Abstract
Background
Breast cancer and HCV are two frequent diseases in Egypt. There is a considerable probability of concurrent affection. This concurrence creates a subpopulation, which needs special evaluation and care.
Objective
To evaluate a subset of Egyptian breast cancer patients receiving Doxorubicin based adjuvant chemotherapy, with HCV seropositivity (group 2) compared to HCV seronegative patients (group 1).
Methods
102 breast cancer patients, planned to receive Doxorubicin based adjuvant chemotherapy, at the Oncology Department, Alexandria Faculty of Medicine, were recruited since June 2009. Pretreatment evaluation included serological testing for HCV. FAC Adjuvant chemotherapy was given for six cycles.
Results
HCV seropositivity was detected in 52 cases. Two cases in the seropositive group developed toxic hepatitis and discontinued treatment and follow up. The remaining 100 patients suffered comparable toxicities, except for more frequent liver enzyme elevations in the seropositive group. Diarrhea was also more frequent in the seropositive group. Treatment delays and dose reductions were more frequently observed in the seropositive group. The 36 month disease-free survival and relapse pattern were not significantly different between the two groups.
Conclusion
Patients receiving chemotherapy should undergo screening for the virus. Most patients with HCV were able to tolerate chemotherapy and continue the initial chemotherapy plan, without a significant change in the toxicity profile or the natural course of their malignancy. Dose or regimen adjustments may be of help to less tolerant patients. A preemptive 10% initial Doxorubicin dose reduction might reduce the frequency of severe toxicity for selected patients. The assistance of a gastroenterologist in HCV positive breast cancer patients, planned for chemotherapy is important.
1 Introduction
Breast cancer is a major medical problem with significant public health and societal ramifications. It is the most common cancer in women.Citation1 In the latest cancer statistics published by the American Cancer Society in early 2014, the incidence of breast cancer in the United States is over 230,000 cases each year and it is responsible for over 40,000 deaths.Citation2 Breast cancer incidence rates are highest in more developed countries with lower incidence in less developed countries.Citation3,Citation4
In a hospital-based Egyptian cancer statistics report of the NCI (Cairo), breast cancer constituted 18.9% of total cancer cases (35.1% in women and 2.2% in men) and was the most common cancer among women in this series.Citation5 The diagnosis was made at a younger median age than in European and North American countries, with a relatively advanced tumor presentation.Citation6 Such a disease profile emphasizes the role of systemic treatment in the multidisciplinary management of breast cancer patients in Egypt.
The Early Breast Cancer Trialists’ Collaborative Group overview of adjuvant chemotherapy trials has shown an “increase in survival benefit over time from polychemotherapy versus no adjuvant chemotherapy, twice as great at 15 years as it was at five years (10 versus 4.7%)”, in women under the age of 50.Citation7 Young and old women have shown similar proportional reductions in recurrence and breast cancer-specific mortality.Citation8
Among the most widely used adjuvant chemotherapy regimens, anthracycline-based therapy has become a standard, unless the patient had a contraindication to anthracyclines.Citation9,Citation10 Toxicities from adjuvant chemotherapy must be weighed in the balance between the survival gains versus quality of life impact in premenopausal and postmenopausal patients.Citation11
1.1 Chemotherapy and liver disease
Chemotherapy can have a direct hepatotoxic effect or may trigger a worsening of preexisting liver disease, especially viral hepatitis. Conversely, a diseased liver will result in alteration of the metabolism and excretion of chemotherapy drugs that rely upon the liver for their clearance. This can result in increased systemic toxicity or worsening of chemotherapy-induced hepatotoxicity.Citation12 Cyclophosphamide is metabolized by the liver to the cytotoxic metabolite. It is infrequently hepatotoxic.Citation13,Citation14 Increased toxicity has not been reported in patients with hepatic dysfunction.Citation15 Doxorubicin is extensively metabolized in the liver; and 80% of each dose is excreted in the bile. Isolated case reports have suggested that Doxorubicin can cause hepatotoxicity.Citation15 Patients with cholestasis have delayed clearance of Doxorubicin and its metabolites and develop greater systemic toxicity from standard doses.Citation16 5-Fluorouracil is metabolized in tissues to its active form. Approximately 10% of administered 5-FU is excreted unchanged in the urine, while the remainder is enzymatically catabolized primarily in the liver. Rare reports of hepatotoxicity have been noted with intravenous 5-fluorouracil.Citation17
Liver injury is primarily assessed by liver function tests. Radiological studies may be needed to differentiate drug toxicity from biliary, vascular, or tumor-related conditions.Citation18 Criteria have been developed to grade the severity of treatment-related abnormalities in liver function tests and adjust the dose in patients undergoing chemotherapy.Citation15
1.2 Hepatitis C virus
HCV infection has become an increasingly serious health problem in different parts of the world.Citation19 Egypt has possibly one of the highest HCV prevalence rates in the world. HCV is the leading cause of HCC and chronic liver disease in the country.Citation20 It is reported that more than 50% of infected people are unaware of their disease, leading to the spread of infection and lost treatment opportunities.Citation21
Modern serological HCV diagnostic tests have good sensitivity and specificity particularly in high risk patient groups and their use for screening in these populations has been recommended.Citation22 Given the prevalence rates of HCV and breast cancer in Egyptians, a significant chance of a concurrent affection is present and warrants screening in breast cancer cases planned for treatment.
This study was conducted to evaluate the experience of Egyptian breast cancer patients receiving Doxorubicin based adjuvant chemotherapy, in association with HCV seropositivity compared to HCV seronegative patients.
2 Patients and methods
This observational cohort study was conducted after the approval of the IRB of Alexandria Faculty of Medicine in April 2009 (IRB No: 00007555-FWA No: 00018699).Footnotea It included a total of 102 pathologically proven breast cancer patients, recruited since June 2009, who were planned to receive Doxorubicin based adjuvant chemotherapy, at the Oncology Department, Alexandria Faculty of Medicine, Alexandria University. They were chosen according to the following criteria:
| 1. | World Health Organization (WHO) performance status 0–2.Citation23 | ||||
| 2. | Adequate bone marrow function (white blood cell count > 3000/dl, | ||||
| 3. | platelet count > 100,000/dl and hemoglobin > 9gm /dl) | ||||
| 4. | Adequate liver and renal functions (⩽1.5 times the upper limit of normal) | ||||
| 5. | Age 20–70 years at the time of inclusion | ||||
| 6. | A signed consent obtained from each patient before inclusion in this study, as required by the IRB. | ||||
| 7. | LVEF more than 50% measured by echocardiography. | ||||
Patients were excluded, if they had one or more of the following conditions:
| 1. | Pregnancy and lactation. | ||||
| 2. | Any cardiac disease, or a serious medical or psychiatric condition. | ||||
| 3. | Previous liver disease or primary or secondary hepatic malignancy. | ||||
| 4. | Refusal of treatment. | ||||
All patients included in this study were subjected to pretreatment clinical, radiological and laboratory evaluation, including peripheral blood count, kidney and liver function tests. HCV antibody testing using Enzyme Linked Immunosorbent Assay (ELISA) was performed on all the patients and accordingly they were classified into 2 groups, the first group included 50 HCV negative patients (group 1) and another group including 52 HCV positive patients (group 2).
All patients were intermediate and high risk breast cancer and were planned to receive anthracycline chemotherapy in the adjuvant setting which started from 4–6 weeks after surgery. The surface area was calculated in both groups by the formula of Mosteller using both the weight and height of each patient to calculate the dose of chemotherapy given to each patient.Citation24 All patients received CAF regimen (cyclophosphamide 500 mg/m2, adriamycin 50 mg/m2 and 5-flurouracil 500 mg/m2) given intravenously on day 1 of each cycle. The cycles were repeated every 21 days for a total of 6 cycles. All patients received antiemetic premedication with Ondansteron, Ranitidine and Dexamethasone by intravenous route, 15 min before starting the chemotherapy.
On day 1 of each cycle, each patient was assessed clinically and by laboratory investigations including peripheral blood count, serum kidney and liver function tests. Toxicities were recorded according to the Common Terminology Criteria Adverse Event version 4.Citation23 Dose modifications and/or treatment delay if needed were done according to the laboratory findings, with the guidelines for each individual drug.Citation25 Ejection fraction was measured after finishing the six cycles. An estimate of the average dose given to each group was calculated, with compensation for the average delay per patient, considering a relative effective dose reduction of 25% of a cycle dose for a 1 week delay of that cycle.Citation26
Statistical analysis was performed using SPSS/Win version 16. Chi square and Fisher’s Exact test (if Chi Square test is not applicable in 2 × 2 tables) were done for non-parametric evaluation of data. Odds ratio and 90% confidence interval were calculated for comparing odds of toxicity and delay endpoints. Multiple linear regression was done on factors affecting liver enzyme levels in each cycle. Analysis included HCV status, age, diabetic status, hypertension and menstrual status. For each cycle there was a model which was analyzed for significance. Kaplan Meier disease-free survival curves were plotted.
3 Results
One hundred and two patients were included in the study. Fifty of them were HCV seronegative (group one) and fifty-two were HCV seropositive (group two). Patient characteristics were comparable in both groups with no statistically significant difference ().
Table 1 Patient characteristics.
3.1 Chemotherapy induced toxicity
3.1.1 Laboratory toxicity
There was no statistically significant difference between both groups regarding the frequency and grade of anemia, neutropenia or thrombocytopenia throughout the course of treatment. All patients experienced anemia but none reached grade 3 throughout the course. Grades 3–4 neutropenia were observed after the fifth cycle in 10% (five patients) and 4% (two patients) of group one and two respectively. Grade 1 thrombocytopenia was observed in four patients, two in each group, and was reversible.
Most patients did not suffer any renal impairment. One patient in group two had elevated serum creatinine and blood urea. It reached grade 2 after the first, fourth and fifth cycles but it was still controlled with increased fluid intake. Four other patients had transient elevated blood urea and creatinine, one of them in group one and none of them exceeded grade 1.
Two patients in group two had grade 2 SGOT and SGPT elevation, with manifestations of severe toxic hepatitis. The liver profile for these two patients was not corrected by liver support and they discontinued their chemotherapy. Differences between the two groups in the grade and frequency of liver enzyme elevations started to be significant after the third cycle of chemotherapy ( and ). Throughout the whole six cycles of chemotherapy, 42% (twenty-one patients) of group two patients developed elevated SGOT compared to 28% in group one (OR = 4.125, 90% CI: 1.8742–9.0788), all of these patients were grade 1 and 2. As regards SGPT level, 34% of patients in group two had elevated SGPT level compared to 18% in group one (OR 3.777 with 90% CI from 1.8846 to 7.5728). None of the study population, who continued for the 6 cycles, experienced any levels of elevated bilirubin except for one patient in group two who had grade 1 transient elevation after the sixth cycle.
3.1.2 Clinical toxicity
Nausea was the most common side effect in the whole studied population. It was more frequent in group one patients. The incidence of high grade nausea was similar in both groups, except after the fourth cycle, where 28% (fourteen patients) and 6% (three patients) had grade 3 nausea in group one and two respectively (p ⩽ 0.001).
Vomiting was the second common side effect among the studied population. The incidence of high grade vomiting was statistically similar in both groups as 44% of the patients in group one had grade 3–4 compared to 40% in group two throughout the whole cycles.
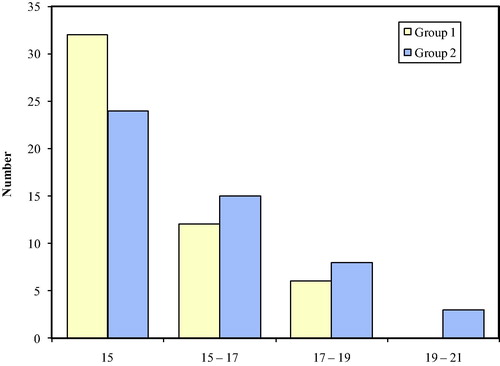
Diarrhea was the clinical toxicity showing the most significant difference between both groups regarding the clinical toxicity profile (). Overall, 28% of the patients in group two experienced grade 3–4 compared to 4% only in group one through the six cycles of chemotherapy. (OR = 14.636, 90% CI 5.5084–38.8902).
Figure 3 The incidence of diarrhea throughout the course of treatment. OR 14.636 (90% CI: 5.5084–38.8902).
Grade 3 stomatitis was more common in group one patients (20% versus 16%). The incidence of stomatitis was higher in group one after cycle V where 62% (thirty-two patients) had stomatitis compared to 44% (twenty-two patients) in group two. This was also observed after the sixth cycle where the incidence of stomatitis was 62% and 48% in group one and two respectively (OR = 5.9341, 90% CI: 2.6278–13.4005).
Mild Skin changes, including skin discoloration and nail changes, not exceeding grade 2, were more frequent in group one and increased steadily throughout the course of treatment. By the end of the sixth cycle, 100% and 98% of patients of group one and group two respectively had grade 2 alopecia.
After six cycles, cardiac toxicity in terms of grade 1 change in ejection fraction was observed in 70% and 58% of group one and group two respectively, but the difference was not statistically significant (p = 0.211).
3.2 Dose delay and modification
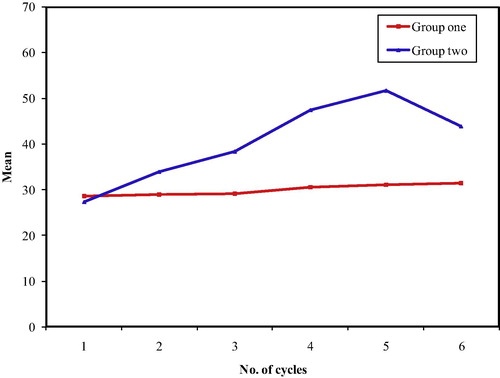
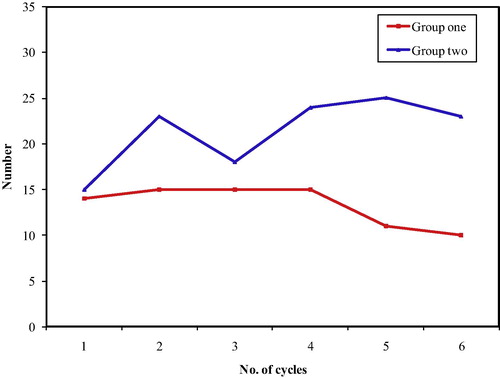
In group one, the range of the overall treatment duration was 15–19 weeks, while in group two the range of the overall treatment duration was a bit wider ranging from 15 to 21 weeks (). The majority of patients in both groups finished their treatment on time. The number of patients with delays was higher in group two (52%) than in group one (36%), excluding the two patients in group two, who had severe toxic hepatitis after the first cycle and discontinued chemotherapy. HCV seronegativity was associated with a lower risk of treatment delays, but the difference is not significant (OR = 0.5192, 90% CI = 0.2651–1.0168).
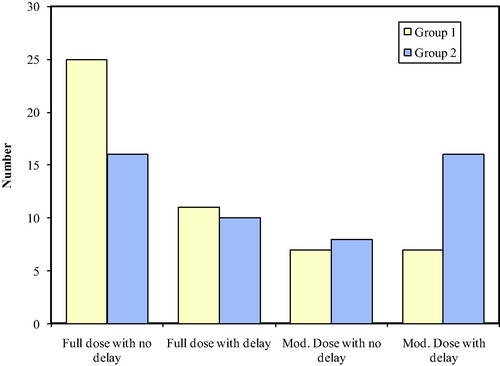
demonstrates that 50% of the patients in group one finished their course without delays or dose modification compared to 32% in group two, with OR = 2.125 (90% CI = 1.0744 to 4.2031). Dose delays without dose modification or dose modification without delay were comparable in both groups (22% vs. 20% and 14% versus 16%). Patients in group one had lower odds for dose delays >17 weeks in spite of dose modification in 14% compared to 32% in group two, with OR = 0.3459 (90% CI = 0.15–0.7977).
Figure 5 Treatment delay > 17 weeks and dose modification OR for no delay and no dose modification = 2.125 (90% CI = 1.0744–4.2031). OR for delay > 17 weeks, in spite of dose modification (in HCV negative patients) = 0.3459 (90% CI = 0.15–0.7977).
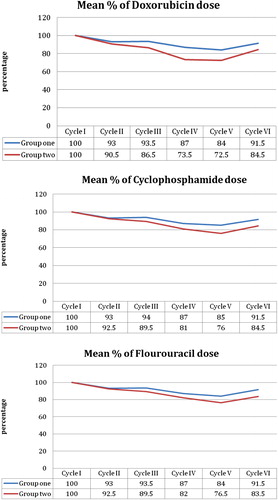
shows the mean percentage of the dose given for each drug throughout the six cycles in both groups. The difference between both groups is best demonstrated in the Doxorubicin dose graph, which is considered to be the most hepatotoxic of the three components of the protocol. The dose given for all the studied population is gradually reduced in each consecutive cycle until reaching the fifth cycle where the difference between both groups is best demonstrated. The estimate of the average dose given to each group calculated, with compensation for the average delay per patient, is reported in . The maximum delay and dose reduction affected the Doxorubicin relative dose. The other 2 drugs were less affected. The average relative DI of FAC in HCV seropositive patients compared to seronegative patients is 92%. Multiple linear regression models were not significant in the first two cycles. After the third cycle and onward, the model was significant for the level of SGOT and SGPT, where p was <0.01. HCV status was the significant predictor, for SGOT and SGPT increased levels, except for SGPT in cycle 6. Age had an inverse impact on SGOT for cycles 4, 5, and 6. The inverse effect of age on SGPT was apparent in cycles 4 and 6 only.
Table 2 Relative dose and relative dose intensity in HCV positive patients versus HCV negative patients.
3.3 Disease-free survival and patterns of recurrence
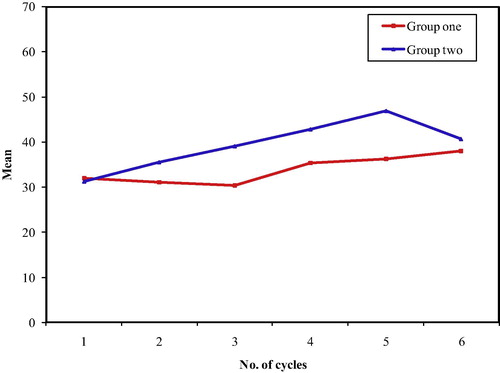
Of the 100 patients who were able to continue the 6 cycles of chemotherapy, 6 patients in the HCV seronegative group and 15 patients in the HCV positive group were untraced and lost follow up. Kaplan–Meier disease free survival curves () were plotted for the remaining patients and showed no significant difference between both groups (Log Rank test: 0.26, p = 0.61), but the pattern of relapse was similar in both groups (), with no statistically significant difference.
Figure 7 Kaplan–Meier 3-year disease-free survival. HCV seronegative (![]()
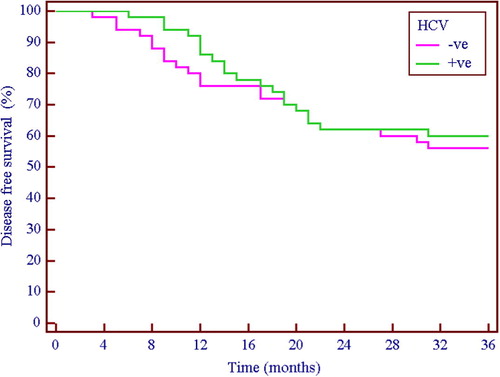
Table 3 Pattern of failure.
4 Discussion
Receiving chemotherapy is a very distressing and concern-provoking stage of the treatment of breast cancer, associated with decrease in overall quality of life.Citation27,Citation28 Endemic HCV infection can be exacerbated with cytotoxic agents.Citation29 HCV positive patients receiving chemotherapy are already burdened with associated comorbidities and may be less tolerant to adverse events of the chemotherapy.Citation30
Fallahian et al. reviewed the literature for viral hepatitis reactivation in immune suppressed patients and patients under chemotherapy. The reviewed studies showed a “less clear relationship between chemotherapy and HCV reactivation than HBV”. Mild abnormalities of liver enzymes were reported. HCV appeared to increase the risk of veno-occlusive disease in transplant patients. The authors advocated the use of HCV antiviral treatment for progressive cases of reactivation.Citation31
Torres and Davila in their review on hepatitis virus reactivation in cancer patients stated that: “HCV reactivation seems to be less common than HBV reactivation and is usually associated with a good outcome and low mortality. However, once severe hepatitis develops, as a result of viral reactivation, mortality rates seem to be similar among patients infected with HBV or HCV. Liver damage owing to viral reactivation frequently leads to modifications or interruptions of chemotherapy, which can negatively affect patients’ clinical outcome”.Citation32
In the current prospective cohort study, the difference in the toxicity profile between HCV seropositive and seronegative patients was mainly observed in the form of more frequent reversible transaminase level elevations and more frequent high grade diarrhea. Two cases developed severe hepatitis precluding any further chemotherapy and were excluded from further analysis. There were more delays in the HCV positive group compared to the HCV negative group (52% versus 36%). High grade neutropenia was more frequent in group 2. There was no clear explanation regarding the high incidence of diarrhea in the HCV positive patients compared to other adverse effects. One reason could be attributed to the findings of Fouad et al. which showed that Irritable bowel syndrome was more frequent in HCV positive patients compared to HBV patients and the normal controls.Citation33
Morrow et al. reported an expansion of their retrospective series analyzing the effects of chronic hepatitis C infection on the treatment of 45 HCV positive breast cancer patients treated in MD Anderson Cancer Center (MDACC). Only 36 patients of the 44 received chemotherapy. Patients received different kinds of regimens including anthracyclines, taxanes, and anthracycline plus taxanes, with or without trastuzumab. This cohort was compared to a HCV negative historical control. Of the 36 patients who received chemotherapy, 33 patients were able to complete the initial planned number of cycles and nine patients experienced elevations in the liver enzymes, graded as 1 or 2 toxicity. Twenty percent of their cases had prior antiviral treatment and 39% received colony stimulating factor support during treatment.Citation34
The toxicity profile in the current series and the ensuing dose reduction/dose delays did not adversely affect the pattern of relapse or disease-free survival. However, this observation should be taken cautiously for two main reasons, firstly is the short term follow up of the patients (36 months), longer follow up is needed to accurately monitor the effect of dose delay and modification especially in the seropositive group, secondly, the number of untraced cases and their unequal distribution between the two groups may distort the results.
4.1 Conclusion
HCV is an endemic infection in Egypt and all patients receiving chemotherapy should undergo screening for the virus. Most patients with HCV were able to tolerate chemotherapy well and continue the initial chemotherapy plan, without significant change in the toxicity profile or the natural course of their malignancy. Close monitoring of the patients would help diagnose and promptly manage those with less tolerance to treatment. Dose or regimen adjustments may be of help to such patients. A preemptive 10% initial Doxorubicin dose reduction might reduce the frequency of severe toxicity and the consequent dose reductions and delays. It is important to seek the assistance of a hepatologist/gastroenterologist in evaluating any HCV positive breast cancer patient who is planned to receive chemotherapy.
Conflict of interest
None declared.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 10 September 2014
a Institutional Review Board Information http://ohrp.cit.nih.gov/search/IrbDtl.aspx
References
- T.J.KeyP.K.VerkasaloE.BanksEpidemiology of breast cancerLancet Oncol232001133140
- R.SiegelJ.MaZ.ZouA.JemalCancer statistics, 2014CA Cancer J Clin6412014929
- A.JemalF.BrayM.M.CenterJ.FerlayE.WardD.FormanGlobal cancer statisticsCA A Cancer J Clin6120116990
- GLOBOCAN 2012, IARC–18.4.2014, (accessed on April 18, 2014) Available online: http://globocan.iarc.fr/Pages/summary_table_site_prev_sel.aspx.
- Elatar I. Cancer Registration, NCI Egypt, 2001. National Cancer Institute; Cairo, Egypt: 2002, (accessed on April 18, 2014). Available online: http://www.nci.edu.eg/Journal/nci2001%20.pdf.
- S.OmarH.KhaledR.GaafarA.R.ZekryS.EissaO.el-KhatibBreast cancer in Egypt: a review of disease presentation and detection strategiesEast Mediterr Health J92003448463
- Early Breast Cancer Trialists’ Collaborative GroupEffects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15-year survival: an overview of the randomised trialsLancet365200516871717
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG)M.ClarkeA.S.CoatesS.C.DarbyC.DaviesR.D.GelberAdjuvant chemotherapy in oestrogen-receptor-poor breast cancer: patient-level meta-analysis of randomised trialsLancet37120082940
- C.J.PooleH.M.EarlL.HillerJ.A.DunnS.BathersR.J.GrieveEpirubicin and cyclophosphamide, methotrexate, and fluorouracil as adjuvant therapy for early breast cancerN Engl J Med355200618511862
- L.BertelsenL.BernsteinJ.H.OlsenL.MellemkjærR.W.HaileC.F.LynchEffect of systemic adjuvant treatment on risk for contralateral breast cancer in the Women’s Environment, Cancer and Radiation Epidemiology StudyJ Natl Cancer Inst10020083239
- B.F.ColeR.D.GelberS.GelberA.S.CoatesA.GoldhirschPolychemotherapy for early breast cancer: an overview of the randomized clinical trials with quality-adjusted survival analysisLancet3582001277285
- W.M.LeeDrug-induced hepatotoxicityN Engl J Med3492003474485
- D.A.AubreyMassive hepatic necrosis after cyclophosphamideBr Med J31970588 (Correspondence)
- L.S.SnyderR.I.HeighM.L.AndersonCyclophosphamide-induced hepatotoxicity in a patient with Wegener’s granulomatosisMayo Clin Proc68199312031209
- P.D.KingM.C.PerryHepatotoxicity of chemotherapyOncologist62001162176
- S.C.PiscitelliK.A.RodvoldD.A.RushingD.A.TewksburyPharmacokinetics and pharmacodynamics of doxorubicin in patients with small cell lung cancerClin Pharmacol Ther531993555564
- J.R.BatemanR.P.PughF.R.CassidyG.J.MarshallL.E.Irwin5-Fluorouracil given once weekly: comparison of intravenous and oral administrationCancer281971907
- D.S.PrattM.M.KaplanEvaluation of abnormal liver-enzyme results in asymptomatic patientsN Engl J Med342200012661279
- Hepatitis C Fact sheet No 164 (Updated April 2014), (accessed on April 18, 2014). Available online http://www.who.int/mediacentre/factsheets/fs164/en/index.html.
- O.G.PybusA.J.DrummondT.NakanoB.H.RobertsonA.RambautThe epidemiology and iatrogenic transmission of hepatitis C virus in Egypt: a Bayesian coalescent approachMol Biol Evol2032003381387
- A.CraxìG.LaffiA.L.ZignegoHepatitis C virus (HCV) infection: a systemic diseaseMol Aspects Med29220088595
- C.ColinD.LanoirS.TouzetL.Meyaud-KraemerF.BaillyC.Trepo HEPATIS Group Sensitivity and specificity of third generation hepatitis C virus antibody detection assays: an analysis of the literatureJ Viral Hepat820018795
- Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 pdf, (accessed on June 28, 2014). Available online, http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf.
- R.D.MostellerSimplified calculation of body-surface areaN Engl J Med317198710981106
- V.NoronhaD.TiedmanE.ChuGuidelines for chemotherapy and dose modificationV.T.DeVitaChuPhysicians cancer chemotherapy manual2007Jones and BartletUSA379391
- W.M.HryniukM.GoodyearThe calculation of received dose intensityJ Clin Oncol8199019351937
- N.K.AroraD.H.GustafsonR.P.HawkinsF.McTavishD.F.CellaS.PingreeImpact of surgery and chemotherapy on the quality of life of younger women with breast carcinoma. A prospective studyCancer92200112881298
- P.A.GanzK.DesmondB.LeedhamJ.RowlandB.MeyerowitzT.BelinQuality of life in long-term, disease-free survivors of breast cancer: a follow-upJ Natl Cancer Inst9420023949
- T.KawataniT.SuouF.TajimaIncidence of hepatitis virus infection and severe liver dysfunction in patients receiving chemotherapy for hematologic malignanciesEur J Haematol6720014549
- H.B.El-SeragH.HampelC.YehL.RabeneckExtrahepatic manifestations of hepatitis C among United States male veteransJ Hepatol36200214391444
- F.FallahianS.M.AlavianV.FallahianF.ZamaniImpact of immunosuppression and chemotherapy on reactivation of Viral hepatitisSaudi J Kidney Dis Transplant212010621627
- H.A.TorresM.DavilaReactivation of hepatitis B virus and hepatitis C virus in patients with cancerNat Rev Clin Oncol92012156166
- Y.FouadM.MakhloufH.KhalafZ.MostafaA.Abdel RaheemW.MeneasiIs irritable bowel syndrome associated with chronic hepatitis C?J Gastroenterol Hepatol25201012851288
- P.MorrowJ.TarrandS.TaylorS.KauR.TheriaultG.HortobagyiEffects of chronic hepatitis C infection on the treatment of breast cancer patientsAnn Oncol21201012331236