?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
To evaluate the role of the detection of ischemia modified albumin (IMA) level in the differentiation between ischemic and hemorrhagic cerebrovascular stroke.
Methods
Sixty elderly persons classified into three groups, 25 patients diagnosed with cerebral infarction, 15 patients diagnosed with cerebral hemorrhage, and 20 elderly healthy persons with matched age as control were enrolled in the study. IMA was measured using the available chemical method and computerized tomography (CT) was done for diagnosis of brain lesions.
Results
IMA was significantly higher in the patient group than in the control group. There was a positive significant correlation between age, albumin with IMA, (P = 0.000 and 0.037 respectively). However there was no statistical significant difference between sex and diagnosis cross tabulation (0.51). It was found that, IMA was statistically higher in the infarction group than the hemorrhage group (P = 0.000) and IMD index was statistically higher in the infarction group than the hemorrhage group (P = 0.013).
Conclusion
Our investigation in elderly patients suggests that IMA assay is a sensitive marker for early detection of ischemic and hemorrhagic stroke.
1 Introduction
A stroke, previously known medically as a cerebrovascular accident (CVA) is the rabid loss of brain function(s) either focal or global due to disturbance in the blood supply to the brain, with symptoms lasting 24 h or longer or leading to death with no apparent cause other than of vascular origin.Citation1 The 24 h limit differentiates stroke from transient ischemic attack, which is a related syndrome of stroke symptoms that resolve completely within 24 h.Citation2
The incidence of stroke increases exponentially from 30 years of age, and etiology varies by age.Citation3 Advanced age is one of the most significant risk factors. 95% of strokes occur in people aged 45 years and older, and two-thirds of strokes occur in those over the age of 65 years. As the patient age increases the risk of dying increases.Citation4 Family members may have a genetic tendency for stroke or share a lifestyle that contributes to stroke.Citation5
Stroke can be classified into two major categories: ischemic and hemorrhagic. About 87% of strokes are caused by ischemia and the remainder by hemorrhage. Some hemorrhages develop inside areas of ischemia (hemorrhagic transformation).Citation6 In ischemic stroke, blood supply to part of the brain is decreased, leading to dysfunction of the brain tissue in that area. There are four main reasons why this might happen: thrombosis (obstruction of a blood vessel by a blood clot formed locally), embolism (obstruction due to embolus formed elsewhere in the body),Citation7 systemic hypo perfusion (general decrease in the blood supply e.g. in shock) (global ischemia),Citation8 and venous thrombosis. Stroke without an obvious explanation is termed cryptogenic stroke and this constitutes 30–40% of all ischemic strokes.Citation9
As regards hemorrhagic stroke, it is caused by accumulation of blood anywhere within the skull vault. A distinction is made between intra-axial hemorrhage (blood inside the brain either intra-parenchymal or intra-ventricular hemorrhages) and extra-axial hemorrhage (blood inside the skull but outside the brain e.g. epidural hematoma, subdural hematoma, and subarachnoid hemorrhage).Citation10
A growing body of investigation supporting the potential of ischemia modified albumin (IMA) as a marker of ischemia is now available. Human serum albumin (HSA) is the most abundant protein in the blood with a mean concentration of 0.63 mmol/L. It is synthesized in the liver and has a half life of about 19 days. HSA has a unique structure and amino acid sequence which is specific to humans at its amino terminus (N-terminus).Citation11 Previous studies have shown the N-terminus of HSA to be the primary binding site for the transitional metals cobalt and copper. The HSA metal binding site is particularly susceptible to biochemical changes during ischemia compared to albumin from other species.Citation12
The precise mechanisms for production of IMA during ischemia are not known, but have localized modification in the amino terminal of HSA during ischemia which leads to reduction in cobalt binding to this modified N-terminus.Citation13 Many reports indicate that the factors involved in ischemia that can induce these in vivo changes to albumin may include: acidosis, free radical damage, membrane energy dependent sodium and calcium pump disruption, reduced oxygen tension and free iron and copper ion exposure.Citation14 These conditions necessary for altering the metal binding site of HSA are known to occur within minutes of the onset of ischemia, and their effect on albumin could be detectable up to 6 h after the ischemic event.Citation15
In the current study we tried to evaluate the detection of ischemia modified albumin level in the differentiation between ischemic and hemorrhagic cerebrovascular stroke.
2 Methods
From May 2011 to January 2013, this study was conducted in the Internal Medicine Department, Alexandria University Hospital, Egypt; after being approved from the local Research Ethics Committee, and informed consent was obtained from all participants. We studied 40 elderly persons recruited from the emergency department with a mean age of 49.0 ± 7.27 (range 40–75) years with cerebrovascular stroke (Group I included 25 patients with cerebral infarction and Group II included 15 patients with cerebral hemorrhage). In addition, 20 apparently healthy elderly persons (Group III) matched for age were included as controls. The exclusion criteria included cardiac diseases, cancer, infections, end stage renal disease, liver disease, uncontrolled diabetes mellitus and history of treatment of thyroid disease. All subjects were subjected to:
| 1. | Full history taking. | ||||
| 2. | Complete clinical examination especially for signs of cerebrovascular stroke (paresis, paralysis, loss of sensation, abnormal speech). | ||||
| 3. | Radiological investigations: Computerized tomography (CT) for diagnosis of brain lesions. | ||||
| 4. | Blood samples were collected by venipuncture tubes within two hours of arrival. These samples were sent to the laboratory and processed for: routine laboratory tests (liver function tests, renal function). Blood samples were collected before any heparin/thrombolytic treatment is started. Patients received routine institutional care according to their diagnosis blinded to the IMA results. IMA was measured using spectrophotometric albumin cobalt binding assay. The assay is based on the fact that ischemia causes changes in human serum albumin that are demonstrated by reduced exogenous cobalt binding. The concentration of IMA can be determined by addition of a known amount of exogenous cobalt (CoCl2) to a serum specimen and measurement of unbound cobalt using a colorimetric assay after adding a coloring substance (dithiothreitol) which binds any excess (unbound) cobalt. An inverse relationship thus exits between the amount of albumin bound cobalt and the intensity of the color formation. All reactions were carried out at room temperature and in duplicate. | ||||
2.1 Test procedure
Addition of 200 μL of the patient serum, 50 μL of CoCl2, followed by good mixing and incubation for 10 min were carried out. Then 50 μL of DTT working solution (1.5 g/L DTT solution) was added and mixed. After a period of 2 min incubation, 1.0 ml of 9.0 g/L solution of NaCl was added. The absorbance of this assay mixture was read at 470 nm using the 5010 Spectrophotometer (after reading the blank). Each sample was read in duplicate, with average absorbance taken and recorded in absorbance units (ABSU0) using PD-3035 Apel Japan. As regards result interpretation: cases with absorbance greater than 0.400 ABSU were considered positive for IMA, while cases with absorbance less than 0.400 ABSU were considered negative for IMA. IMA index was calculated by using the following equation ((IMA index = serum albumin concentration (g/dl) × 23 + IMA (U/ml) − 100)).
2.2 Statistical analysis
The clinical and laboratory results obtained are statistically analyzed using SPSS/PC (version 4) (Statistical Package for Social Science for Personal Computer). The value of LSD (least significant difference) indicates the difference within the group, if LSD is more than the difference between the mean of any two groups, it is an indication of significance.
3 Results
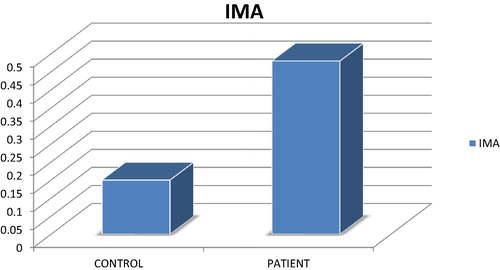
shows diagnosis in relation to groups. There was a statistical difference between control and patients regarding diagnosis (P = 0.000). shows IMA ranged between 0.10 and 0.30 with the mean of 0.1872 ± 0.049 and 0.5083 ± 0.111 for control and patients respectively. The patient group has statistically higher values than the control group (P = 0.000) as shown in . IMA index ranged between 87.52–124.42 and 87.79–124.80 with the mean of 104.7223 ± 11.81 and 110.3908 ± 10.019 for control and patient groups respectively. The patient group had statistically higher values than the control group (P = 0.047) (see ).
Case processing summary (ROC curve);
Groups (b) positive (a) negative;
Valid N (list wise) 40 20.
Table 1 Diagnosis in relation to groups.
Table 2 IMA and IMA index in the studied groups.
Large values of the test result variable(s) indicate stronger evidence for a positive actual state. (a) The positive actual state is patients, (b) the test result variable: IMA has at least one tie between the positive actual state group and the negative actual state group.
Area under the curve
Test result variable(s): IMA;
Area .996;
Std error (a) 005;
Asymptomatic;
Sig. (b) .000;
Asymptomatic 95% Confidence Interval
Lower bound .987;
Upper bound 1.005;
(a) Under the nonparametric assumption. (b) Null hypothesis: true area = 0.5;
Coordinates of the curve;
Test result variables: IMA;
Positive if greater than or equal to (a) .2800
Sensitivity 975;
Specificity 050.
(a) The smallest cutoff value is the minimum observed test value minus 1, and the largest cutoff value is the maximum observed test value plus 1. All the other cutoff values are the averages of two consecutive ordered observed test values.
Sex in relation to diagnosis cross-tabulation is found in ; it showed that there were no statistical significant differences between sex and diagnosis cross tabulation.
Table 3 Sex in relation to diagnosis cross-tabulation.
IMA ranged between 0.43–0.80 ABSU and 0.23–0.49 ABSU with the mean of 0.5698 ± 0.0087 and 0.4059 ± 0.06033 for infarction and hemorrhage respectively. The infarction group has statistically higher values than the hemorrhage group (P = 0.000). As regards IMA index, it ranged between 87.79–122.0 and 88.0–124.8 with the mean of 112.6 ± 6.98 and 107.6 ± 11.3 for infarction and hemorrhage respectively. The infarction group has statistically higher values than the hemorrhage group (P = 0.013). These findings are represented in .
Table 4 Shows relations between the diagnosis and IMA, IMA index.
4 Discussion
Gunduz et al.Citation17 evaluated time course of IMA in acute ischemic stroke (AIS) patients to validate its prognostic value. IMA level was estimated in serum samples collected from five AIS patients at admission, 24 h, 48 h, 72 h, and 144 h after admission and also from five control subjects. There was a significant increase in IMA level in AIS samples at admission, 24 h, 48 h and 144 h respectively when compared with control. On comparing IMA levels in follow up AIS samples with that of admission values we found that it decreased in follow up samples till 72 h, and a significant (P < 0.05) decrease was observed at 24 h and 72 h. Their findings show that follow up estimation of IMA level in AIS may help in the prediction of the clinical status and outcome. Also there was a positive significant correlation between IMA and age in this study. IMA ranged between 0.43–0.80 and 0.23–0.49 with the mean of 0.4059–0.06033 for infarction and hemorrhage respectively. The infarction group has values statistically higher than the hemorrhage group. Although IMA is a sensitive marker for ischemia, as seen in our study, its sensitivity decreases especially in conditions associated with transient and reversible ischemia. Another factor responsible for these false negative IMA values is the presence of lactic acid in these patients secondary to prolonged ischemia and acidosis. Elevated lactic acid levels have been shown to be associated with a decrease in IMA levels, the cause of which is not known. The third possible cause for false negative IMA, may be delayed presentation to the ER.Citation17
Few studies had assessed the role of IMA in stroke patients. In the study of Gunduz et al. 8 patients in group B had an elevated IMA in the absence of cardiac ischemia. Six of these 8 patients presented with features of transient ischemic attack and progressed to develop ischemic stroke. However, their IMA levels were elevated at the time of presentation. Chi-square analysis revealed a significant association of elevated IMA levels with a diagnosis of ischemic stroke (P = 0.0029). Our results were agreement in with a preliminary study by Sameer et al. which showed that IMA is a biomarker for early identification of acute ischemic stroke.Citation16
Mentese et al.Citation18 reported that IMA concentrations are significantly lower immediately after exercise-induced leg ischemia in patients with peripheral vascular disease. Two previous studies have assessed the effect of skeletal muscle ischemia on serum IMA levels in apparently healthy individual. A transient decrease in IMA concentration has been observed immediately after exercise and/or skeletal muscle ischemia, followed by a delayed increase after 24–48 h. It has been hypothesized that the immediate decrease may be attributed to interference in the IMA measurement by lactate produced during skeletal muscle ischemia.
Since susceptibility of the cells to ischemia may vary from one organ to another, it would be critical to determine the optimal IMA level for diagnosis of ischemia in various organs especially the heart and the brain. Mentese et al.Citation18 showed that an increasing number of studies have shown that IMA levels rise in a number of acute ischemic conditions such as cerebral infarction, myocardial infarction, pulmonary infarction and mesenteric infarction, suggesting that IMA may be useful as a diagnostic marker. IMA is a promising marker to be considered for use in emergency department in conjunction with CT brain for the diagnostic assessment of suspected stroke to exclude stroke in patients with low clinical probability.Citation19
5 Conclusion
IMA assay is a sensitive marker for early detection stroke. The level of IMA is higher in ischemic stroke than hemorrhagic stroke.
Conflict of interest
We have no conflict of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 6 March 2015
References
- G.A.DonnanM.FisherM.MacleodS.M.DavisStrokeLancet3719624200816121623
- C.S.KidwellS.WarachAcute ischemic cerebrovascular syndrome: diagnostic criteriaStroke3412200329952998
- B.IndredavikA.TarentEpidemiology of stroke in inhered, Norway, 1994 to 1996: incidence and 30-day case fatality rateStroke2811199821802184
- T.BongersM.De MaatM.Van GoorHigh Von Willbrand factor levels increase the risk of first ischemic stroke: influence of ADAMTS 13, inflammation and genetic variabilityStroke3711200626722677
- N.R.SimsH.MuydermanMitochondria, oxidative metabolism and cell death in strokeBiochim Biophys Acta1802120098091
- J.StamThrombosis of cerebral veins and sinusesN Engl J Med35217200517911798
- J.M.BamfordThe role of clinical examination in the subclassification of strokeCerebrovas Dis10Suppl 4200024
- A.ShuaibV.C.HachinskiMechanisms and management of stroke in the elderlyCMAJ14551991433443
- F.GuerciniM.AcciarresiM.PaciaroniCryptogenic stroke: time to determine etiologyJ Thromb Haemost642008549554
- L.P.GoldsteinD.L.SimelIs this patient having a stroke?JAMA29319200523912402
- N.V.BhagavanE.M.LaiP.A.RiosEvaluation of human serum albumin cobalt binding assay for the assessment of myocardial ischemia and myocardial infarctionClin Chem492003581585
- B.ChanN.DodsworthJ.WoodrowA.TuckerR.HarrisSite specific N-terminal auto degradation of human serum albuminEur J Biochem2271995524528
- R.H.ChristensonS.H.DohW.R.SanhaiV.HoltmanCharacteristics of an albumin cobalt binding test for assessment of acute coronary syndrome patients: a multicenter studyClin Chem472001464470
- E.BerenshteinB.MayerC.GoldbergN.KitrosskyM.ChevionPatterns of mobilization of copper and iron following myocardial ischemia. Possible predictive criteria for tissue injuryJ Mol Cell Cardiol29199730253034
- EftihiaSbarouniPanagiotaGeorgiadouT.H.DimitriosIschemia modified albumin: is this marker of ischemia ready for prime time use?Hellenic J Cardiol492008260266
- H.Sameer AbboudJ.LabreucheE.MeseguerIschemia modified albumin in acute strokeCerebrovasc Dis232–32008216220
- A.GunduzS.TurdiA.MeneteseS.C.KarahanIschemia modified albumin levels in cerebrovascular accidentsAm J Emerg Med26112011874878
- A.MenteseS.TurdiM.TophasEffect of deep vein thrombosis on ischemia modified albumin levelsEmerg Med J25122008811814
- L.KeatingJ.R.BengerR.BeethamThe PRIMA study: presentation ischemia modified albumin in the emergency departmentEmerg Med J232006267268