 5 Immunohistochemical expression of D2-40 in (A) Rat skin incisional wound on day 21. B and C correspond to part figures B and C. (B) Day 21: higher magnification demonstrating absence of lymphatics in the wound area. (C) Day 21: higher magnification demonstrating D2-40 positive lymphatics in the adjacent non-wounded skin (arrows). (D) Rat skin incision wound on day 35. Lymphatics were absent in the wound area, and present in the adjacent non-wounded skin Original magnifications: (A, D) ×100; (B,C) ×200.
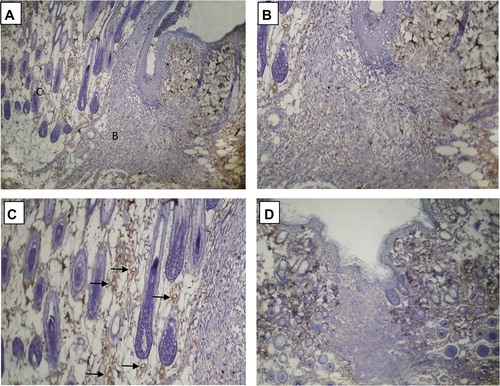
In the center of the wound area, no D2-40 positive lymphatic vessels could be detected in the tissues obtained 1, 2, 3, 4, 5, 7, 10, 14, 21, 28, 35, 48, 54 and 60 days after the skin incision.
On the other hand, at the edge of the wound and at the depth, transient appearance of D2-40 positive lymphatic vessels and lymphatic vessel-like structures (D2-40 positive cells arranged as cords) was observed. Lymphatics appeared to grow from the subcutaneous tissue to the depth of the wound area, as evidenced by a condensation of D2-40 lymphatic vascular elements along the junction between the subcutaneous tissue and the dermis at the wound depth ().
In sutured wounds, these lymphatic vascular elements appeared on day 3, very few remained on day 7 and none could be detected on day 10. In unsutured wounds, these D2-40 positive lymphatics, appearing on day 5, were detectable in lower numbers on day 10 and none remained on day 14 ().
Figure 6 Mean numbers of D2-40 positive vessels at ×200 magnification at the edge and depth of sutured and unsutured skin wounds from 1 through 21 days after the incision. (n = 4 at each time point) ∗: statistically significant difference at P < 0.05.
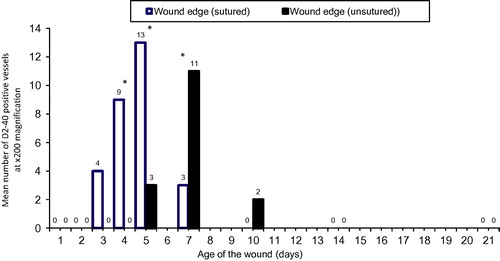
In order to assess the relationship between the age of the wound and the appearance of lymphatics, the D2-40 positive vessels were counted at the wound edge/depth in sutured and unsutured wounds. Lymphatic vessels were only detectable at the wound edge/depth in the early days after the injury. The mean numbers of D2-40 positive vessels were significantly higher in sutured than unsutured wounds on days 4 and 5, whereas the mean number was significantly higher in unsutured wounds on day 7 ().
In the adjacent non-wounded skin, D2-40 positive lymphatic vessels were seen in the dermis and subcutaneous tissue all through the duration of the study ( and ).
summarizes different events observed during wound healing in sutured and unsutured wounds in relation to wound age.
Table 1 Observations during the wound healing process in sutured and unsutured incisional wounds in relation to wound age.
4 Discussion
Wound examination is one of the most important and indispensable areas for forensic pathologists, who are always required to determine how long before death a wound was sustained; and to discriminate antemortem wounds from postmortem damage to correctly evaluate the relationship between death and any wounds.Citation1 Determination of wound age plays a role in connection with traumatic deaths due to sharp and blunt force injuries, burns, strangulation, suffocation or drowning, thus, it is mandatory to estimate wound age in cases of murder, manslaughter, bodily harm with fatal consequences, (primarily survived) accidents and further constellations.Citation20
Incisional skin wounds heal by ingrowth of granulation tissue and re-epithelialization.Citation18 In the present work, a difference was observed in the timing of the sequential steps of wound healing between sutured and unsutured wounds. In sutured wounds, 2 days after the skin incision, re-epithelialization of the wound surface was achieved, and early granulation tissue with angiogenic blood vessels appeared in the dermis. On the 5th day, neovascularization was maximal, with the blood vessels appearing vertical, and collagen fibrils started to appear. Wound contraction was observed on day 10. On day 14, the blood vessels were less vertical, and gradually became similar to the vessels in the adjacent non-wounded skin areas thereafter. Unsutured wounds showed a lag of 1 to 4 days behind the sutured wounds, where early granulation tissue appeared on day 3, re-epithelialization occurred on day 4, collagen fibrils started to appear on day 7 and wound contraction was noted on day 14. From day 14 onwards, the picture was not different from that of sutured wounds. Paavonen et al.Citation18 studied healing in sutured incisional wounds in pigs, and found that angiogenic blood vessels appeared on day 4, and were most abundant on days 5 and 6. Their results were comparable to ours except for the earlier appearance of angiogenic blood vessels in our study, possibly due to the difference in species of the experimental animals used. Nogami et al.Citation5 reported that the blood vessels that regenerated in the granulation tissue of the rat skin incision wound were vertically running 5 days after the incision, and changed into regular-shaped vessels by 28 days. Again, their findings agree with our observations.
In our work, postmortem wounds showed simple tissue separation, without evidence of tissue reaction. This is in accordance with the findings reported by Obac et al.,Citation21 who observed absence of fibrin in the borders of the postmortem incision inflicted 30 min after death in striking contrast with the fibrin accumulation seen in case of in vivo 30 min vital reaction.
Despite their essential physiological role and their obvious medical importance, until recently the lymphatic vessels have received much less attention than blood vessels, and the recovery process of lymphatics in injured tissues remains poorly understood.Citation22,Citation23 In the present study, we examined lymphatic growth and morphology at different time points during the wound healing process using anti-D2-40 immunohistochemistry. In the center of the wound area, no D2-40 positive lymphatic vessels were detected in the tissues obtained up to 60 days after the skin incision. In the peripheral part of the granulation tissue, at the edge of the wound, and at the depth, transient appearance of lymphatic vessels was observed. In addition, some D2-40 positive cells appeared to assemble into cords that represent lymphatic vessel-like structures. In sutured wounds, these D2-40 positive lymphatic elements appeared on day 3, very few lymphatics remained on day 7 and none could be detected on day 10. In unsutured wounds, these vessels appeared on day 5, were still detectable on day 10, and none remained on day 14.
Quantitation of the number of D2-40 expressing vessels at the wound edge at different time points revealed that the lymphatic vessels were significantly more in sutured than unsutured wounds on days 4 and 5, whereas the reverse was noted on day 7. Furthermore, D2-40 expression was detectable in the pre-existing lymphatics in the dermis and subcutaneous tissue of the non-wounded skin adjacent to the wound area all through the duration of the study.
Controversial results have been reported in the literature regarding lymphatic regeneration in wounds. Greco et al.Citation23 evaluated the lymphatic flow in skin incisional wounds with sutures using lymphoscintigraphy. They reported restoration of lymphatic flow within 14 days, regaining the ability to drain fluid and particulate material from the tissue. Meanwhile, Nogami et al.,Citation5 using podoplanin immunohistochemistry, found no recovery of lymphatic vessels up to 84 days after the skin incision in open wounds. Paavonen et alCitation18 demonstrated the appearance of vascular endothelial growth factor receptor (VEGFR)-3-positive lymphatic endothelial cells in sutured incisional wounds on the 5th day, especially in the peripheral parts of the granulation tissue, and their disappearance on day 14. Moreover, Ji et al.Citation22 reported that lymphatic vascular structures which stained for 5′-nucleotidase, extended irregularly along the wound edge between days 7 and 15 of the wounds. The transient appearance of lymphatics and their location at the wound edge that are reported in the last two studies agree with our findings.
The origin of the regenerating lymphatic vessels in the skin wound tissue remains obscure.Citation19
Maruyama et al.Citation19 observed that cells that were double-positive for the macrophage marker F4/80 and the lymphatic marker LYVE-1 appeared in the granulation tissue from the 5th till the 14th day after wounding. They also reported that lymphatics in the wound at day 14 express low levels of F4/80 suggesting that new lymphatic vessels that assemble transiently during the acute phase of wound healing are derived in situ from macrophages.Citation19
In a skin regeneration model using the rat tail, a band of skin was removed and the gap was filled with collagen, where lymphatic endothelial cells were found to migrate as single cells that later join together into vessels, that is, lymphatics do not sprout in the region.Citation6 Conversely, another study group reported that lymphatic elements appeared to sprout from pre-existing lymphatic vessels at the wound edge.Citation18 In the present work, we did not observe direct sprouting of lymphatics from pre-existing lymphatic vessels at the wound edge, but we definitely observed lymphatic vessels and lymphatic vessel-like structures at the wound depth, together with a condensation of lymphatic vessels at the junctional area between the dermis and subcutaneous tissue in the vicinity of the wound. Our findings are in line with those of Ji et al.,Citation22 who declared that lymphangiogenesis occurs from the subcutaneous area to the dermis along the wound healing edge, and mentioned that the dermis and subcutaneous tissue transitional area is favorable to the growth of regenerating lymphatic vessels.
The notable absence of lymphatics in the center of the wound which was observed throughout the duration of our study needs explanation. It is known that interstitial fluid flow plays an important role in lymphatic function and lymphangiogenesis.Citation7 Rutkowski et al.Citation6 declared that migrating lymphatic endothelial cells populate the regenerating region and organize unidirectionally (in the direction of interstitial fluid flow) into vessels, and that when the interstitial fluid flow is shunted lymphatic endothelial cells fail to organize into functional vessels. It is possible that the interstitial fluid flow may be disrupted in the central region of the wound, resulting in interference with lymphangiogenesis in this area.
Another possibility is that the interstitial fluid pressure in the center of the wound may be increased (due to the leaky nature of the newly-formed blood vessels in the granulation tissue), which may impede lymphatic formation in a manner similar to what occurs in tumors, which may induce lymphangiogenesis, yet the lymphatic vessels cannot penetrate the tumor stroma due to increased interstitial pressure.Citation24 The process of tumor lymphangiogenesis has been suggested to operate via similar mechanisms to the wound lymphangiogenesis,Citation18 which may support the latter view.
The transient formation of lymphatic vessels and their rapid regression when inflammation is resolved, as demonstrated in the current work, suggest that lymphatics may play a role in the process of wound healing. Although Hong et al.Citation25 suggested that lymphangiogenesis is not essential for closure of excisional skin wounds, several other researchers have declared that the early formation of lymphatic vessel-like structures in granulation tissue seems to be an integral part of normal wound healing.Citation18,Citation19 In addition, the induction of the growth of lymphatic vessels was found to be beneficial in conditions of wound healing or lymphedema.Citation23 Furthermore, the reduced development of lymphatics seen in infections,Citation26 and their relative absence in the chronic woundsCitation18 may, at least partly, account for delayed wound healing in these conditions.
To sum up, transient lymphangiogenesis occurs in parallel with angiogenesis in healing skin incisional wounds, yet lymphatics regress earlier than blood vessels. This process is delayed in unsutured wounds. Identification of lymphatic elements in the region of the wound may help to estimate the wound age in the early days after the injury. At later times, it may help to recognize the injured area of the skin, being the area where the dermis and subcutaneous tissue are devoid of lymphatics.
Conflict of interest
There is no conflict of interest to declare.
Notes
Available online 20 June 2014
Peer review under responsibility of Alexandria University Faculty of Medicine.
References
- T.KondoTiming of skin woundsLegal Med922007109114
- G.C.GurtnerS.WernerY.BarrandonM.T.LongakerWound repair and regenerationNature4532008314
- T.OhshimaForensic wound examinationForensic Sci Int1132000153164
- Tissue renewal, regeneration and repairV.KumarA.K.AbbasN.FaustoJ.AsterCotran pathologic basis of disease8th ed.2010Saunders ElsevierPhiladelphia79108
- M.NogamiT.HoshiT.AraiY.ToukairinM.TakamaI.TakahashiMorphology of lymphatic regeneration in rat incision wound healing in comparison with vascular regenerationLegal Med1152009213218
- J.M.RutkowskiK.C.BoardmanM.A.SwartzCharacterization of lymphangiogenesis in a model of adult skin regenerationAm J Physiol Heart Circ Physiol29132006H1402H1410
- K.C.BoardmanM.A.SwartzInterstitial flow as a guide for lymphangiogenesisCirc Res922003801808
- T.TammelaK.AlitaloLymphangiogenesis: molecular mechanisms and future promiseCell1402010460476
- B.OdenA micro-lymphangiographic study of experimental wounds by second intentionActa Chir Scand1201960100114
- F.SchierA.UnerJ.WaldschmidtMicrolymphography of spontaneous lymph vessel anastomosis in small bowel transplantation in the ratJ Pediatr Surg26199112391242
- J.P.AnthonyR.D.FosterD.C.PriceM.MahdavianY.InoueLymphatic regeneration following microvascular limb replantation: a qualitative and quantitative animal studyJ Reconstr Microsurg131997327330
- G.OliverM.DetmarThe rediscovery of the lymphatic system: old and new insights into the development and biological function of the lymphatic vasculatureGenes Dev162002773783
- Y.KatoM.K.KanekoA.KunoInhibition of tumor cell-induced platelet aggregation using a novel anti-podoplanin antibody reacting with its platelet aggregation-stimulating domainBiochem Biophys Res Commun3494200613011307
- A.N.KalofK.CooperD2-40 immunohistochemistry–so far!Adv Anat Pathol16120096264
- M.FakunagaExpression of D2-40 in lymphatic endothelium of normal tissue and in vascular tumorsHistopathology462005396402
- B.BandarchiL.MaC.MargineanS.HafeziJ.ZubovitsG.RastyD2-40 a novel immunohistochemical marker in differentiating dermatofibroma from dermatofibrosarcoma protuberansMod Pathol2332010434438
- H.J.KahnD.BaileyA.MarksMonoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi’s sarcoma and a subset of angiosarcomasMod Pathol1542002434440
- K.PaavonenP.PuolakkainenL.JussilaT.JahkolaK.AlitaloVascular endothelial growth factor receptor-3 in lymphangiogenesis in wound healingAm J Pathol1565200014991504
- K.MaruyamaJ.AsaiM.IiT.ThorneD.W.LosordoP.A.D’AmoreDecreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healingAm J Pathol170200711781191
- W.GrellnerB.MadeaDemands on scientific studies: vitality of wounds and wound age estimationForensic Sci Int1652007150154
- A.R.ObacE.G.CarvalhoP.C.S.SilvaN.Fenerich-VeraniM.AlmeidaHistological analysis of short-term vital reactions in skin wounds: potential applications in forensic workBraz J Biol714201110111014
- R.C.JiM.MiuraP.QuS.KatoExpression of VEGFR-3 and 5′-nase in regenerating lymphatic vessels of the cutaneous wound healingMicrosc Res Tech6432004279286
- K.V.GrecoP.F.LaraR.M.Oliveira-FilhoR.V.GrecoL.S.Sudo-HayashiLymphatic regeneration across an incisional wound: inhibition by dexamethasone and aspirin, and acceleration by a micronized purified flavonoid fractionEur J Pharmacol5511–32006131142
- Y.BoucherM.LeunigR.K.JainTumor angiogenesis and interstitial hypertensionCancer Res56199642644266
- Y.K.HongB.Lange-AsschenfeldtP.VelascoS.HirakawaR.KunstfeldL.F.BrownVEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrinsFASEB J18200411111113
- R.C.JiCharacteristics of lymphatic endothelial cells in physiological and pathological conditionsHistol Histopathol202005155175