Abstract
Background
The metabolic imbalance in the articular cartilage following meniscectomy includes an increase in cartilage degradation with an insufficient reparative or anabolic response resulting in structural, biochemical and mechanical changes that can progress from pre-clinical, to pre-radiographic, to radiographic damage of the joint.
Purpose
To evaluate combinations of imaging and biochemical biomarkers for cartilage breakdown, synthesis and quantity in the early period of post-arthroscopic meniscectomy.
Subjects and methods
Twenty young adults (three of them were females) who underwent unilateral arthroscopic partial meniscectomy were evaluated. The patients had a mean age of 32.5 years (range, 24–39), mean BMI of 28.5 kg/m2 (range, 24–34). Preoperative and six months postoperative US and MRI-based markers (cartilage thickness and volume, respectively) were quantified for medial and lateral tibio-femoral compartments for both knees. Preoperative, three and six months postoperative biochemical markers serum assays were measured; COMP and Col II (cartilage matrix breakdown) and PIICP (cartilage synthesis). These three markers were measured in an age, sex and BMI matched twenty healthy subjects for comparison.
Results
The meniscectomized knees had significantly lower total knee cartilage volume, P < 0.05 but non-significant mean thickness than the intact contralateral knees. Among the individual biochemical markers, PIICP had the highest significant diagnostic accuracy quantified as the area under the receiver-operator characteristics curve (AUC) of 0.75 (95% confidence interval: 0.509–0.912) higher than all others, P < 0.05 to distinguish subjects with progressive cartilage loss from non-progressors. Diagnostically, ratio of COMP and Col II to PIICP scored AUC of 0.90 (0.69–0.98, higher than PIICP: P = 0.0001). For prediction of cartilage loss, none of the individual markers could be used.
Conclusion
Cartilage volume loss by MRI combined with changes in cartilage matrix turnover detected by molecular biomarkers may reflect the initial changes associated with cartilage degeneration that account for early OA.
Abbreviations:
1 Introduction
Knee trauma, such as cruciate ligament or meniscus injury, is a strong predictor of subsequent knee osteoarthritis (OA). Considering the young age at which many of these injuries occur, knee joint replacement at a relatively young age is a distinct possibility.Citation1 So, secondary prevention of OA is important. The hallmark of OA is loss of articular cartilage which is characterized by high wear resistance, and poor regenerative qualities.Citation2 Osteoarthritis is a disease with a long silent period. Early identification of OA is crucial to improve clinical decision making and advancing treatment options. Thus preventing further cartilage destruction and joint failure, especially in athletes, in whom unnecessary treatment or intervention may be detrimental to a competitive future. Hence, diagnostic tests that can detect and monitor molecular events early in the pathogenesis of OA would be potentially very useful.Citation3
A variety of risk factors work together to incite a cascade of pathophysiologic events within joint tissues leading to OA including advancing age, gender, genetics, obesity and trauma. The repetitive nature of stress on a previously healthy, yet traumatized, articular surface, has also been related with early cartilage changes and degeneration that may stay invisible for a long period of time before evolving into irreversible cartilage lesion.Citation4
The menisci are two semilunar-shaped fibrocartilaginous structures, containing water, collagen (mainly type I), and proteoglycans. Their unique anatomy is comprised of circumferentially oriented collagen fibers which provide resistance to hoop stresses and radially oriented fibers which resist shear forces.Citation5 Long-term follow-up studies showed that virtually all meniscectomized knees develop arthritic changes with time.Citation1 The severity of these changes seems to be proportional to the amount of meniscus removed.Citation6 The frequent bone and cartilage changes found after partial meniscectomy are thought to be due to the decrease in contact area up to 50–70% with a resulting increase in contact stresses,Citation6 and loss of the load distributing function which prevents the menisci from extruding out the joint during axial loading and decreased stability of the knee.Citation7 Moreover, as 74% of the total weight of the meniscus is water which could be squeezed out into the joint space during compression forces adding to the joint lubrication, thus increased coefficient of friction between the gliding joint surfaces is one of the consequences of meniscectomy.Citation7
The increased intra-articular contact stresses within the knee are thought to ‘overload’ the articular cartilage, with associated structural, biochemical and mechanical changes. The macroscopic and microscopic signs of failure of articular cartilage after meniscectomy have also been demonstrated by animal models, ranging from fibrillation of the surface to necrosis and loss of the cartilage layer.Citation8
Cartilage is a connective tissue made of cells (chondroblasts/chondrocytes) that produce an extracellular matrix of proteoglycans and collagen fibers with high water content. The tensile strength of cartilage is due to the collagen component. Its resistance to compression is due to the ability of proteoglycan to attract and hold water.Citation9
Biomarker is a detectable biologic parameter, whether biochemical, genetic, histologic, anatomic, physical, functional, or metabolic.Citation10 Biomarkers are used in diagnosis of disease, in addition they allow classification of disease severity, risk of onset and progression, as well as assessment of the efficacy of a treatment.Citation10
Cartilage imaging biomarkers have gained a significant role in various aspects of OA. Magnetic resonance imaging (MRI) is the most promising imaging modality to detect structural changes in cartilage tissue, as it is direct and noninvasive.Citation11 MR-based morphological cartilage biomarkers are superior to radiographs in characterizing disease burden. Their role in characterizing prognosis and risk of OA is showing great promise. Many techniques enable imaging of fissuring and focal or diffuse cartilage loss. The 3D-spoiled gradient recalled echo imaging with fat suppression either using selective fat suppression or selective water excitation provide higher spatial and contrast resolution, and are the current standard for morphological imaging of cartilage.Citation12 The articular cartilage has a very high signal intensity, joint fluid has an intermediate to low signal intensity, and subchondral bone and bone marrow are dark. Reported sensitivity and specificity for directly assessing knee structural alterations, such as cartilage volume, cartilage defects, subchondral bone changes and meniscal lesions are 75–85% and 95–97%, respectively, which has increased our understanding of early joint changes.Citation13
Morphologic changes in the cartilage matrix and chondrocytes could be detected on the molecular level. The molecules released could be measured by biochemical markers. Measurement of biochemical markers in blood, urine or synovial fluid samples could reflect dynamic and quantitative changes in joint remodeling and therefore disease progression. Because type II collagen is the most abundant protein of cartilage matrix, the assessment of its synthesis and degradation is an attractive approach for the investigation of OA and can be assessed by several markers.Citation14
The procollagen molecule of type II collagen has large amino and carboxyl-terminal regions (N-propeptides and C-propeptides, respectively) at each end. During synthesis, these propeptides are removed by specific proteases before the mature molecules are incorporated into fibrils. The C-propeptide can be detected by a PIICP assay. Two assays that recognize N-propeptides; the N-propeptide of type IIA procollagen (PIIANP) and the total PIINP have been developed. The serum levels of these propeptides are thus believed to represent an adequate index of the rate of type II collagen synthesis.Citation15
Other biomarkers are related to cartilage matrix turnover and degradation including collagenous (e.g. C-terminal telopeptide of collagen type II, human collagen type II-specific neoepitope (C2M) and cartilage cleavage products) and non-collagenous proteins (e.g. cartilage oligomeric matrix protein, COMP). Serum levels of COMP, a member of the thrombospondin family of glycoproteins, have been shown to be increased in individuals with knee OA and synovitis, compared to those without synovitis.Citation16
So, there are a number of promising imaging and biochemical cartilage biomarkers although none is sufficiently discriminating to differentiate between individual patients and controls (diagnostic) or between patients with different disease severities (burden of disease), predict prognosis in individuals with or without osteoarthritis (prognostic) or perform so consistently that it could function as a surrogate outcome in clinical trials (efficacy of intervention). Combining imaging and biochemical biomarkers is likely to increase the predictive power of future ‘combination biomarkers’.Citation17
The aim of this study was to evaluate combinations of imaging and biochemical biomarkers for articular cartilage breakdown, synthesis and quantity in the early period post-arthroscopic meniscectomy among Egyptians performing partial meniscectomy.
2 Subjects
Twenty young adults who underwent arthroscopic partial meniscectomy were evaluated. The patients with a mean age of 32.5 years (range, 24–39) and mean body mass index of 28.5 kg/m2 (range, 24–34) comprised 17 men and three women. All patients were given a preoperative diagnosis of torn medial meniscus by history, physical examination, and confirmatory MRI.
General criteria for exclusion from the study were as follows:
| - | Age older than 50 years at the time of evaluation (to exclude idiopathic osteoarthritis according to the ACR criteriaCitation18). | ||||
| - | The presence of inflammatory arthritides. | ||||
| - | Surgery at any joint before. | ||||
| - | Diabetes mellitus, neurological or muscular disease (because these comorbid conditions will affect the patient relevant outcome). | ||||
| - | Varicose veins of the lower limbs. | ||||
| - | Any contraindication to MRI examination. | ||||
Knee-specific criteria for exclusion (either knee) were:
| - | Previous knee surgery (other than meniscus surgery). | ||||
| - | Osteochondritis dissecans. | ||||
| - | Osteochondromatosis. | ||||
| - | Fracture in or adjacent to the knee. | ||||
| - | Septic arthritis. | ||||
| - | Osteonecrosis. | ||||
| - | Chondromalacia patellae. | ||||
| - | Patellar subluxation. | ||||
| - | Radiographic changes indicating knee OA at the time of index surgery. | ||||
In summary, these were otherwise healthy patients with previously healthy knees who experienced relatively recent onset of knee symptoms caused by meniscal pathology.
Controls for imaging modalities were the “non-operated” knees of the same patients. The control group for the clinical and biochemical markers evaluation was twenty healthy individuals of matched age and gender to the patients.
A full informed consent was signed from all participants and this study was formally approved by the Ethics Committee of the Faculty of Medicine, Alexandria University with the following ID:[IRB No. 00007555-FWA No. 00015712,November 2011.
2.1 Methods
All patients were subjected to: (1) thorough general and meniscectomy related history taking focusing on articular cartilage status, amount of resection and the knee injury and osteoarthritis outcome score (KOOS) questionnaire, Swedish version LK 1.0,Citation19 which was used to quantify knee-related symptoms. The KOOS was developed for short and long-term follow up studies of knee injury and knee OA. KOOS comprises five subscales: pain, symptoms, activities of daily living, sports and recreation function, and knee-related quality of life. A score from 0 to 100 is calculated for each subscale, with 100 representing the best result. Patients were instructed to complete the KOOS form by considering their operated knee. Control subjects were asked to consider their knees in general. (2) General and local knee joint examination.
(3) MR imagesCitation10 were acquired with a 1.5-T superconducting MRI-system (Gyroscan Intera, Philips Medical Systems) using a dedicated phased-array knee coil. A positioning device was used to ensure uniformity of positioning among patients. The subjects were scanned in a supine position with no load-bearing during or prior to scanning. Sagittal and coronal, three-dimensional (3D), water selective (WatS), balanced steady-state free precession (3D-WS-bSSFP) sequence; Fast Field Echo (FFE) technique was done, (TR 20 ms [shortest]; TE 8 ms [shortest]; 2.0-mm slice thickness; slice overlap 1 mm; 75 slices; 130 mm FOV; 256 × 256 acquisition matrix; voxel size 0.51 × 0.51 × 2.00 mm; flip angle: 25°; Number of Signals Averaged (NSA) 2; automatic shim; 1:1 water excitation [Proset: a frequency selective and spatially selective binomial shaped water excitation pulse with a pulse duration of 3.41 ms]; bandwidth/pixel: 98.6; acquisition time five minutes). Cartilage is visible as a light gray or white band between the dark1 subchondral endplate and the gray joint fluid (a).
Figure 1 Cartilage volume quantification framework. (a) sagittal WS-bSSFP sequence with optimal contrast. Cartilage is visible as a light gray or white band between the dark subchondral endplate and the gray joint fluid. (b) The outlines of the knee cartilage plates. (c) 3D geometric model of the medial tibial cartilage plate.
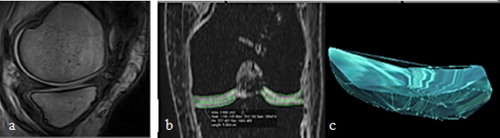
The sagittal and coronal WS-bSSFP sequence images were analyzed quantitatively. Cartilage volumes for the medial and lateral femoral condyles were measured on the anterior and posterior cartilage plates, and on the medial and lateral plates of the tibia. This was performed twice; preoperative and six months postoperative for the operated knees and once for the normal knees at the postoperative scan time. All cartilage contours were drawn manually (b). 3D geometric models of cartilage plates of the knee joints were constructed using these contours (c). Morphometric data were acquired from computer-assisted segmentation of MR images.
(3) High resolution ultrasonography (US),Citation20 using Siemens Prima apparatus, utilizing high resolution multi-frequency probe (3.5–7 MHz) was used to assess knee joint for cartilage thickness, irregularities and effusion using transverse suprapatellar scans tangential to the upper patellar pole at 90° knee flexion. The cartilage thickness was measured within the weight-bearing area from four anatomical locations; namely, the medial femoral condyle (MFC), the lateral femoral condyle (LFC), the medial tibial condyle (MTC), and the lateral tibial condyle (LTC). The femoral cartilage typically appears as a clear-cut, wavy hypo-anechoic layer, with upper concavity. Measurements were done two times preoperative and six months postoperative for the operated knees and once for the normal knees at the postoperative scan time.
(4) Biochemical markers assay: serum samples were obtained thrice preoperatively, three and six months postoperatively for the patients and once for the controls. The serum was thawed and aliquoted to 0.5-ml volumes, and then stored at −80 °C until analyzed. From the available markers, a combination of human cartilage oligomeric matrix protein (COMP) and collagen type II-specific neoepitope (Col II); (for cartilage matrix breakdown) and procollagen molecule of type II collagen C-propeptides (PIICP); (for cartilage synthesis) were assayed. The three markers were measured with a commercial sandwich enzyme-linked immunosorbent assay (ELISA) (Cusabio Biotech Co, Hubei Province, China). For all ELISA determinations, samples, standards, and controls were assayed according to the manufacturer’s instructions.Citation21 The samples were assayed in a blinded manner, independent of group assignment. The mean intra-assay precision was <8% coefficient of variation (CV) and inter-assay CV <10% for all assays.
Data were analyzed using the Statistical Package for Social Sciences (SPSS ver. 17, Chicago, IL, USA). The data were reported as mean and standard deviation. T-tests and chi-square tests were used to compare differences in means and proportions where appropriate. The data were compared between the operated and contralateral control knees using paired t-test. Morphometric cartilage change was reported as mean change. The percentage change in pre- and post-operative scans in cartilage volume and thickness was compared with biochemical marker assay change using independent two-sample t-test. Statistical significance level was set at 0.05.
3 Results
There was no significant difference between patients and controls regarding age (32.5 ± 4.7 vs. 30.8 ± 3.5; n.s.), sex (v = 0.305; n.s.), or BMI (28.5 ± 3.2 vs. 26.7 ± 4; n.s.). The mean time from injury was 6.4 ± 2.6 months. The mechanism of injury was significant trauma in 50% of patients. The amount of meniscus removed was >60% of the medial meniscus in 88% of patients. Articular chondral lesions were defined arthroscopically in two patients (10%). There was a significant improvement in the KOOS scores 3 and 6 months post-operatively compared to pre-operatively (81 ± 7 and 86 ± 5.4 vs. 64 ± 10; P < 0.05).
3.1 Imaging biomarkers
The meniscectomized knees demonstrated a statistically significant decrease in total ‘medial’ tibiofemoral cartilage volume compared with the control side post-operatively (5.04 ± 1.19 cm3 vs. 6.15 ± 0.92 cm3; P = 0.001) but no significant difference was found between both pre-operatively 6.05 ± 0.9 cm3 vs. 6.15 ± 0.92 cm3; n.s.). Additionally, there was a significant reduction in total ‘lateral’ tibiofemoral cartilage volume compared with the control side post-operatively (6.1 ± 0.5 cm3 vs. 6.9 ± 0.9 cm3; P = 0.0020) but no significant difference was found between both pre-operatively (7 ± 0.1 cm3s. vs. 6.9 ± 0.9 cm3; n.s.). The percentage of decrease in the total knee cartilage volume post-operatively from pre-operatively was >10% in 13 patients (65%), this indicates progressors of cartilage loss based on MRI measurements of cartilage volume as a biomarker.
Compared with the control side, the meniscectomized side demonstrated no statistically significant difference in total knee cartilage thickness measured by US in this cohort (2.0 ± 0.2 mm vs. 1.9 ± 0.3 mm, n.s.). In addition, no significant difference was observed for the cartilage thickness in each part of the cartilage.
3.2 Biochemical markers
A significant difference was found between the patients at the preoperative markers assay and controls in both the COMP (588.8 ± 566.7 vs. 133.8 ± 65 ng/ml; P < 0.05) and PIICP (72.5 ± 27.9 vs. 64.6 ± 18.6 ng/ml; P < 0.05) but not in Col II (29.8 ± 28.7 vs. 45.1 ± 31.3 ng/ml; P < 0.05).
There was an increase in the COMP and Col II assays between 3 and 6 months postoperatively, however, this was not significant. A significant decrease in PIICP was found between 3 and 6 months post-operatively in all patients ().
Table 1 Change in biochemical markers assays at 3 and 6 months post-operative.
Diagnostically, with the exception of PIICP, none of the individual biochemical biomarkers was a statistically significant indicator of cartilage volume loss by MRI. PIICP had the highest diagnostic accuracy quantified as the area under the receiver-operator characteristics curve (AUC) of 0.75 (95% CI: 0.509–0.912) higher than all others, P < 0.05, to distinguish subjects with progressive cartilage loss from non-progressors. However on combinations, the ratio of COMP and Col II to PIICP scored AUC of 0.906 (0.69–0.98, was higher than PIICP.
The prognostic performance was defined as the ability of the percentage of change in the three measured biochemical markers from pre-operative to 3 months and from 3 to 6 months post-operative values to separate non-progressors (no or <10% change in the cartilage volume pre- and post-operatively) from early progressors (>10% change), and was evaluated by the P value from multivariate analysis of variation (based on Hotelling’s T2 test, none of the markers nor their change could predict progression of volume loss by MRI. Multivariable logistic regression revealed significant associations of increased COMP and Col II (95% CI = 0.85–7.82) and decreased PIICP (95% CI = 1.2–9.3) with the presence of cartilage volume loss >10%, independent of age and duration after injury. The combined impact of increased COMP and Col II and decreased PIICP was 20.9 (95% CI = 1.97–235.3), far exceeding the impact of each independent biomarker.
4 Discussion
In this study serum levels of COMP, Col II and PIICP are modestly associated with cartilage volume loss by MRI, but not with its progression, in a group of twenty young Egyptian adults who underwent unilateral arthroscopic partial meniscectomy.
Knee cartilage is an important structure in the human body and plays a pivotal role in knee joint activity. It is known that cartilage morphology is influenced by many factors, such as age, gender, genetics, BMI, level of physical activity, osteoarthritis, pain and ACL or meniscal injuries.Citation22 Among these factors, meniscal damage appears to be an important factor to induce cartilage morphological changes. Meniscal damage or loss alters the in vivo cartilage contact biomechanics by shifting the contact location to smaller regions of thinner cartilage, and by increasing the magnitude of the cartilage contact deformation. It is presumed that cartilage morphological alteration is accelerated in the knees with meniscal pathology; with altered knee joint kinematics, the mechanical demand eventually exceeds the ability of the joint to repair itself, setting the stage for OA development.Citation23
In this study the mechanism of injury was non-significant trauma in 50% of patients. This finding is in agreement with previous studies that reported that 52–92% of patients with symptomatic knee osteoarthritis present with meniscal damage when assessed by MRI to the contralateral normal knees.Citation23
Quantitative MRI (qMRI), like 3D-WS-bSSFP sequence used in this study, provides non-invasive and reliable data on cartilage morphology in healthy subjects with different age groups and different genders.Citation24 Comprehensive knowledge of the biochemical and biomechanical changes that occur with OA may require a combination of various qMRI techniques. The application of these qMRI techniques to the identification and monitoring of cartilage damage in individual patients for the early diagnosis and treatment of OA would be clinically significant. However, assessment of other joint structures is not possible. In fat-suppressed 3D-WS-bSSFP, the articular cartilage has very high signal intensity, joint fluid has an intermediate to low signal intensity, and subchondral bone and bone marrow are dark. Reported sensitivity and specificity for detection of cartilage loss are 75–85% and 95–97%, respectively.Citation25
The cartilage morphology (including cartilage volume by MRI and thickness by US) was investigated in the meniscectomized knees and the contralateral intact knees with a mean injury time of 6.4 months. Using the 3D cartilage models (), the mean total cartilage volume was calculated for the femur and tibia. The cartilage thickness was measured within the weight-bearing area by US. In this study, the contralateral knees were used as the control side for analyzing the cartilage morphological changes in the post-meniscectomy knees. It was found that the meniscectomized side demonstrated a statistically significant lower total knee cartilage volume compared with the control side. The percentage of decrease in the total knee cartilage volume post-operatively from pre-operatively was >10% in 13 patients (65%), this value progressors of cartilage loss based on MRI measurements of the cartilage volume as a biomarker.Citation11 However, this did not correlate with KOOS scores. As the articular cartilage is avascular and aneural, early changes could be asymptomatic or affecting the functional knee activity.Citation25 Significant rates of cartilage loss are seen also in other studies; in subjects of post partial meniscectomy compared with healthy controls (difference 6.5% per year, 95% CI 3.7–9.3% per year; P < 0.001).Citation26 Even greater losses were observed at the central medial tibial cartilage and medial femoral condyle (15% and 12% respectively).Citation27 Greater losses are observed in this cohort of Egyptian adults, this may have a genetic basis. The same genes that promote healing after cartilage damage also appear to protect against OA due to wear-and-tear processes. Osteoarthritis, like several other disorders, involves many genes that each contribute in a small way to the disease process. It is proved that there is a subtle genetic influence on OA risk, while other genes are protective.Citation28
There was no statistically significant difference in total cartilage thickness of the knee between the meniscectomized side and the control side. As cartilage layers exhibit a mean thickness of only 1.3–2.5 mm throughout the human knees,Citation28 so small changes could not be accurately measured. Moreover, it was measured at a single point.Citation27
Joint injury, including meniscal injuries, triggers a lengthy remodeling process in the cartilage and surrounding tissues that has adverse biomechanical and biochemical consequences that encourage joint degeneration. The metabolic sensitivity of chondrocytes to such mechanical environment changes, combined with the low adaptation potential of mature cartilage, could lead to cartilage degeneration and premature osteoarthritis after meniscal injury.Citation29 A significant difference was found between the patients at the preoperative markers assay and controls in both the COMP and PIICP but not in Col II. This could be explained by the articular cartilage trauma associated with the initial knee trauma that caused meniscal injury,Citation23 however, chondral lesions were defined arthroscopically in only two patients (10%).
Diagnostically, assays for type II collagen degradation, when used alone or in combination with markers of collagen synthesis, can distinguish populations with cartilage lesion which exhibit progression of joint damage from non-progressors.Citation30 In this study, with the exception of PIICP a marker of cartilage synthesis, none of the individual biochemical biomarkers was a statistically significant indicator of cartilage volume loss by MRI. The diagnostic accuracy of combinations; the ratio of COMP and Col II to PIICP was high even higher than PIICP alone. This may be due to the short follow up duration, as COMP and Col II are promising in the evaluation of knee OA severity.Citation17 Progression was identified by the increase in type II collagen degradation products compared to a decrease in the propeptide marker of synthesis.Citation31 Measurement of biochemical marker change to predict MRI progression in patients revealed none of the individual or combined biomarkers was a statistically significant predictor of cartilage loss. This may be a β error due to small sample size. It is hoped that the availability of assays to measure degradative, synthetic, and turnover products of cartilage matrix metabolism in body fluids offers opportunities to try and monitor cartilage turnover in vivo.Citation17
5 Conclusion
Cartilage volume loss as an MRI-based biomarker combined with changes in cartilage matrix turnover detected by molecular biomarkers appears to reflect the initial changes associated with cartilage degeneration and so identifies a subgroup of patients whose losses could be a significant initiation that account for early OA. Genetics and gene therapy is another new avenue which must be explored to treat non- or poorly-healing cartilage defects. It is found that one-time measurement of biochemical markers could not be reliable to predict MRI progression in patients following meniscectomy, with the possible exception of PIICP.
There is increasing evidence that the best solution may be a panel of biomarkers, covering the range of physiological effects, or indeed a combination of tissue biomarkers with other parameters (e.g., US or MRI) into single diagnostic test. Measuring markers of these processes would seem promising as predictors of early and subsequent cartilage degeneration at the individual level. Moreover, genetic markers might be also useful predictors of prognosis. Genetic mapping may reveal differences in diverse healing responses in multiple tissues giving a way to new therapeutic targets designed to induce or enhance regeneration and, potentially, protect from OA.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 15 August 2014
References
- M.EnglundL.LohmanderRisk factors for symptomatic knee osteoarthritis fifteen to twenty-two years after meniscectomyArthritis Rheum149200428112819
- G.KarsentyE.WagnerReaching a genetic and molecular understanding of skeletal developmentDev Cell242002389406
- D.BursteinM.GrayT.MosherMeasures of molecular composition and structure in osteoarthritisRadiol Clin North Am472009675686
- M.EnglundA.GuermaziF.RoemerMeniscal tear in knees without surgery and the development of radiographic osteoarthritis among middle-aged and elderly persons: the multicenter osteoarthritis studyArthritis Rheum1432009831839
- B.ColeT.CarterS.RodeoAllograft meniscal transplantation: background, techniques, and resultsInstr Course Lect522003383396
- T.FukubayashiH.KurosawaThe contact area and pressure distribution pattern of the knee. A study of normal and osteoarthritic knee jointsActa Orthop Scand5161980871879
- K.MessnerJ.GaoThe menisci of the knee joint. Anatomical and functional characteristics and a rationale for clinical treatmentJ Anat19321998161178
- M.SalataA.GibbsJ.SekiyaA systematic review of clinical outcomes in patients undergoing meniscectomyAm J Sports Med3892010 Sep19071916
- V.MartinekAnatomy and pathophysiology of articular cartilageDtsch Z Sportmed542003166170
- M.ThomasCartilage as a BiomarkerM.ThomasSignificance, Techniques, and New Developments152011Springer Science+Business Media, LLCNew York Dordrecht Heidelberg London205212
- D.DevaP.CoreyProbing articular cartilage damage and disease by quantitative magnetic resonance imagingJ R Soc Interface620131078
- G.GoldB.HargreavesS.ReederControversies in protocol selection in the imaging of articular cartilageSemin Musculoskelet Radiol922005161172
- M.HuberS.TrattningF.LintnerAnatomy, biochemistry and physiology of articular cartilageInvest Radiol352000573580
- F.NelsonL.DahlbergS.LavertyEvidence for altered synthesis of type II collagen in patients with osteoarthritisJ Clin Invest102199821152125
- J.RousseauP.DelmasBiologic markers in osteoarthritisNat Clin Pract Rheumatol32007347356
- V.VilimR.VytásekM.OlejárováSerum cartilage oligomeric matrix protein reflects the presence of clinically diagnosed synovitis in patients with knee osteoarthritisOsteoarthritis Cartilage92001612618
- M.LotzJ.Martel-PelletierC.ChristiansenValue of biomarkers in osteoarthritis: current status and perspectivesPostgrad Med J9010612014171178
- R.AltmanE.AschD.BlochD.BoleK.BorensteinK.BrandtDevelopment of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the kneeArthritis Rheum29198610391049
- Knee injury & Osteoarthritis Outcome Score (KOOS), English version LK1.0; available at http://www.KOOS.nu.
- M.BackhausG.BurmesterT.GerberGuidelines for musculoskeletal ultrasound in rheumatologyAnn Rheum Dis602001641649
- Human cartilage oligomeric protein, Human collagen type II, Human carboxy terminal propeptide of type II procollagen ELISA Kits; available at http://www.cusabio.com.
- L.HongA.HosseiniL.Jing-ShengQuantitative magnetic resonance imaging (MRI) morphological analysis of knee cartilage in healthy and anterior cruciate ligament-injured kneesKnee Surg Sports Traumatol Arthrosc20201214961502
- M.BerthiaumeJ.RaynauldJ.Martel-PelletierMeniscal tear and extrusion are strongly associated with progression of symptomatic knee osteoarthritis as assessed by quantitative magnetic resonance imagingAnn Rheum Dis642005556563
- H.ImhofI.NobauerC.KrestanMRI of the cartilageEur Radiol12200227812793
- T.LinkS.MajumdarFuture Perspective and Significance of Cartilage Imaging and QuantificationT.LinkCartilage Imaging: Significance, Techniques, and New Developments182011SpringerNew York Dordrecht Heidelberg London229238
- J.PelletierJ.RaynauldM.BerthiaumeRisk factors associated with the loss of cartilage volume on weight-bearing areas in knee osteoarthritis patients assessed by quantitative magnetic resonance imaging: a longitudinal studyArthritis Res Ther92007R74
- F.CicuttiniA.ForbesW.YuanyuanRate of knee cartilage loss after partial meniscectomyRheumatology299200219541956
- D.HeinegårdMolecular events in cartilage formation and remodelingArthritis Res3A200123
- M.StiebelL.MillerJ.BlockPost-traumatic knee osteoarthritis in the young patient: therapeutic dilemmas and emerging technologiesOpen Access J Sports Med520147379
- J.David.Hunter1L.JiangCartilage markers and their association with cartilage loss on magnetic resonance imaging in knee osteoarthritis: the Boston Osteoarthritis Knee StudyArthritis Res Ther92007108
- P.GarneroX.AyralJ.RousseauUncoupling of type II collagen synthesis and degradation predicts progression of joint damage in patients with knee osteoarthritisArthritis Rheum46200226132624
