Abstract
Background
Pesticides are widely used in order to enhance the food protection by controlling the unwanted insects and disease vectors in agriculture.
Aim
The aim of this study was to investigate the impact of repeated sublethal doses (0.2 mg kg−1 day−1 for two weeks) of the insecticide parathion on some biochemical parameters in rabbit.
Methods
The activity of some enzymes; cholinesterase (ChE), adenosine triphosphatase (ATPase), carboxylesterase (CE), glutathione-S-transferase (GST), acid phosphatase (ACP), alkaline phosphatase (ALP), aspartate aminotransferase (AST) and alanine aminotransferase (ALT) was determined in the brain, liver, kidney and heart.
Results
Parathion administration markedly inhibited the activity of ChE. The activities of CE, GST, ALP, AST, and ALT in all organs of treated rabbits were increased. ATPase fluctuation was recorded in its activity, whereas ACP activity showed a significant reduction in the liver and kidney.
Conclusion
The results indicated that changes in body and organ weights have been used as indicators of adverse effects of parathion and also alteration in tested enzymes activity can be used as relevant biomarkers for monitoring toxicity due to parathion exposure in non target organisms.
1 Introduction
Pesticides have played a vital role in controlling agricultural, industrial, home and public health pest worldwide.Citation1 However, their use poses animal and human health concerns because of their toxicity, widespread use and release into the environment.Citation2 According to the World Health Organization, 3 million cases of pesticide poisoning occur every year, resulting in more than 250,000 deaths.Citation3 Despite this alarming figure, there is currently no global system to track and stem poisoning or diseases associated with pesticide use.Citation4 Real world exposure to pesticides normally occurs through lower level single or repeated exposures (for example, as residues in food products). The organophosphorus pesticides (OPs) are a group of highly toxic compounds and they are readily available commercially for domestic, agricultural, and industrial purposes.Citation5 They account for over 50% of all insecticides applied world-wide.Citation3 Parathion (O,O-diethyl-O-(4-nitrophenyl) phosphorothioate) is one of the most acutely toxic pesticides registered by the EPA 1986. Because of its highly toxic nature, parathion is classified as a Restricted Use Pesticide (RUP).Citation6 It is a well-known AChE inhibitor just like other OPs which leads to the accumulation of acetylcholine and results in excessive stimulation of postsynaptic receptors and consequent signs of toxicity.Citation7,Citation8
The general physiology of rabbits is similar to humans; therefore, the rabbit has been used as a model for human diseases. The rabbit is large enough to provide adequate quantities of tissue for experimental work without pooling of samples but is small enough to be economical for most studies. However, rabbits do have many advantages and serve to bridge the gap between these small animal models, which are perhaps best suited for discovery phases of research, and larger animal models are often required for preclinical, translational research.Citation9 Changes in body weight have been used as an indicator of adverse effects of drugs and chemicals. Also, organ weight changes have long been accepted as a sensitive indicator of chemically induced changes to organs and in toxicological experiments.Citation10
A biomarker may be any measurable biochemical, cellular, physiological or behavioral change in an organism or population that indicates exposure to chemical pollutants.Citation11 By focusing on the intermediate, sublethal effects of a pollutant, this developing field aims to reveal environmental threats before obvious toxic effects such as death of organisms are observed. An ideal biomarker will also be nondestructive to the indicator organism. Biomarkers and rabbits have been employed as useful tools for risk assessment of chemical compounds that are exposed in the environment.Citation12
The aim of the present work was to evaluate the distribution pattern of ChE, ATPase, CE, GST, ACP, ALP, AST, and ALT in different organs of male rabbits. Besides the effect of repeated sublethal doses of parathion on some biological and biochemical parameters, consequently these parameters can be used as potential biomarkers of damage caused by parathion exposure.
2 Materials and methods
2.1 Animals
Male New Zealand white rabbits (six months old, 3–4 kg weight), were purchased from the Abbis Farm, Faculty of Agriculture; Alexandria University. Animals were individually housed in stainless steel cages at 22–26 °C temperature, 40–70% humidity and controlled environment with a 12 h light/dark cycle. Food and water were given ad libitum. The animals were accommodated to laboratory conditions for two weeks before being experimented. All maintenance and care were in accordance with the animal welfare guidelines established at the university.
2.2 Chemicals
Technical material (99.6%) parathion was obtained from EPA, Research Triangle Park, N.C. All other chemicals used were of the highest purity grade available from Sigma and Merck Chemical Companies.
2.3 Experimental design
The rabbits were divided into two groups (5 animals per each). Animals in the first group were daily given corn oil (1 ml/kg bw) for two weeks and used as control. Rabbits in the second group orally received parathion (0.2 mg/kg/day). Animals were examined during the 6 weeks of study period (2 weeks of treatment + another 4 weeks without treatment). The body weights of control and treated animals were recorded weekly. At the end of the experiment the animals were sacrificed and dissected, then the brain, liver, kidney and heart were removed, rinsed in saline solution (0.9% NaCl), dried on filter paper and weighted individually in all rabbits and the relative organ weight was calculated (organ weight: body weight).
2.4 Biochemical analysis
Each organ was minced and homogenized separately in ice cold saline solution (10% w/v) in a polytron homogenizer (Tekmar tissumizer). The homogenate was centrifuged at 10,000×g for 30 min at 4 °C using a cooling centrifuge. The resultant supernatant was used for different enzymes’ assay.
ChE activity was measured spectrophotometrically according to Ellman et al.,Citation13 using acetyl thiocholine iodide as a substrate. ATPase activity was measured by the colorimetric method of Koch.Citation14 CE activity was assayed using p-nitrophenyl butyrate as substrate according to Verschoyle et al.Citation15 GST activity was assayed using 1-chloro-2,4-dinitrobenzene as substrate by the method of Vessey and Boyer.Citation16 The assay was conducted by monitoring the appearance of the conjugated complex CDNB and GSH at 340 nm. Data were expressed as u mole/mg protein/minute. The activities of ACP and ALP were determined according to Bessey et al.Citation17 with sodium p-nitrophenyl phosphate as a substrate in the acid and alkaline medium, respectively. AST and ALT activities were measured by the procedure of Reitman and FrrankelCitation18 using a commercially available kit from Bio-Merieux. The total protein content was determined by the method of Lowry et al.Citation19 using bovine serum albumin as the standard.
2.5 Statistical analysis
All data were expressed as mean ± standard error (SE). Data were analyzed using one-way analysis of variance (ANOVA) followed by the Student–Newman–Keuls test to determine significance between different groups. The criterion for statistical significance was set at p < 0.05.
3 Results
3.1 Body weight and relative organ weight
The physiological status of control and treated animals was noticed as the change in the body weight gain and relative organ weights. Male rabbits orally administrated with parathion in a dose of 0.2 mg/kg/day for 2 weeks have shown that no mortality occurred during the experimental period. It is clear that the body weight of the control group was gradually increased during the 6 weeks by 106.45, 108.65, 113.09, 116.65, 121.99, and 125.34% after 1, 2, 3, 4, 5 and 6 week, respectively. On the other hand the body weight of treated animals was gradually decreased until the fourth week and then it was increased (). From this result it is noticed that there is a significant difference (p < 0.05) of mean body weight gains between control and treated animals. No significant change in the relative weights of all organs except the liver in treated animals when compared with control was observed (). The percentage relative weights of the liver in control and treated animals were 3.52 ± 0.3 and 4.53 ± 0.31, respectively.
Figure 1 Body weight pattern of rabbits orally administrated to 0.2 mg/kg/day of parathion for two weeks. ∗Significantly different from the control value at p < 0.05.
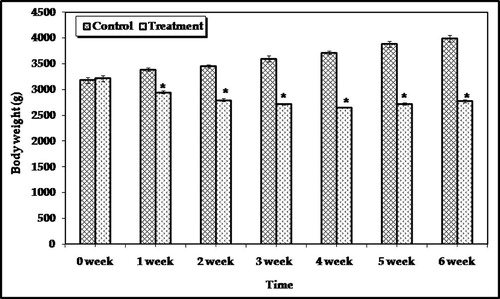
Table 1 Relative organ weights of rabbits after four weeks of last treatment with parathion.
3.2 Biochemical studies
The distribution pattern of the enzyme activities; ChE, ATPase, CE, GST, ACP, ALP, AST and ALT in the different organs of rabbits was carried out.
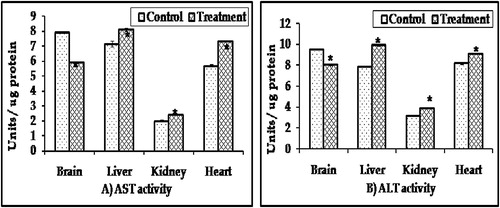
ChE activity: The results of this survey detected that the brain contained extremely high activity of ChE, followed by the liver (87.32 and 54.67 u moles acetylthiocholine iodide hydrolyzed/mg protein/min., respectively). Parathion resulted in a significant inhibition of ChE activity in the brain, liver, and kidney by 22.71, 27.02, and 22.42%, respectively ().
Figure 2 ChE and ATPase activities in different organs of male rabbits treated with 0.2 mg/kg/day for two weeks of parathion after four weeks of last treatment. Values represent the mean ± SE of three replicates from each animal. ∗Significantly different from the control value at p < 0.05.
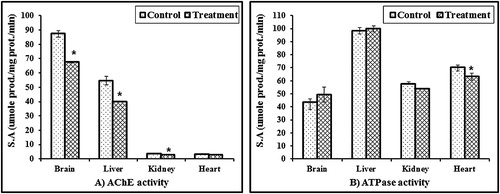
ATPase activity: The data showed that the liver contained the highest activity of ATPase, which is almost twice as much as the activity in the brain and kidney. It is clear from that parathion has different effects on ATPase in different organs. Activities oscillating were between activation and inhibition.
CE activity: The CE activities were 27.7 ± 1.3, 18.73 ± 0.75, 13.7 ± 0.9 and 7.2 ± 0.52 m mole/mg protein/min. in the kidney, liver, heart, and brain, respectively (). The activity in the kidney was about 3.6 times more than that in the brain. CE of treated rabbits in all organs was higher than that of control. The activation power of the tested organs was in the following order: liver > heart > kidney > brain.
Figure 3 CE and GST activities in different organs of male rabbits treated with 0.2 mg/kg/day for two weeks of parathion after four weeks of last treatment. Values represent the mean ± SE of three replicates from each animal.
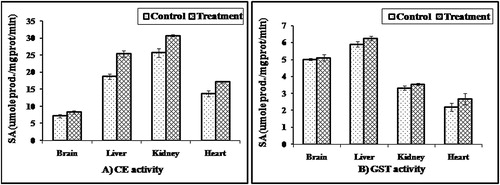
GST activity: It was noticed from that the liver contained the highest GST activity followed by the brain, while the heart contained the least level of enzyme activity. Parathion had slightly stimulatory effects on GST activities in the tested organs.
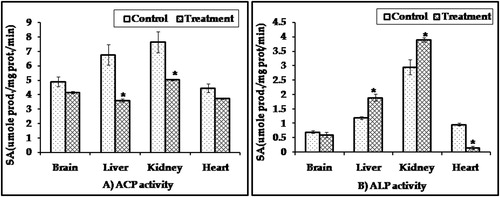
ACP and ALP activities: Kidney contained the highest activity of ACP and ALP followed by the liver. Parathion reduced ACP activity in the kidney, liver, brain and heart with percentage of control; 29.6, 41.0, 15.1 and 16.6, respectively, while ALP activities in all organs of treated animals were activated except in the heart ().
Figure 4 ACP and ALP activities in different organs of male rabbits treated with 0.2 mg/kg/day for two weeks of parathion after four weeks of last treatment. Values represent the mean ± SE of three replicates from each animal. ∗Significantly different from the control value at p < 0.05.
AST and ALT activities: The activity of AST and ALT in the brain was the highest one, while the kidney contained the least activity. AST and ALT levels in all organs were markedly elevated in the treated animals when compared with the control group except in the brain ().
4 Discussion
Pesticides are widely used in order to enhance the food protection by controlling the unwanted insects and disease vectors in agriculture. It is known that the OPs among other pesticides are the most toxic pesticides to the vertebrates. OPs toxicity is an important problem in many countries of the world due to easy accessibility, extensive and unconscious use.Citation5
4.1 Body weight and relative organ weight
In toxicological studies body and organ weights are important criteria for the evaluation of toxicity. In the present study, the body weight of parathion treaded rabbits was significantly (p < 0.05) lower than that of the control group. This may be attributed to a decreased food intake (anorexia or food avoidance), poor food palatability or increased degradation of lipids and protein due to treatment-related toxicity.Citation20 Also, weight loss observed in the parathion treatment group may be a result of the combination of oxidative stress and adrenal-mediated stress caused by the inhibition of cholesterol ester hydrolase. Parathion is known to show its toxic effects by inhibiting cholinesterase activity which could be due to the less food consumption and/or fluid and electrolyte loss. Our data are in consonance with those obtained by Ambali et al.Citation21 and Al-Othman et al.Citation22 Results showed that there is a significant increase in the relative liver weight in rabbits exposed to parathion compared with control rabbits (). The increase in relative liver weight in rabbits exposed to parathion seems to be due to toxic potential of pesticide, and it is in agreement with Al-Sarat et al.Citation23 in rabbits, Institoris et al.Citation24 in rats.
4.2 Biochemical studies
The classical target of organophosphorus compounds is cholinesterase, which is one of the hydrolytic enzymes for acetylcholine. The inhibition of cholinesterase action is leading to cholinergic hyperactivity resulting in the death of the animal.Citation7,Citation8 In our study it was found that the brain contained extremely high enzyme activity followed by the liver. Administration of sublethal dose of parathion (0.2 mg/kg/day) for successive two weeks into rabbits elicited highly significant inhibition of ChE in all tested organs. It is well known that there is a good relationship between exposure to OPs and inhibition of ChE activity. Therefore, the level of ChE activity has been considered a good biomarker exposure to this pesticide.Citation25
ATPase CE, GST, phosphatases, and transaminases are important indices in the biological processes. They are responsible for detoxification processes, metabolism, and biosynthesis of energetic macromolecules for different essential functions. Any interference with these activities out of the normal range should lead to biochemical impairment and lesions of tissues and cellular function.Citation26 ATPase plays an important role in the maintenance of cell permeability and energy transformation in the biological system. ATPase activity can be taken as a meaningful index of cellular activity and forms a useful toxicological tool and is considered to be sensitive indicator of toxicity.Citation27 Data showed that the activity of the liver and brain ATPase was slightly stimulated in rabbit treated with parathion. On the contrary, heart ATPase was significantly inhibited in treated animals than in the control group. It is well known that ATPase is essential for the regulation of ionic content and membrane excitability of myocardial cells, impairment of its function would lead to the coronary artery vasospasm, arrhythmias, ischemic damage, and cardiac failure. This contention is in concert with some findings that certain cardiovascular diseases, such as cardiomyopathy, and hypertension, associate with decreased ATPase activity.Citation28 On the other hand, Sushma and RaoCitation27 found that aluminum acetate decreased the total ATPase activity in the brain, liver, kidney, heart, muscle and testis of albino mice. From the results of this study, it is quite clear that ATPase sensitivity toward parathion differs depending on the type of the tissues.Citation29
CE is found in almost all organisms, it has been proposed as a possible biomarker for OP and carbamate exposure, but it has not been studied to the extent that AChE has.Citation30 In this study, it was found that the kidney contained the highest activity of CE followed by the liver. CE level in treated rabbits has been slightly stimulated by parathion. These data are in agreement with the results of El-Gendy et al.Citation31 who found that hepatic CE was slightly increased in alachlor and butachlor treated mice.
It was noticed that the liver contained the highest activity of GST, while the heart contained the least enzyme (). We also observed that parathion possesses slightly stimulatory effects on GST in all tested organs. This induction may be due to glutathione and glutathione dependent enzyme systems that provide major protection against the toxic agent.Citation32 GST is thought to play a physiological role in the detoxification and elimination of toxic and undesirable foreign compounds. This enzyme catalyzes the conjugation of a variety of electrophilic substrates to the thiol group of GSH, producing less toxic forms.Citation33 Increased GST activity in tissues may indicate the development of a defensive mechanism to counteract the effects of parathion and may reflect the possibility of a more efficient protection against pesticide toxicity.
Regarding ACP and ALP activities in different organs of rabbit, it is clear that the kidney contained the highest activity of ACP and ALP followed by the liver. Treatment with parathion significantly decreased ACP activity, while increased ALP in both the kidney and liver as compared to controls. Also, Igwenyi et al. 2014 reported a significant increase in alkaline phosphatase activity, in serum and liver of experimental animals treated with baygon.Citation34 ACP is known to be localized in lysosomes and surrounded by a lipoprotein membrane. The decrease in ACP may be related either to leakage of the enzyme into extracellular compartments or to tissue damage.Citation21 ACP was used as a marker for lysosomal functions in liver cells in order to estimate the interference with catabolic and autophagic processes in the liver. Also, the change in the ACP activity may be related to the biotransformation and elimination of the tested pesticide.Citation35 The significant elevation in the activity of ALP indicated damage to any or all of the organs producing this enzyme such as the liver and kidney is due to leakage of lysosomal enzymes into the cytoplasm and renal necrosis and pyknotic nuclei. This result is paralleled by the result of Ambali et al.Citation21 Rahman et al.Citation36 suggested that the decrease in the activities of ALP and ACP in different tissues might be due to the increased permeability of plasma membrane or cellular necrosis, showing the stress condition of the treated animals.
It is quite clear that the liver contained the highest activity of AST and ALT followed by the brain and heart, while the kidney contained the least activity. AST and ALT are considered to be the liver markers that are used to study varying cell viability and changes in cell membrane permeability.Citation37 The levels of these enzymes in treated animals were significantly higher than those in the control group in all organs except the brain. The disruption of transaminases from the normal values denotes biochemical impairment and lesions of tissues and cellular function because they are involved in the detoxification process, metabolism and biosynthesis of energetic macromolecules for different essential functions.Citation21 The liver plays a crucial role in the detoxification process and faces the threat of maximum exposure to xenobiotics and their metabolic by-products, which lead to liver damage and hepatotoxicity.Citation22 The susceptibility of liver tissues to the stress resulting from exposure to pesticides is a function of overall cellular balance between the degree of oxidative stress and the antioxidant capacity.Citation38 In the case of liver injury resulting from hepatotoxicity, cellular contents are released, thereby increasing the level of liver enzymes above the normal threshold. In this context, activity levels of liver enzymes (i.e. CE, GST, ALP, ALT, and AST) are primarily employed as biomarkers of hepatic damage and injury. These results are consistent with previous findings indicating that OP exposure correlates with elevation of the enzymatic activity of CE, GST, ALP, ALT, and AST.Citation33 Our data demonstrated that repeated sublethal doses of parathion caused a significant alteration in the level of tested enzymes especially in the liver, which is the main vital organ of all biochemical processes in the body.Citation22
In conclusion: The present study demonstrated that excessive exposure to parathion caused cytotoxic changes in the hepatic biochemical markers. Hence these biomarkers might be used in addition to ChE activity to monitor the non target organisms’ exposure to pesticides to take effective measures to avoid serious adverse health effects.
Conflict of interest
None declared.
Notes
Available online 16 September 2014
Peer review under responsibility of Alexandria University Faculty of Medicine.
References
- M.Bjørling-PoulsenH.R.AndersenP.GrandjeanPotential developmental neurotoxicity of pesticides used in EuropeEnviron Health7502008123
- G.M.CalvertJ.KarnikL.MehlerJ.BeckmanB.MorrisseyJ.SievertAcute pesticide poisoning among agricultural workers in the United States, 1998–2005Am J Ind Med512008883898
- C.YangJ.DengIntermediate syndrome following organophosphate insecticide poisoningJ Chin Med Assoc70112007467472
- S.M.AliS.E.ChiaInterethnic variability of plasma paraoxonase (PON1) activity towards organophosphates and PON1polymorphisms among Asian population. A short reviewIndus Health462008309317
- H.AardemaJ.H.J.M.MeertensJ.J.M.LigtenbergO.M.Peters-PolmanJ.E.TullekenG.ijlstraOrganophosphorus pesticide poisoning: cases and developmentsNeth J Med6642008149153
- R.T.MeisterFarm Chemicals Handbook1992Meister Publishing CompanyWilloughby, OH
- Y.M.A.Al-BadranyF.K.MohammadEffects of acute and repeated oral exposure to the organophosphate insecticide chlorpyrifos on open-field activity in chicksToxicol Lett1741–32007110116
- I.A.HundekariA.N.SuryakarD.B.RathiAcute organo-phosphorus pesticide poisoning in North Karnataka, India: oxidative damage, haemoglobin level and total leukocyteAfr Health Sci1312013129136
- Z.BoszeL.M.HoudebineApplication of rabbits in biomedical research: a reviewWorld Rabbit Sci142006114
- J.T.MukindaP.F.EaglesAcute and sub-chronic oral toxicity profiles of the aqueous extract of polygala fruticosa in female mice and ratsJ Ethnopharmacol1282010236240
- M.H.DepledgeThe Rational Basis for the Use of Biomarkers as Ecotoxicological ToolsM.C.FossiC.LeonzioNondestructive Biomarkers in Vertebrates1994LewisBoca Raton, Florida271295
- I.M.YousefAluminium-induced changes in hemato-biochemical parameters, lipid peroxidation and enzyme activities of male rabbits: protective role of ascorbic acidToxicology19920044757
- G.L.EllmanK.D.CourtneyV.AndreessR.M.FeatherstoneA new and rapid colorimetric determination of acetylcholinesterase activityBiochem Pharmacol719618895
- R.B.KochChlorinated hydrocarbon insecticides: inhibition of rabbit brain ATPase activitiesJ Biol Chem1931969265275
- R.D.VerschoyleE.ReinerE.BaileyW.N.AdrigeDimethyl phosphorothioate, reaction with malathion and effects on malathion toxicityArch Toxicol491982293301
- D.A.VesseyT.D.BoyerDifferential activation and inhibition of different forms of rat liver glutathione-S-transferase by the herbicides 2,4-dichloro phenoxy acetate 2,4-D) and 2,4, trichloro phenoxy acetate (2,4, S-T)Toxicol Appl Pharmacol731984492499
- D.A.BessyO.H.LowryM.I.BrockDetermination in serum with P-nitrophenylphosphateJ Biol Chem1641946321329
- S.ReitmanS.A.FrrankelA colorimetric method for the determination of serum glutamic oxaloacetic and glutamic pyruvictransaminasesAm J Clin Pathol2819575663
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem1931951265275
- S.A.MansourA.T.H.MossaOxidative damage, biochemical and histopathological alterations in rats exposed to chlorpyrifos and the antioxidant role of zincPest Biochem Physiol9620101423
- S.AmbaliD.AkanbiN.IgbokweM.ShittuM.KawuJ.AyoEvaluation of sub-chronic chlorpyrifos poisoning on hematological and serum biochemical changes in mice and protective effect of vitamin CJ Toxicol Sci3222007111120
- A.M.Al-OthmanZ.A.Al-OthmanG.B.El-DesokyK.YusufM.A.M.Aboul-SoudAmeliorative effect of α-tocopherol and selenium on effects of malathion on plasmatic biochemical indices and lesions in the liver of ratsCurr Pharm Anal82012214218
- A.S.Al-SaratA.M.HafizA.E.BayoumiH.I.HusseinY.AboBaerImpact of fenitrothion thermal fogging on some biological and parameters in New Zealand rabbits as non-target organismsInt J Agric Biol132011435438
- L.InstitorisO.sirokiU.UndegerN.BasaranI.DesiImmunotoxicological investigation in rats dosed repeatedly with combinations of cypermethrin, As(III), and Hg(II)Toxicology17220025967
- A.P.RemorC.C.TottiD.A.MoreiraG.P.DutraV.D.HeuserJ.M.BoeiraOccupational exposure of farm workers to pesticides: biochemical parameters and evaluation of genotoxicityEnviron Int352009273278
- L.A.LatnerAminotransferasesW.B.SaundresClinical Biochemistry Seventh1978Comp. PhiladelphiaLondon, Toronto553568
- N.J.SushmaK.J.RaoTotal ATPases activity in different tissues of albino mice exposed to aluminium acetateEnviron Biol282007483484
- C.C.ChenS.Y.Lin-ShiauATPase activities in the kidney and heart of alloxan induced diabetic miceAsia Pac J Pharmacol319885155
- D.A.FoxS.D.RubinsteinP.HsuDevelopment lead exposure inhibits adult rat retinal, but not kidney, Na, K-ATPaseToxicol Pharmacol1091991482493
- K.J.P.YoonC.L.MortonP.M.PotterM.K.DanksR.L.LeeSynthesis and evaluation of esters and carbamates to identify critical functional groups for esterase specific metabolismBioorg Med Chem11200332373244
- K.S.EL-GendyN.M.AlyF.S.SabraBiochemical effects of a single oral dose of two acetanilide herbicides alachlor and butachlor on female miceAlex J Pharm Sci1219988790
- N.M.AlyK.S.EL-GendyF.H.MahmoudA.H.El-SebaeProtective effect of vitamin C against chlorpyrifos oxidative stress in male micePest Biochem Physiol972010712
- Y.KalenderS.KayaD.DurakF.G.UzunF.DemirProtective effects of catechin and quercetin on antioxidant status, lipid peroxidation and testis-histoarchitecture induced by chlorpyrifos in male ratsEnviorn Toxicol Pharmacol332012141148
- I.O.IgwenyiN.AbohN.NwachukwuU.A.IbiamC.E.OfforP.M.AjaEffect of baygon insecticide on the activities of total, alkaline and acid phosphatases of selected tissues of albino ratsJ Pharm Biol Sci920144143
- S.KhanEndosulphan concentration alters biomarkers in albino ratIndian J Pharm Biol Res220141417
- M.F.RahmanM.K.J.SiddiquiC.K.JamilInhibition of acetylcholinesterase and different ATPases by a novel phosphorothionate (RPR-II) rat brainEcotoxicol Environ Saf472000125129
- E.L.B.NovelliE.P.VieriraN.L.RodriguesB.O.RibasRisk assessment of cadmium toxicity on hepatic and renal tissues of ratsEnviron Res79 A1998102105
- S.KalenderF.G.UzunD.DurakF.DemirY.KalenderMalathion-induced hepatotoxicity in rats: the effects of vitamins C and EFood Chem Toxicol482010633638