Abstract
Introduction
Angiogenesis is known to play a pivotal role in most of malignancy, including HCC, and in chronic inflammation.
Aim
To investigate the angiogenic output in HCV and HBV infection and its implication in the development of HCV associated HCC.
Materials and methods
Blood samples were collected and grouped as; HS healthy subjects control group; HCC–HCV; chronic HCV infected patient group (HCV+ve) who are positive for serum anti-HCV antibodies and HCV–RNA; anti-HCV antibody positive and HCV–RNA negative patient group (HCV−ve); patients with positive HBsAg and HBV-DNA group (HBV+ve); and HBsAg positive and HBV-DNA negative patient group (HBV−ve). Serum levels of vascular endothelial growth factor, angiopoietin-2, endostatin and angiostatin were assessed in different studied groups.
Results
The level of sVEGF was insignificantly elevated in both HCV+ve and HCV−ve groups when compared with controls, while Ang-2, sES and sAS were significantly elevated in both groups as compared with healthy controls. The studied parameters were significantly elevated in HBV-+ve patients when compared with the control. However, HBV−ve patients showed significantly elevated levels in sAng-2, sES and sAS when compared with the control while the level of sVEGF was equal to that of controls. In patients with HCC, the studied parameters showed a significant elevation when compared with healthy controls and patients either with HBV or HCV infection except for sAS in the case of HCV-+ve patients and VEGF for HBV-+ve patients who were also higher but not significant.
Conclusion
The increased hepatic angiogenesis in chronic HCV and HBV could provide the molecular basis for liver carcinogenesis and contribute to the increased risk of HCC in patients with cirrhosis due to HCV and/or HBV.
KEYWORDS:
1 Introduction
Hepatocellular carcinoma (HCC) comprises nearly 6% of all incident cancer cases worldwide, with the overwhelming majority occurring in the developing world.Citation1 With more than half a million new cases each year, HCC is the fifth most common tumor worldwide and the third cause of cancer-related deaths.Citation2 Chronic infection with HBV and HCV has been cited as by far the most important etiologic agent.Citation3 Annual incidence of HCC in patients with cirrhosis due to HBV infection exceeds 2%, and in those with cirrhosis due to HCV infection, it is estimated between 3% and 8%. Association of the two viruses or chronic exposure to alcohol substantially increases these figures.Citation4
In Egypt, the increasing importance of HCV infection in the etiology of HCC has been shown, now estimated to account for 40–50% of cases.Citation5 On the other hand, the prevalence of HBsAg in Egypt is of intermediate endimicity (2–8%). Nearly 2–3 million Egyptians are chronic carriers of HBV.Citation6 It appears that the cause of HBV-associated HCC is a combination of HBV-encoded oncogenic activities along with the synergistic effect of chronic inflammation.Citation7
Angiogenesis is known to play a pivotal role in almost every kind of malignancy favoring growth and metastasis of several types of cancer including HCC,Citation8 and in chronic inflammation, where, accumulation of the inflammatory infiltrate and the development of fibrosis increases resistance of the tissue to blood flow and delivery of oxygen, resulting in hypoxia.Citation9 Tumor angiogenesis is a complex phenomenon and is regulated by a net balance between the proponents and the inhibitors of the process.Citation10
The difference in the pathogenesis between chronic HBV and HCV viral infection and their progression into HCC may be a consequence of the angiogenic processes. Accordingly, the aim was to investigate the angiogenic output in HCV and HBV infection and its implication in the progression into HCC. The objective is approached by assessing the serum levels of pro-angiogenic parameters; endothelial growth factor (VEGF), angiopoitein-2 (Angio-2) and anti-angiogenic parameters; endostatin (ES) and angiostatin (AS) as. The presence or absence of the active replicating virus was taken in consideration.
2 Materials and methods
This work included 100 patients admitted to the Hepatology Units, Medical Research Institute and Faculty of Medicine, Alexandria University in addition to 20 healthy volunteers with no evidence of liver disease and negative for both HCV and HBV. None of the patients had a history of alcohol abuse or previous interferon treatment. The study was approved by the local Ethics Committees of the Medical Research Institute and Faculty of Medicine, Alexandria University. A signed informed consent was obtained from all the participants in the present study.
The study enrolled six groups; (1) healthy subjects (HS) group; (2) HCC–HCV patient group; (3) chronic HCV infected patient group (HCV+ve) who are positive for serum anti-HCV antibodies and HCV–RNA; (4) anti-HCV antibody positive and HCV–RNA negative patient group (HCV−ve); (5) patients with positive HBsAg and HBV–DNA group (HBV+ve); and (6) HBsAg positive HBV-DNA negative patient group (HBV−ve).
Originally, the study was designed to enroll HCC cases associated with HBV, and HCC cases with no previous history of HBV or HCV infection (spontaneous HCC) but unfortunately those cases were hard to find. However, HCC patients enrolled in the present study have no previous history of other malignant diseases. Eighty percent of those patients were at stage II/III. Also, careful subsequent exclusion of any patient with a possible alternative cause of chronic liver disease was considered.
Venous blood samples were collected and sera separated and stored at −20 °C until used. Serum samples were tested for anti-HCV Ab and HBsAg according to the manufacturer’s instructions (EIA; Abbott GmbH, Delkenheim, Germany). Quantitative detection of HCV-RNA and HBV-DNA was carried out by real time PCR.Citation11
Serum level of VEGF was determined using Bender Medsystems GMBH ELISA Kits, Vienna, Austria, Europe. Serum levels of Agiopoietin-2 (Angio-2), endostatin (ES) and angiostatin (AS) were determined using RayBio® Human ELISA kits Raybiotech, Inc.
2.1 Statistical analysis
SPSS 11.5 statistical package (SPSS Inc., Chicago, IL, USA) was used for data analysis. Mann Whitney U test was used after examining the significance of Kruskal–Wallis H test (p < 0.05). The p values of less than 0.008 were considered statistically significant according to Bonferroni adjustment. Correlations were explored using Spearman correlation coefficient (p < 0.05).
3 Results
3.1 HCV patients
The mean values of VEGF in HCV+ve and HCV−ve were insignificantly different from that in healthy control subjects p = 0.114 and 0.461, respectively (). Meanwhile, the mean levels of sAng-2, sES and sAS were significantly elevated in HCV+ve and HCV−ve patients when compared to that in the healthy control subjects p = 0.0001 ( and ).
Table 1 Serum levels of proangiogenic factors, VEGF and Angio-2 (pg/ml), mean ± S.E., in the different studied groups.
Table 2 Serum levels of anti-angiogenic factor, ES and AS (pg/ml), mean ± S.E., in the different studied groups.
The mean values of sVEGF, sAng-2 and sAS in HCV+ve patients were insignificantly different from that in HCV−ve patients p = 0.583, 0.102 and 0.060, respectively ( and ), while, the mean level of sES in HCV+ve patients was significantly lower from that in HCV−ve patients p = 0.0001 ( and ).
3.2 HBV patients
The mean levels of sVEGF, sAng-2, sES and sAS in HBV+ve patients were significantly higher than the healthy control subjects (p = 0.001 and 0.0001) ( and ). In HBV−ve patients, the mean levels of sVEGF showed to be insignificantly different from that in healthy control subjects p = 0.968 (), while the mean levels of sAng-2, sES and sAS showed a significant elevation p = 0.0001 ( and ). It should be noted that the mean levels of all angiogenic markers in HBV+ve patients were significantly higher than those in HBV−ve patients p = 0.0001 and 0.004, except for sAS which was high but not significant p = 0.242.
While the mean levels of sVEGF and sES in HBV+ve were significantly higher than those in HCV+ve p = 0.0001 ( and ), however, Ang-2 and As showed insignificant difference p = 0.127 and 0.023, respectively ( and ). Meanwhile, the mean levels of sVEGF and sES in HBV−ve patients were insignificant different from those in HCV−ve patients p = 0.718 and 0.013, respectively ( and ). While the mean level of sAng-2 and sAS in HCV−ve patients was significantly lower than that in HBV−ve patients, p = 0.003 and 0.0001, respectively ( and ).
3.3 HCC patients
A highly significant increase in the mean levels of pro- and anti-angiogenic factors were noticed when compared to that in healthy subjects, p = 0.0001, ( and ) and to that in HCV+ve patients p = 0.0001, except sAS was high but not significant p = 0.277 (). Moreover, serum levels of sVEGF, sAng-2, sES and sAS were significantly higher in HCC patients than that in HBV+ve patients p = 0.003 and 0.0001, except sVEGF was high but not significant p = 0.017 ( and ).
3.4 Bio-statistical correlation
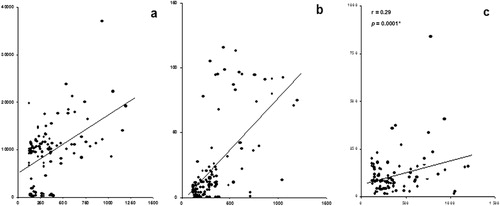
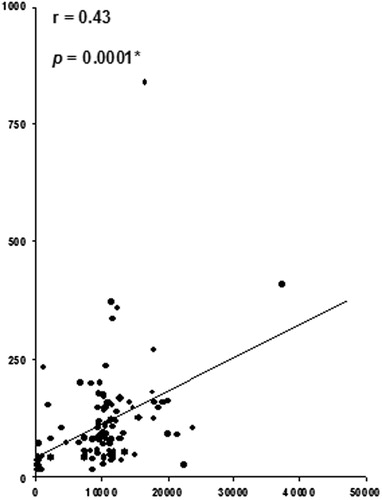
Data of the present study showed that sVEGF is significantly correlated with sAng-1; r = 0.48, p = 0.0001, sES; r = 0.60, p = 0.0001, and sAS; r = 0.29, p = 0.0001, in a positive manner (). Also, sES was found to be significantly correlated positively with sAng-2; r = 0.62, p = 0.0001, and sAS; r = 0.41, p = 0.0001, in a positive manner (). Finally, the data showed a significant positive correlation between sAng-2 and sAS; r = 0.43, p = 0.0001 ().
4 Discussion
The formation of fibrotic septa and capillarization of sinusoids due to deposition of fibrillar extracellular matrix (ECM) in the space of disease, result in an increase in resistance to blood flow and oxygen delivery.Citation12 These are premises for hypoxia and the transcription of hypoxia-sensitive proangiogenic genes.Citation13 Moreover, the mediators of inflammatory response in chronic liver diseases may stimulate other cells in the surrounding microenvironment to express other pro-angiogenic factors to sustain angiogenesis.Citation14 Vespasiani–Gentilucci et al.,Citation2 demonstrated that in chronic HCV patients significant increased levels of Ang-2 and equivalent VEGF levels with respect to controls were seen. Also, they reported an association of the stage of fibrosis with sAng-2 levels. These were in agreement with results of the present study where HCV+ve and HCV−ve patients showed significantly increased levels of sAng-2 and equivalent VEGF levels with respect to controls. The insignificant elevated level of sVEGF could be attributed to significant elevation in levels of both endostatin and angiostatin, which have been reported to down-regulate the production of sVEGF.Citation15 This in turn, may reflect the role of Ang-2 in enhancement of angiogenesis that is associated with HCV infection.
A significant elevation in both sAS and sES levels in HCV+ve and HCV−ve patients was observed when compared to that in control. Notably, an insignificant difference was shown in sVEGF, sAng-2 and sAS levels in HCV+ve patients when compared to that in HCV−ve patients. Thus, the absence of significance in those angiogenic markers may be explained by the fact that just the attack of the HCV may initiate the process of angiogenesis. This can be supported by Hoare et al.,Citation16 who reported that fibrosis was observed in liver biopsy of nonviremic HCV antibody-positive patients which was similar to the inflammatory infiltrate in viremic cases. They have suggested the observed immune response in the liver, supporting the view that HCV may persist in the liver in the majority of HCV RNA-negative cases.
On the other hand, HBV encoded proteins as HBx protein which stabilizes and facilitates the transactivation function of the hypoxia inducible factor-1αCitation17,Citation18 which regulates the expression of multiple genes involved in angiogenesis such as VEGF. VEGF could also account for the induction of Ang-2 observed in chronic hepatitis B, contributing to pathological angiogenesis and hepatocellular carcinoma progression.Citation19 These could be the molecular basis that may explain the significant elevation in sVEGF and sAng-2 levels in HBV+ve group. In HBV+ve infection, the significant elevation in endostatin and angiostatin levels could be an attempt to counteract the action of significantly elevated levels of pro-angiogenic factors. In HBV−ve patients, an insignificant elevation in the level of sVEGF and a significant elevation in sAng-2 were observed. Meanwhile, the levels of sES and sAS were significantly elevated in those patients. The initiation of angiogenic response in those carrier HBV patients could be attributed to the production of HBx protein very early after infection which mediates the establishment and maintenance of the chronic carrier state.Citation20
The virulent nature of HCV might explain the observation that sVEGF and sES levels in HBV+ve patients were significantly higher than those in HCV+ve patients while Ang-2 level was insignificantly raised.Citation21 Unlike previously stated, these observations indicate that angiogenesis is particularly linked to HBV infection, suggesting a possible contribution to HBV-related liver oncogenesis ( and ).Citation22
It has been reported that the expression pattern of Ang-2 is strongly associated with the expression of VEGF in the process of tumor angiogenesis, and subsequently, in tumor progression.Citation23 In agreement with previous study,Citation24 significantly elevated levels of sVEGF and sAng-2 were expected in HCC patients. It was suggested that sAng-2 may allow vessels to be more responsive to sprouting signals provided by VEGF.Citation25 It was revealed that over expression of Ang-2 blocks Ang-1 mediated Tie receptor signaling and causes loss of the tight vascular structure, which results in the exposure of endothelial cells to VEGF.Citation26 These findings may suggest that an advantageous environment for angiogenesis may be created by the HCC itself, secreting not only VEGF but also Ang-2. Accordingly, angiostatic response is not tumor-specific, but is a consequence of angiogenic normal regulatory mechanisms that couple pro and anti-angiogenic signaling to the induction of an opposing response. This obligatory coupling of pro- and anti-angiogenic signaling is advantageous since excessive angiogenesis is associated with a variety of disease statesCitation14 and uncontrolled angiogenesis would compromise normal tissue architecture in growth, morphogenesis, and wound healing.Citation27 It was reported that s-ES level was significantly higher in HCC patients and has a significantly inverse correlation with the angiogenic score of HCC.Citation20 Also, it was confirmed that the source of s-endostatin is the tumor. In our study, sES and sAS levels were significantly higher in the HCC group than all other included groups. However, it is still controversial that endostatin expression or its serum level has a significant role in predicting the prognosis of patients with HCC.Citation20,Citation25
Thus, in HCC, the elevated anti-angiogenic markers could be attributed to the fact that tumor hypoxia produced a homeostatic feedback up-regulation of VEGF since its expression is believed to be modulated by hypoxia. In addition, angiostatin is generated through the action of matrix metalloproteinases (MMP), that are activated by proangiogenic signaling cascades such as hypoxia inducible factor induction, to oppose the angiogenic response.Citation20 As a result, the release of anti-angiogenic factors may explain the control exerted by primary tumors over metastasis.Citation28
The sequence of pathological insult of the liver in viral infection usually starts with inflammation; cirrhosis till the end stage of HCC may necessitate more pro/anti-angiogenic output. This may explain the elevation levels of the studied parameters in the HCC group when compared with HCV+ve patients in particular and HBV+ve patients in general.
In conclusion, there is a difference in angiogenic/anti-angiogenic outputs between HBV and HCV infection and HCC development. The increased hepatic angiogenesis in chronic HCV and HBV could provide the molecular basis for liver carcinogenesis and contribute to the increased risk of HCC in patients with cirrhosis due to HCV and/or HBV. Furthermore, anti-angiogenic therapeutic agents should be targeted to counteract the action of proangiogenic factors rather than enhancement of antiangiogenic factors.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 4 July 2014
References
- E.M.LehmanM.L.WilsonEpidemiology of hepatitis viruses among hepatocellular carcinoma cases and healthy people in Egypt: a systematic review and meta-analysisInt J Cancer1242009690697
- U.Vespasiani-GentilucciG.GalatiC.MazzarelliD.D’AvolaS.SpataroP.GalloAngiogenic cytokines in patients undergoing antiviral treatment for chronic hepatitis C virus infectionJ Interferon Cytokine Res312011207210
- Y.BarazaniJ.R.HiattM.J.TongR.W.BusuttilChronic viral hepatitis and hepatocellular carcinomaWorld J Surg31200712431248
- D.SemelaJ.F.DufourAngiogenesis and hepatocellular carcinomaJ Hepatol412004864880
- A.R.El-ZayadiH.M.BadranE.M.BarakatD.Attia MelS.ShawkyM.K.MohamedHepatocellular carcinoma in Egypt: a single center study over a decadeWorld J Gastroenterol11200551935198
- A.El-ZayadiB.HepatitisVirus infection: the Egyptian situationArab J Gastroenterol820079498
- E.M.D.MargaretM.KarlViruses associated with human cancerBiochim Biophys Acta17822008127150
- R.MazzantiL.MesseriniC.E.CominL.FedeliN.Gannè-CarrieM.BeaugrandLiver angiogenesis as a risk factor for hepatocellular carcinoma development in hepatitis C virus cirrhotic patientsWorld J Gastroenterol1337200750095014
- A.D.AmarapurkarD.N.VibhavN.D.PatelAngiogenesis in chronic liver diseaseAnn Hepatol632007170173
- S.RamanujanG.C.KoenigT.P.PaderaB.R.StollR.K.JainLocal imbalance of proangiogenic and antiangiogenic factors: a potential mechanism of focal necrosis and dormancy in tumorsCancer Res60200014421448
- S.MitsunagaK.FujimuraC.MatsumotoR.ShiozawaS.HirakawaK.NakajimaHigh-throughput HBV DNA and HCV RNA detection system using a nucleic acid purification robot and real time detection PCR: its application to analysis post transcription hepatitisTransfusion422002100106
- S.L.FriedmanMechanisms of hepatic fibrogenesisGastroenterology134200816551669
- B.L.CoppleJ.J.BustamanteT.P.WelchN.D.KimJ.O.MoonHypoxia-inducible factor-dependent production of profibrotic mediators by hypoxic hepatocytesLiver Int29200910101021
- P.CarmelietAngiogenesis in health and diseaseNat Med92003653660
- E.RaymondTumor angiogenesis inhibitors: media and scientific aspectsPresse Med27199812211224
- M.HoareW.T.H.GelsonS.M.RushbrookD.M.MartinM.D.CurranT.WoodallHistological changes in HCV antibody-positive, HCV RNA-negative subjects suggest persistent virus InfectionHepatology48200817371745
- E.J.MoonC.H.JeongJ.W.JeongK.R.KimD.Y.YuS.MurakamiHepatitis B virus X protein induces angiogenesis by stabilizing hypoxia-inducible factor-1-alphaFASEB J182004382384
- P.PichiuleJ.C.ChavezJ.C.La MannaHypoxic regulation of angiopoietin-2 expression in endothelial cellsJ Biol Chem27920041217112180
- E.SzabC.PaskaP.Kaposi NovakZ.SchaffA.KissSimilarities and differences in hepatitis B and C virus induced hepatocarcinogenesisPathol Oncol Res12004511
- S.Martin-VilchezE.Lara-PezziM.Trapero-MarugánR.Moreno-OteroP.Sanz-CamenoThe molecular and pathophysiological implications of hepatitis B X antigen in chronic hepatitis B virus infectionRev Med Virol212011315329
- D.K.DharT.OnoA.YamanoiY.SodaE.YamaguchiM.A.RahmanSerum endostatin predicts tumor vascularity in hepatocellular carcinomaCancer95200221882195
- R.MazzantiL.MesseriniL.MonsacchiG.BuzzelliA.L.ZignegoM.FoschiChronic viral hepatitis induced by hepatitis C but not hepatitis B virus infection correlates with increased liver angiogenesisHepatology251997229234
- J.HolashP.C.MaisonpierreD.ComptonP.BolandC.R.AlexanderD.ZagzagVessel cooption, regression, and growth in tumors mediated by angiopoietins and VEGFScience284199919941998
- R.YamaguchiH.YanoO.NakashimaJ.AkibaN.NishidaM.KurogiExpression of vascular endothelial growth factor-C in human hepatocellular carcinomaJ Gastroenterol Hepatol212006152160
- N.FerraraVascular endothelial growth factor as a target for anticancer therapyOncologist92004210
- A.StratmannW.RisauK.H.PlateCell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesisAm J Pathol153199814591466
- M.L.WahlD.J.KenanM.Gonzalez-GronowS.V.PizzoAngiostatin’s molecular mechanism: aspects of specificity and regulation elucidatedJ Cell Biochem962005242261
- D.RibattiA.VaccaB.NicoD.SansonnoF.DammaccoAngiogenesis and anti-angiogenesis in hepatocellular CarcinomaCancer Treat Rev322006437444