Abstract
Background
Alzheimer’s disease (AD) is a complex neurodegenerative disease characterized by progressive decline in memory, language and other cognitive functions. Recent studies provide convincing evidence on the role of vitamin D3 on the nervous system.
Aim
To investigate the effect of the active form of vitamin D3 (1,25-dihydroxycholecalciferol) as a neuroprotective agent in experimentally induced AD in rats.
Methods
40 adult male Wistar (albino) rats weighing 180 to 200 g were included in this study. Rats were divided into four groups (each of 10 rats): Group I: normal healthy rats receiving intracerebroventricular injection (icv) of artificial cerebrospinal fluid (ACSF) and serving as a control group. Group II: rats with induced AD by icv colchicine injection of 15 μg/rat bilaterally and receiving no treatment. Group III: rats pre-treated with active form of vitamin D3 42 IU/kg/day subcutaneously (s.c.) for one week followed by induction of AD then post-treated with vitamin D3 in the same dose for 3 weeks. Group IV: rats with induced AD then post-treated with vitamin D3 for 3 weeks.
The following parameters were evaluated in rats of all studied groups:
| 1- | Behavioral assessment: Morris water maze and open field tasks were performed at days 13, 14 and 21 post-icv injection for assessing cognitive, gross behavioral and motor activities of studied groups. | ||||
| 2- | Biochemical tests: Hippocampal tissue levels of brain derived neurotrophic factor (BDNF), amyloid beta (Aβ) peptide, and antioxidant system; glutathione reductase (GR) and glutathione peroxidase (GPX). | ||||
Results
The present study revealed a significant increase in time latency of water maze test and hippocampal tissue level of Aβ peptide concomitant with significant reduction of hippocampal tissue levels of BDNF, GR and GPX, in untreated AD rats (group II) versus control ACSF-injected rats (group I) and vitamin D3-treated AD rats (groups III and IV). However, group III (AD rats pre- and post-treated with vitamin D3) showed a significant decrease in time latency and Aβ peptide, and a significant elevation of BDNF, GR and GPX, versus group IV (AD rats post-treated with vitamin D3).
Conclusion
Prophylactic use of active form of vitamin D3 (1,25(OH)2D3) appears to possess a neuroprotective effect in AD involving various mechanisms. Hence, vitamin D3 or its analogues can be considered as promising agents for development of new prophylactic and therapeutic neuroprotectors.
1 Introduction
Alzheimer’s disease (AD) is a complex neurodegenerative disease characterized by progressive decline in memory, language and other cognitive functions. Several etiologic hypotheses have been advanced for AD as genetic defects, appearance of neurofibrillary tangles, altered amyloid precursor processing, deficiency of neurotropic factors, mitochondrial defect, trace element neurotoxicity, energy metabolism deficit and oxidative stress.Citation1
Figure 1 Effect of icv injection of colchicine, vitamin D3 pre- and post-AD treatment and vitamin D3 post-AD treatment on cognitive performance in Morris water maze (seconds) in comparison with the control ACSF-injected rats; IAL, initial acquisition latency; 1st RL, 2nd RL, first and second retention latency respectively. Data are expressed as mean ± standard error of the mean. Number of rats/group = 10.
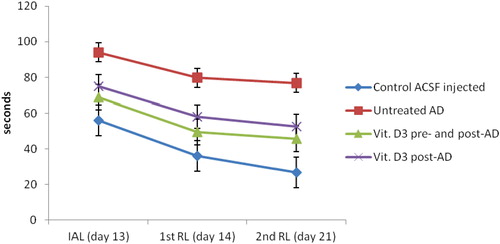
Figure 2 Hippocampal tissue levels of BDNF (pg/mg protein) in the studied groups. Group I: control ACSF injected, Group II: untreated AD, Group III: vitamin D3 pre- and post-AD treated, Group IV: vitamin D3 post-AD treated. Values are expressed as mean ± SD.
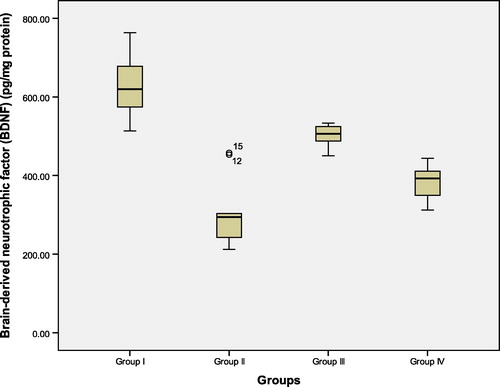
Figure 3 Hippocampal tissue levels of amyloid beta peptide (pg/mg protein) in the studied groups. Group I: control ACSF injected, Group II: untreated AD, Group III: vitamin D3 pre- and post-AD treated, Group IV: vitamin D3 post-AD treated. Values are expressed as mean ± SD.
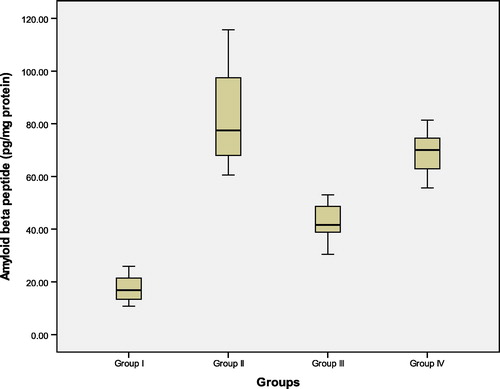
Figure 4 Hippocampal tissue levels of glutathione reductase (GR) (nmol/min/mg protein) in the studied groups. Group I: control ACSF injected, Group II: untreated AD, Group III: vitamin D3 pre- and post-AD treated, Group IV: vitamin D3 post-AD treated. Values are expressed as mean ± SD.
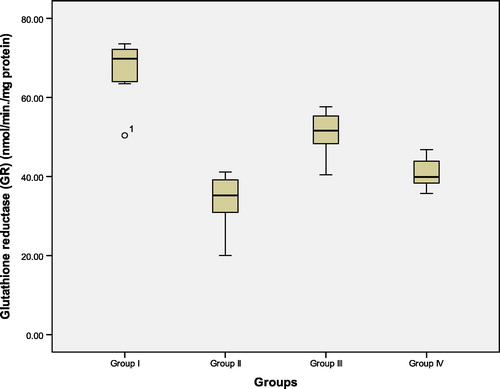
Figure 5 Hippocampal tissue levels of glutathione peroxidase (GPX) (nmol/min/mg protein) in the studied groups. Group I: control ACSF injected, Group II: untreated AD, Group III: vitamin D3 pre- and post-AD treated, Group IV: vitamin D3 post-AD treated. Values are expressed as mean ± SD.
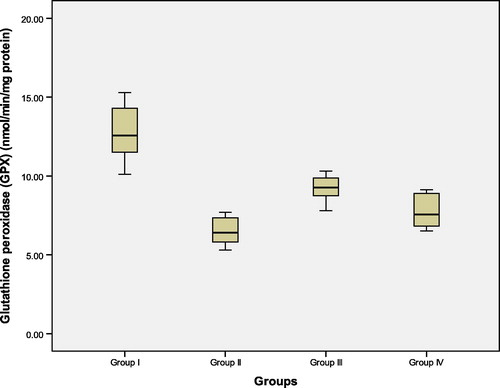
Table 1 Effect of icv injection of colchicine, vitamin D3 pre- and post-AD treatment and vitamin D3 post-AD treatment on the cognitive performance in Morris water maze (seconds) in the studied groups.
Table 2 Hippocampal tissue levels of brain derived neurotrophic factor (BDNF) (pg/mg protein), amyloid beta (Aβ) peptide (pg/mg protein), and activity of both glutathione reductase (GR) (nmol/min/mg protein) and glutathione peroxidase (GPX) (nmol/min/mg protein) in the studied groups.
Table 3 Correlations between hippocampal tissue levels of brain derived neurotrophic factor (BDNF) and amyloid β (Aβ) peptide and glutathione reductase (GR) and glutathione peroxidase (GPX) in different studied groups.
The hippocampus, a key region of the medial temporal lobe, is a frequent target in many neurological diseases and most forms of dementia. It is well established that damage to the hippocampus accounts for many of the cognitive deficits observed in AD, particularly those concerned with long term memory.Citation2
Alzheimer’s disease is associated with microtubule dysfunction and characterized by the appearance of specific cytoskeletal cellular abnormalities, which are associated with cognitive impairment.Citation3 Colchicine is a microtubule-disrupting agent that produces marked destruction of hippocampal granule cells, mossy fibers and septo-hippocampal pathways. It induces neurofibrillary degeneration by binding to tubulin, the structural protein of the microtubule, which is associated with loss of cholinergic neurons and decrease in acetylcholine transferase, thereby, resulting in impairment of learning and memory. Colchicine-induced cognitive impairment has been established as an animal model of sporadic dementia of Alzheimer’s type.Citation4
Oxidative stress, due to increased free radical generation and impairment of the endogenous antioxidant mechanisms, is an important factor that has been implicated in neuronal damage of AD and cognitive defects seen in elderly.Citation5
An abnormally elevated level of the amyloid beta (Aβ) peptide in the brain is one of the prominent features of AD. Aβ peptide is normally produced by neurons and cleared through its degradation by the proteinase enzyme within the brain tissues, as well as, through its elimination from the brain to the circulating blood via an efflux transport system at the blood brain barrier (BBB). It has been proposed that impairment of cerebral clearance of Aβ peptide leads to abnormal elevation of its brain level in late onset AD, which accounts for more than 90% of all cases of AD.Citation6 Indeed, cerebrovascular dysfunction has been implicated to cause impairment of Aβ peptide clearance across BBB; likewise, low levels of vitamin D3 are associated with increased risk of AD, as well as, vascular disorders.Citation7
Traditionally, vitamin D3 was considered as a hormonal regulator of calcium and phosphate homeostasis; however, results from recent studies provide a convincing evidence on the role of vitamin D3 in other biochemical processes in various tissues, including the nervous system.Citation8
Therefore, the aim of the present work was to investigate and assess the possible neuroprotective effect of the active form of vitamin D3 (1,25-dihydroxycholecalciferol) in colchicine-induced AD in rats.
2 Materials and methods
This study was carried out on 40 adult male Wistar (albino) rats weighing 180–200 g. The animals were housed under standard laboratory conditions with natural light/dark cycle with free access to standard diet and water throughout the experimental period. All the experiments were held between 9:00 and 15:00 h. The study protocol was approved by Ethics Committee of Faculty of Medicine, Alexandria University, and conducted in Physiology, Clinical Pharmacology and Medical Biochemistry Departments, Faculty of Medicine, Alexandria University.
Animals were divided into 4 groups (each of 10 rats); as follows:
| • | Group I (Control ACSF-injected): included normal healthy rats that received intracerebroventricular (icv) artificial cerebrospinal fluid (ACSF) 5 μl/site/rat. ACSF constituents in milli mole (mM): 147 NaCl, 2.9 KCl, 1.6 MgCl2, 1.7 CaCl2 and 2.2 dextrose.Citation9 | ||||
| • | Group II (Untreated AD): included rats with induced AD by icv injection of colchicine dissolved in ACSF 15 μg/rat (7.5 μg in 5 μl/site), bilaterally into the lateral ventricle using Hamilton microsyringe.Citation10 | ||||
| • | Group III (Vitamin D3 pre- and post-AD treated): rats in this group received active form of vitamin D3 (1,25-dihydroxycholecalciferol) (1,25(OH)2D3) in a daily dose of 42 IU/kg subcutaneously (s.c.) for one week,Citation11 followed by induction of AD by icv injection of colchicine as previously mentioned.Citation10 Then, rats received the same daily dose of 1,25(OH)2D3 for 3 weeks.Citation11 | ||||
| • | Group IV (Vitamin D3 post-AD treated): included rats with induced AD, as previously mentioned, followed by administration of the same daily dose of 1,25(OH)2D3 for 3 weeks (42 IU/kg s.c.).Citation11 | ||||
Animals were anesthetized with thiopental sodium 45 mg/kg, intraperitoneally (i.p.), then surgery was performed according to the previously described protocol using a stereotaxic apparatus (Kopf, Germany).Citation9,Citation10 The coordinates of the icv cannula implantation were 0.8 mm posterior to the bregma, 1.8 mm lateral to the sagittal suture and 3.6 mm beneath the cortical surface. After surgery, rats received gentamycin (5 mg/kg, i.p.) to prevent sepsis and were provided with special care and were monitored until spontaneous movement occurred.
Colchicine was obtained from Sigma–Aldrich, St. Louis, USA and was dissolved in ACSF. Vitamin D3 (1,25(OH)2D3) was obtained from Calbiochem, CA, USA and was prepared daily and dissolved in 1% ethanol (diluted with sterile saline). All other chemicals were analytical grade commercial products.
2.1 Behavioral assessment
2.1.1 Assessment of cognitive performanceCitation4–Citation5Citation6Citation7Citation8Citation9Citation10
The acquisition and retention of a spatial navigation task were assessed using Morris water maze. Animals were trained to swim to a platform in a circular pool (180 cm diameter × 60 cm), filled with water (28 ± 2 °C) to a depth of 40 cm. The pool was divided into 4 quadrants by 4 starting points marked on its wall: North, South, East and West (N–S–E–W). A circular platform 9 cm in diameter was placed in the pool 2 cm below water level mounted on a column. The platform provided the only escape from water and was fixed in the center of one of the 4 quadrants for the duration of experiment. A colored flag was placed outside the pool in a fixed position relative to the pool to help the rat to locate the position of the platform hidden below the water.
| (a) | Maze acquisition phase (training): On day 13 post-icv injection, rats received a training session consisting of 4 trials with an interval of 10 min. In all 4 trials, the starting positions were different. The time taken by a rat to reach the platform was recorded as initial acquisition latency (IAL) up to a maximum of 120 s (s). | ||||
| (b) | Maze retention phase (testing for retention of the learned task): Following 24 h (day 14) and 8 days (day 21) after IAL, each rat was randomly released at any one of the starting points (N–S–E–W) facing the wall of the pool and tested for the retention of the learnt task. The latency to reach the hidden platform on days 14 and 21 was recorded and termed as first and second retention latency (1st RL and 2nd RL) respectively. | ||||
2.1.2 Assessment of gross behavioral activityCitation12
On days 13, 14 and 21 post-icv injection, the gross behavioral, and spontaneous motor activities of rats were assessed in open field arena. The open field chamber is a wooden box (72 × 72cm × 36 cm height) with one wall formed of glass and its floor is divided into equal-sized squares. Open field task was performed immediately before water maze test.
2.2 Biochemical tests
Next day after the last behavioral test, all rats were decapitated under ether anesthesia and the brains were rapidly removed and rinsed with ice-cold phosphate buffered saline (PBS) solution, pH 7.4 to remove any red blood cells and clots. Then, the hippocampus was dissected and the following measurements were done:
2.2.1 Hippocampal tissue levels of brain derived neurotrophic factor (BDNF)
One half of the hippocampal tissues was homogenized in 1 ml of the homogenization buffer (30 mM Tris–HCl, 150 mM NaCl, 1 mM EDTA, 1 mM EGTA and 1% triton X-100 supplemented with protease inhibitor cocktail). Then, the homogenates were centrifuged at 3000 rpm for 20 min. The supernatants were collected and stored at −80 °C after protein level estimation by Lowry’s method.Citation13 Level of BDNF was determined according to the manufacturer’s ELIZA kit (Boster Biological Technology, Fermont, California, USA).Citation14
2.2.2 Hippocampal tissue levels of amyloid beta (Aβ) peptide
The other half of hippocampal tissues was homogenized in 1 ml cold PBS (with a pH of 7.4), to which a protease inhibitor cocktail was added. Homogenates were centrifuged at 3000 rpm for 20 min. The supernatants were collected and stored at −80 °C after protein level estimation by Lowry’s method.Citation13 The Aβ peptide was determined according to the manufacturer’s ELIZA kit (WKEA Med Supplies Corp., New York, USA (rat β-amyloid 1–42).Citation15
2.2.3 Hippocampal tissue levels of antioxidant system: glutathione reductase (GR), and glutathione peroxidase (GPX)
GR and GPX activities were measured in hippocampal tissues according to the method described by Carlberg et al. Citation16 and Wheeler et al.Citation17 respectively.
GR activity was assayed spectrophotometrically, using Trevigen Glutathione Reductase Assay Kit, wherein the oxidation of NADPH to NADP was monitored as a decrease in absorbance at 340 nm. This rate of decrease in absorbance was directly proportional to the glutathione reductase activity in the sample.Citation16
Quantitative colorimetric glutathione peroxidase determination was performed using EnzyChromTM Glutathione Peroxidase Assay Kit (EGPX-100), wherein Cumene hydroperoxide is reduced while reduced glutathione (GSH) is oxidized to oxidized glutathione (GSSG). The generated GSSG is reduced to GSH with consumption of NADPH by GR. The decrease of NADPH (measured at 340 nm) was proportional to GPX activity.Citation17
2.3 Statistical analysis
The changes in the studied parameters were expressed as mean ± SD. Differences between groups were compared by one way ANOVA using the statistical package of social sciences (SPSS) version 20. Association between different parameters was determined using Pearson’s correlation coefficient (r). Statistical significance was set at p value less than 0.05.
3 Results
3.1 Alteration in cognitive performance: initial acquisition latency (IAL), first and second retention latency (1st and 2nd RL) in water maze task (seconds) ( – )
On day 13, control ACSF-injected, vitamin D3 pre- and post-AD treated, and vitamin D3 post-AD treated groups of rats quickly learned to swim directly to the platform in the Morris water maze. Untreated AD rats showed an initial increase in IAL, which declined with continued training during the acquisition of a spatial navigation task on day 13. Indeed, there was a significant difference in the mean IAL of untreated AD group versus control group, on day 13, indicating that icv injection of colchicine induced impaired acquisition of spatial navigation task. In contrast, vitamin D3 pre- and post-AD treated, and vitamin D3 post-AD treated rats significantly decreased the IAL to reach the platform in the pre-trained rats versus untreated AD rats, on day 13 (F = 81.29, P < 0.0001).
Following training, the mean retention latencies (1st and 2nd RL) to escape onto the hidden platform were significantly decreased in control, untreated AD, vitamin D3 pre- and post-AD treated, and vitamin D3 post-AD treated rats on days 14 and 21, respectively, as compared to IAL on day 13. However, the mean of 1st and 2nd RL of untreated AD rats on days 14 and 21, respectively, were significantly higher versus those of the control, vitamin D3 pre- and post-AD treated, and vitamin D3 post-AD treated rats. Regarding the AD rats, vitamin D3 pre-and post-AD treated rats significantly decreased both 1st and 2nd RL, on days 14 and 21, respectively, versus those of the untreated AD and vitamin D3 post-AD treated rats (F = 174.54, P < 0.0001 and F = 154.84, P = <0.0001, respectively).
3.2 Hippocampal tissue levels of brain derived neurotrophic factor (BDNF), amyloid beta (Aβ) peptide (pg/mg protein) ( – and )
At the end of the experiment, biochemical measurement of BDNF and Aβ peptide were estimated in rat hippocampal tissue. There was a significant reduction in the mean values of BDNF and a significant increase in Aβ peptide tissue levels in untreated AD rats of group II, as compared to control (ACSF injected) group I. On the other hand, pre-AD treatment for one week and post-AD treatment for 3 weeks with vitamin D3 in group III, as well as, vitamin D3 post-AD treatment for three weeks in group IV resulted in a significant elevation of BDNF and a significant reduction of Aβ peptide tissue levels versus untreated AD rats. However, the significant differences of both parameters were higher in vitamin D3 pre- and post-AD treated rats of group III versus vitamin D3 post-AD treated rats of group IV. Nevertheless, the mean values of BDNF and Aβ peptide in rats of groups III and IV did not reach their baseline values in control group I. (F = 62.91, P = 0.0001 and F = 45.49, P = 0.0001, respectively).
3.3 Hippocampal tissue levels of glutathione reductase (GR) and glutathione peroxidase (GPX) (nmol/min/mg protein) (, and )
At the 22nd day after colchicine AD induction, start of the experiment, GR and GPX were measured in rat hippocampal tissue. There was a significant reduction in the mean values of GR and GPX in untreated AD rats of group II, as compared to control (ACSF injected) group I. In contrast, pre-AD treatment for one week and post-AD treatment for 3 weeks with vitamin D3 in group III, as well as, vitamin D3 post-AD treatment for three weeks in group IV resulted in a significant elevation of the levels of these enzymes versus their levels in the untreated AD rats. However, the tissue levels of GR and GPX in group III were significantly higher than those of group IV. Yet, their levels in both groups III and IV were still significantly lower than those of control group I (F = 52.42, P = 0.0001 and F = 22.77, P = 0.0001, respectively).
3.4 Correlations between hippocampal tissue levels of BDNF, Aβ peptide, GR and GPX ()
The present study revealed significant positive correlations between the values of hippocampal tissue level of BDNF and those of GR and GPX. On the contrary, there were significant negative correlations between the values of hippocampal tissue level of Aβ peptide and those of BDNF, GR and GPX (P = 0.0001).
4 Discussion
Alzheimer’s disease (AD) is a progressive neurodegenerative disorder characterized by early cognitive dysfunction associated later by behavioral and social deterioration. The hippocampus is the primary neuronal injury region involved in the disease pathophysiology.Citation18 One of the prominent features of AD, is the abnormal accumulation of β amyloid plaque.Citation19
Brain-derived neurotrophic factor (BDNF) is a key target in the pathophysiology of several neurological diseases.Citation20 Animal models are essential tools in the elucidation of the disease pathophysiology. One of these models is the central injection of colchicine in the lateral ventricles, which is considered relevant to cases of sporadic dementia of Alzheimer’s type in humans.Citation21 Hence; the goal of the present study was to clarify the possible neuroprotective role of vitamin D3 in an animal model of AD induced by intracerebroventricular (icv) injection of colchicine.
Relevant findings in the present study revealed that administration of vitamin D3, pre- and post-AD induction by icv colchicine, improved the impaired cognition, restored the reduced hippocampal tissue level of BDNF and antioxidants (glutathione reductase GR and glutathione peroxidase GPX), as well as, reduced the elevated hippocampal Aβ peptide tissue level. These findings illustrate that icv injection of colchicine deteriorates learning and memory, and induces central neuronal damage and oxidative stress. Indeed, the present study cleared a significant memory impairment in Morris water maze task, as evident by the significant increase of initial acquisition latency (IAL), as well as, the first and second retention latencies (1st and 2nd RL) to reach the platform in untreated rats with AD versus control ACSF-injected rats. Furthermore in the untreated AD rats, there were significant reductions of the hippocampal tissue levels of BDNF and antioxidants, and a significant increase in Aβ peptide versus control.
Emerich et al. Citation22 had reported that colchicine injection produces time and dose dependent anatomical, behavioral and neurochemical changes maximum at 2–3 weeks following its administration. As a tubulin inhibitor; colchicine prevents microtubule assembly causing neurofibrillary degeneration and synaptic loss that contribute to impairment of intracellular trafficking of neurotrophic factors, axonal excitotoxicity and oxidative damage.Citation9,Citation23 In the meantime, the neuronal cytoskeleton disruption has been linked to neuro-degeneration in AD implementing a deleterious effect on the function and survival of neurons.Citation24 Meanwhile, the icv injection of colchicine does not seem to induce significant changes in gross behavioral and locomotor activities in rats. This was evident from the current results of the open field task, where the mean scores of gross behavioral and locomotor activities for each rat were relatively stable throughout the days of the task and showed no significant variation among different groups.
On the other hand, the present study revealed that administration of vitamin D3 led to a significant improvement of the retention performance of the spatial navigation task in Morris water maze, as evident by the significant decrease of IAL, 1st and 2nd RL in vitamin D3 pre- and post-AD treated and vitamin D3 post-AD treated rats versus untreated AD rats, yet, being more prominent in vitamin D3 pre- and post-AD treated rats. This finding illustrates the importance of the prophylactic use of vitamin D3 in the improvement of learning and memory performance. In line, Mohsen et al.Citation25 reported that vitamin D-deprived rats had a significantly lower performance and highest failures of navigation in Morris water maze versus control rats and rats receiving vitamin D3 supplementation.
On clinical basis, some clinical studies suggest that serum 25-hydroxyvitamin D3 (25-OHD3) concentration; as an indicator of vitamin D status, may be associated with dementia and impaired cognitive function. For example, Breitling et al.Citation26 in a study on cognition and vitamin D3 in elderly Germans, observed that low serum levels of vitamin D3 may be associated with reduced cognitive functioning in old age. Indeed, Oudshoorn et al.Citation27 found that higher serum levels of vitamin D3 are associated with better cognitive performances in patients with AD. Similarly, Annweiler et al.Citation28 investigated whether the dietary intake of vitamin D could be a predictor of the onset of dementia among elderly women and they found that high vitamin D dietary intake was associated with a lower risk of developing AD among older women. On the contrary, other clinical studies observed no linear association between serum 25 (OH) D or vitamin D dietary intakes and incidence of dementia or memory performance.Citation29–Citation30Citation31 This contradiction could be due to diversity of the populations studied and lack of standardized neurocognitive screening tests.
While focusing on the significant decrease of BDNF, hippocampal tissue levels in untreated AD rats and its significant rise by vitamin D3 in pre- and post-AD treated rats, it reflects the possible enrollment of BDNF as a diagnostic and prognostic biomarker in AD, as well as, the potential neuroprotective role of vitamin D3 through neurotrophin induction.
Aly et al.Citation32 reported that BDNF levels were significantly reduced in rat model of AD induced by intraperitoneal administration of aluminum chloride (AlCl3), while Kirk et al.Citation33 reported that changes in serum level of BDNF might be contributing to shrinkage of the hippocampus that is associated with age related memory decline in late adulthood. Moreover, it was found that a novel polymorphism in BDNF gene was associated with late onset AD.Citation34 On the contrary, Sid et al.Citation35 found that BDNF levels did not distinguish between AD cases and normal controls and did not significantly predict AD severity or global cognitive functioning in participants from the Texas Alzheimer’s Research Consortium.
Regarding the current speculation of the possible neuroprotective role of vitamin D3, it is in line with evidence declaring that vitamin D3 stimulates expression of other neurotrophic factors as nerve growth factor, glial neurotrophic factor and low-affinity neurotrophin receptor in the brain neurons, and glial and schwann cells.Citation36 However, chronic administration of vitamin D3 in rats decreased the hippocampus degenerative processes during aging.Citation37 Furthermore, the development of AD is characterized by a significant reduction of nuclear vitamin D3 receptors.Citation38 All these evidences suggest the importance of the anti-degenerative activity of vitamin D3 as an underlying mechanism for its neuroprotective role.
Conversely, regarding the current significant increase of Aβ peptide hippocampal tissue levels in untreated AD rats, this finding indicates that the neuronal cytoskeleton disruption caused by icv injection of colchicine lead to accumulation of Aβ peptide in the hippocampus. Indeed, the abnormal accumulation of Aβ peptide is one of the prominent features of AD and consequently their clearance is considered a primary therapeutic target for managing AD. Interestingly here in, vitamin D3 administration showed a significant decrease in Aβ peptide hippocampal tissue levels in pre- and post-AD treated rats, reflecting the possible role of vitamin D3 in enhancement of hippocampal Aβ peptide clearance and this could contribute in the mechanism of its neuroprotective action.
In line, Shingo et al.Citation39 found that the active form of vitamin D appears to enhance brain to blood Aβ efflux transport at the blood brain barrier (BBB) leading to enhancement of its cerebral clearance. Similarly, Teresita et al.Citation11 reported that vitamin D3 supplementation modulated age-related increase in the hippocampal amyloid burden by controlling the pro-inflammatory state and increasing the activity of the amyloid degrading enzyme in cases of age-related cognitive decline in rats and suggested that vitamin D3 could be a useful therapeutic option to alleviate the effect of aging on cognitive function. Also, Masoumi et al.Citation40 assuming that brain amyloidosis in AD is related to defective clearance of Aβ by innate immune system, studied the immune stimulation of AD patients’ macrophages by vitamin D3 in combination with curcuminoids. They reported that vitamin D3 strongly stimulated Aβ phagocytosis and clearance while protecting against apoptosis.
On the other hand, the neuroprotective role of vitamin D3, currently assumed in the present study, could be resorted to its possible antioxidant effect as evidenced by the significant rise of hippocampal tissue levels of the antioxidant enzymes GR and GPX in vitamin D3 pre- and post-AD treated rats. As evidenced in the literature, oxidative stress had been implicated in the neurodegenerative process in AD, which may be either due to excessive production of free radicals or loss of antioxidant defenses or both.Citation41 Loss of glutathione had been suggested as an early signaling event in mitochondrial dysfunction and apoptotic cell death involved in neurodegeneration and aging process. Hence, glutathione replenishment had been proposed as one of the therapeutic targets in some of the neurodegenerative disorders, but as it does not cross BBB, thus, glutathione enhancers or analogues are discussed.Citation42 Vitamin D3 is in concern; as it had been related to up-regulation of the activity and expression of brain gamma glutamyl transpeptidase; a key enzyme involved in brain glutathione cycle, thus it ameliorates the antioxidant defense by increasing brain glutathione.Citation43
Moreover, it had been reported that vitamin D3 inhibits the expression of inducible nitric oxide synthase (iNOS) that is thought to play a role in AD pathogenesis; as iNOS-positive neurons were detected in 100% of the AD brains studied and were linked to Aβ peptide.Citation44,Citation45 In the meanwhile, vitamin D3 had been demonstrated to interact with reactive oxygen and nitrogen species in various models of brain oxidative challenges, suggesting its role in brain detoxification pathways.Citation46–Citation47Citation48
It is noteworthy to mention that, in the present study, the prophylactic use of vitamin D3 in rats before induction of AD had yielded more significant findings in all parameters versus rats receiving vitamin D only after AD induction. This finding could highlight the importance of replenishment of brain vitamin D3 stores to guard against substantial neuronal damage; including aging process, ischemic insult, oxidative stress or degenerative illnesses. Indeed, the association between serum vitamin D3 level and cognitive impairment emphasizes the importance of micro-nutrients in the elderly population.Citation49 Likewise, Yu et al.Citation50 reported that vitamin D3 enriched diet correlates with a decrease in the number of amyloid plaques and inflammation, and increase in nerve growth factor in the brain of amyloid-beta protein precursor (AβPP) transgenic mice.
Generally, vitamin D receptors are widely distributed in the cortex and hippocampus, and accumulating data provide evidence for unpredicted roles for vitamin D in brain development and function.Citation8–Citation9Citation10Citation11 Since causal relationship between vitamin D level and pathogenesis of AD had been largely speculated, it is possible that vitamin D supplement, in elderly or in individuals at risk of AD, could allow more circulating hormone providing neuroprotection and guard against cognitive decline. Further research is warranted to inquire the safety and cost-effectiveness of vitamin D supplement in elderly to reduce safe way of reducing the incidence of cognitive impairment in the growing elderly population around the world.
5 Conclusion
Based on data presented in this study, it can be concluded that the active form of vitamin D3 could have a neuroprotective effect in AD that involves many mechanisms; including increase of hippocampal neurotrophic factors as BDNF, rise of the antioxidant defense system, as well as, reduction of the burden of Aβ peptide leading to improvement of the cognitive functions. Hence, vitamin D3 or its analogues could be considered as promising agents for the development of new prophylactic and therapeutic neuroprotectors.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 14 June 2014
References
- J.C.De la TorreIs Alzheimer’s disease a neurodegenerative or a vascular disorder? Data, dogma, and dialecticsLancet Neurol32004184190
- J.GotzM.IttnerAnimals models of Alzheimer’s disease and frontotemporal dementiaNat Rev Neurosci972008532544
- A.KumarS.DograA.PrakashProtective effect of narining, a citrus flavonoid against colchicine- induced cognitive dysfunction and oxidative damage in ratsJ Med Food1342010976984
- A.KumarN.SeghalP.S.NaiduS.S.PadiR.GovalColchicine-induced neurotoxicity as an animal model of sporadic dementia of Alzheimer’s typePharmacol Rep5932007274283
- L.L.TorresN.B.QuaglioG.T.de SouzaR.T.GarciaL.M.DatiW.L.MoreiraPeripheral oxidative stress biomarkers in mild cognitive impairment and Alzheimer’s diseaseJ Alzheimer’s Dis26120115968
- J.HardyD.J.SelkoeThe amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeuticsScience2972002353356
- T.ShiikiS.OhtsukiA.KuriharaH.NaganumaK.NishimuraM.TachikawaBrain insulin impairs amyloid- beta clearance from the brainJ Neurosci24200496329637
- D.J.LlewellynK.M.LangaI.A.LangSerum 25-hydroxyvitamin D concentration and cognitive impairmentJ Geriatr Psychiatry Neurol222009188195
- M.H.Kumar VeerendaY.K.GuptaIntracerebroventricular administration of colchicine produces cognitive impairment associated with oxidative stress in ratsPharmacol Biochem Behav732002565571
- Kumar A, Dogra S, Prakash A. Neuroprotective effect of Centella Asiatica against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress. Int, J Alzheimers Dis 2009.pii:972178.
- L.B.TeresitaH.DarwishVitamin D mitigates age-related cognitive decline through the modulation of pro-inflammatory state and decrease in amyloid burdenJ Neuroinflammation92012244
- M.JähkelO.RilkeR.KochJ.OehlerOpen field locomotion and neurotransmission in mice evaluated by principal component factor analysis-effects if housing condition, individual activity disposition and psychotropic drugsProg Neuropsychopharmacol Biol Psychiatry2420006184
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the folin phenol reagentJ Biol Chem1931951265275
- T.DekelL.AssafG.RomanHiSharonC.AlonResilience to chronic stress is mediated by hippocampal brain-derived neurotrophic factorJ Neurosci3112201144754483
- Z.ZhaoL.HoJ.WangW.QinE.D.FestaC.MobbsConnective tissue growth factor(CTGF) expression in the brain as a downstream effector of insulin resistance-associated promotion of Alzheimer’s disease beta-amyloid neuropathologyFASEB J1914200520812082
- I.CarbergB.MannerviekGlutathione reductase levels in rat brainJ Biol Chem250197554755480
- C.R.WheelerJ.A.SalzmanN.M.ElsayedS.T.OmayeD.W.KorteAutomated assay for superoxide dismutase, catalase, glutathione peroxidase and glutathione reductase activityAnal Biochem1841990193199
- S.E.ArnoldB.T.HymanJ.FloryA.R.DamasioG.W.Van HoesenThe topographical and neuroanatomical distribution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with Alzheimer’s diseaseCereb Cortex11991103116
- V.M.LeeM.GoedertJ.Q.TrojanowskiNeurodegenerative tauopathiesAnnu Rev Neurosci24200111211159
- B.S.DinizA.L.TeixeraBrain-derived neurotrophic factor and Alzheimer’s disease: physiopathology and beyondNeuromol Med1342011217222
- G.BensimonR.ChermatMicrotubule disruption and cognitive defects: effect of colchicine on learning behavior in ratsPharmacol Biochem Behav381991141145
- D.F.EmerichT.J.WalshCholinergic loss and cognitive impairment following intraventricular or intradentate injections of colchicinesBrain Res517199057167
- M.SharmaY.K.GuptaIntracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairmentLife Sci19 69200110211029
- V.M.LeeJ.Q.TrojanowskiThe disordered neuronal cytoskeleton in Alzheimer’s diseaseCurr Opin Neurobiol251992653656
- T.MohsenA.T.SayyedS.MahmoudVitamin D deficiency impairs spatial learning in adult ratsIran Biomed J17120134248
- L.P.BreitlingL.PernaH.MullerE.RaumeM.KliegelH.BrennerVitamin D and cognitive functioning in the elderly population in GermanyExp Gerontol4712012122127
- C.OudshoornF.U.Mattace-RasoN.van der VeldeE.M.ColinT.J.van der CammenHigher serum vitamin D3 levels are associated with better cognitive test performance in patients with Alzheimer’s diseaseDement Geriatr Cogn Disord2562008539543
- C.AnnweilerY.RollandA.M.SchottH.BlainB.VellasF.R.HerrmannHigher vitamin dietary intake is associated with lower risk of Alzheimer’s disease: a 7- year follow upJ Gerontol A Biol Sci Med Sci6711201212051211
- J.McGrathR.ScraggD.ChantD.EylesT.BurneD.ObradovicNo association between serum 25-hydroxyvitamin D3 level and performance on psychometric tests in NHANES IIINeuroepidemiology2920074954
- M.NesS.W.SemB.RousseauG.E.BjørneboeK.EngedalK.TryggDietary intakes and nutritional status of old people with dementia living at home in OsloEur J Clin Nutr4271988581593
- R.JordeK.WaterlooF.SalehE.HaugJ.SvartbergNeuropsychological function in relation to serum parathyroid hormone and serum 25-hydroxyvitamin D levels. The Tromso studyJ Neurol2532006464470
- H.F.AlyF.M.MetwallyH.H.AhmedNeuroptective effect of dehydroepiandrosterone (DHEA) in rat model of Alzheimer’s diseaseActa Biochim Pol5842011513520
- L.KirkS.RuchikaW.MichelleC.LauraH.SuiseM.MollyBrain derived neurotrophic factor is associated with age-related decline in hippocampal volumeJ Neurosci3015201053685375
- H.KunugiA.UekiM.OtsukaK.IsseN.KatoT.NabikaA novel polymorphism of the Brain derived neurotrophic factor (BDNF) gene associated with late-onset Alzheimer’s diseaseMol Psychiatry6120018386
- E.SidH.ValerieR.JamesC.StephenC.WenyanW.ChanBrain-derived neurotrophic factor levels in Alzheimer’s diseaseJ Alzheimers Dis1722009337341
- D.EylesJ.BrownA.Mackay-SimJ.MacgrathF.FeronVitamin D3 and brain developmentNeuroscience1182003641653
- A.V.KalueffK.O.EreminP.TuohimaaMechanisms of neuroprotective action of vitamin D3Biochemistry (Moscow)6972004907911
- M.K.SutherlandM.J.SomervilleL.K.YoongC.BergeronM.R.HausslerD.R.McLachlanReduction of vitamin D hormone receptor mRNA levels in Alzheimer as compared to Huntington hippocampus: correlation with calbindin-28k mRNA levelsBrain Res Mol Brain Res131992239250
- L.ShingoO.SumioN.YasukoK.YusukeM.ShoT.Testsuya1α,25-dihydroxyvitamin D3 enhances cerebral clearance of human amyloid-β peptide(1–40) from mouse brain across the blood-brain barrierFluids Barriers CNS820112029
- A.MasoumiB.GoldensonS.GhirmaiA.AvagyanJ.ZaghiK.Abel1 alpha, 25-dihydroxyvitamin D3 interacts with curcuminoids to stimulate amyloid-beta clearance by macrophages of Alzheimer’s disease patientsJ Alzheimer’s Dis1732009703717
- G.PerryA.D.CashM.A.SmithAlzheimer’s disease and oxidative stressJ Biomed Biotechnol22002120123
- J.B.SchulzJ.LindenauJ.SeyfriedJ.DichgansGlutathione, oxidative stress and neurodegenerationEur J Biochem267200049044911
- E.GarcionN.Wion-BarbotC.Montero-MeneiF.BergerD.WionNew clues about vitamin D in the nervous systemTrends Endocrinol Metab132002100105
- S.NatafE.GarcionF.DarcyD.ChabannesJ.Y.MullerP.Brachet1,25-Dihydroxyvitamin D3 inhibits the expression of inducible nitric oxide synthase in rat nervous system during experimental allergic encephalomyelitisMol Brain Res451997255267
- Y.VodoyotzM.S.LuciaK.C.FlandersL.CheslerQ.W.XieT.W.SmithInducible nitric oxide synthase in tangle-bearing neurons of patients with Alzheimer’s diseaseJ Exp Med184199614251433
- L.R.HarmesH.BrunetD.W.EylesT.J.McGrathVitamin D and the brain: best practice & researchClinical Endocrinol Metab2542011657669
- K.ShinpoS.KikuchiH.SasakiF.MoriwakaK.TashiroEffect of 1,25-dihydroxyvitamin D(3) on cultured mesencephalic neurons to the combined toxicity caused by L-buthionine sulfoximine and 1-methyl-4-phenylpyridineJ Neurosci Res622000374382
- I.L.BrewerV.ThibauliK.ChenM.LangubP.LandfieldN.porterVitamin D hormone confers neuroprotection in parallel with down regulation of L-type calcium channels expression in hippocampusJ Neurosci21200198108
- J.C.McCannB.N.AmesIs there convincing biological or behavioral evidence linking vitamin D deficiency to brain dysfunction?FASEB J2220089821001
- J.YuC.M.GattoniH.ZhuK.SambamurtiC.S.GattoniM.S.KindyVitamin D3 enriched diet correlates with a decrease of amyloid plaques in the brain of Aβ PP transgenic miceJ Alzheimers Dis2522011295307
