?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A simple methodology was developed to evaluate binding efficiency of antibiotic members of fluoroquinolones, namely ciprofloxacin, ofloxacin and enorfloxacin, complexed with 99mTc, against Escherichia coli, Salmonella and Pseudomonas aeruginosa bacterial strains. Radioactivity in the pellet, tips supernatant and micro-centrifugation tubes was counted separately for 5 s in a sodium iodide well-counter with dedicated nuclear medicine software. The overall percentage activity of the live and killed bacteria was found in the range of 5–46% with different types of labelled quinolones and bacteria. Activity of the labelled enorfloxacin and ciprofloxacin indicated acceptable results for both live and killed E. coli and Ps. aeruginosa. However, ofloxacin was found to be moderate for all the live bacterial stains. This developed methodology has achieved more than 95% labelling efficiency of 99mTc with derivatized quinolones and also the observed results indicated that these complexes may be used as an infection specific imaging agents.
1 Introduction
Scintigraphic imaging of infection and inflammation is a powerful diagnostic tool in the management of patient with infectious diseases. Most infections and inflammatory foci may be seen accurately with radio labelled autologous leukocytes. The preparation of these radiopharmaceuticals is laborious and requires the handling of contaminated blood. Few radiopharmaceuticals are available that could be used instead of radio-labelled leukocytes to scintigraphically visualize infections and inflammation foci, such as 99mTc labelled antigranulocyte antibody and 67Ga-citrate.Citation1 Various agents labelled with 99mTc have been developed for this application. Most of these newly developed agents are those that bind receptors on white blood cells subpopulations, i.e. monoclonal antibodies, chemotactic peptides and cytolines.Citation1 The ideal radiopharmaceutical for imaging infection/inflammation should be efficiently accumulated and has good retention in inflammation foci. Moreover, it should have rapid clearance from the background with no accumulation in non-inflamed tissues, no side effects, low cost (99mTc), easy preparation (kit formation), and should have the ability to discriminate infection from non-microbial inflammation cells.
67Ga-citrate has been widely used for imaging infection and inflammation ever since its discovery in 1971. On intravenous injection, 67Ga-citrate binds to transferring and this complex extravagates at the site of inflammation because of the locally enhanced vascular permeability.Citation2–Citation4 This use of radio-labelled autologous leukocytes for imaging infection and inflammation has been a major breakthrough in radio-labelled imaging because they rapidly clear from the blood and migrate actively from the circulation into the infected tissues.Citation5–Citation7Also the use of radio-labelled monoclonal antibodies against surface antigens of granulocytes was one of the first attempts to accomplish in-vivo labelling of leukocytes.
Now several monoclonal antibodies reactive with antigens expressed on granulocytes have been developed,Citation1 and each of these anti-granulocyte antibodies with 99mTc allowed the accurate delineation of infection and inflammation. Rubin et al.Citation8 used radio-iodinated IgG as a control in an experiment to image Pseudomonas aeruginosa infection with a radio-labelled monoclonal antibody against a surface epitope of the Pseudomonas bacterium and observed that the control antibody visualized the good infectious focus as the anti-Pseudomonas antibody did. They proposed the use of non-specific IgG as an infection and inflammation imaging agent. The literatureCitation1, Citation5–Citation9 indicated that different radio-labelled compounds like 67Ga-Citrate, radio-labelled leukocytes (white blood cells-WBCs), labelled antigranulocyte antibodies, radio-labelled non-specific human IgG, anti-e-selection antibodies, radio-labelled liposomes, bacterial chemotactic peptides, Interleukin-1, 2 and 8, Tuftsin, platelet factor-4, and antimicrobial peptides have been investigated for imaging infections and inflammations.
None of the agents discussed above can discriminate between infection and inflammation, because of their binding to the DNA gyrase enzyme present in all dividing bacteria. Fluoroquinolones does not accumulate in dead bacteria and non-inflammatory sites. Ciprofloxacin is a fluoroquinolone antimicrobial agent that binds with the gyrase enzyme, even to those resistant to ciprofloxacin. It is statedCitation9 that 99mTc-labelled ciprofloxacin can distinguish between infection and sterile inflammation. Another studyCitation10 indicated that it also shows high accuracy in the detection of bacterial infection. Preliminary studies with sparfloxacin, norfloxacin, pefloxacin, and lomefloxacin derivatives have shown significant in-vitro uptake in bacteria as well as accumulation in sterile inflammatory sites. Critical evaluation of these investigated compoundsCitation11–Citation15 was warranted to ultimately prove their value for specific imaging of infections.
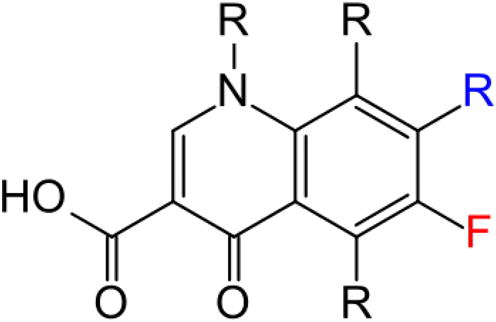
Quinolones are a family of synthetic broad spectrum antibacterial drugs. The essential structure of all quinolone antibiotics is given in . Fluoroquinolones are antibiotics that are commonly used to treat a variety of illnesses such as respiratory and urinary tract infections. These medicines include ciprofloxacin (Cipro), gemifloxacin (Factive), levofloxacin (Levaquin), moxifloxacin (Avelox), norfloxacin (Noroxin), and ofloxacin (Floxin). In the present work, 99mTc labelling has been carried out with derivatized ciprofloxacin, ofloxacin and enorfloxacin to investigate their accumulation in both live and dead Escherichia coli, Salmonella and Ps. aeruginosa bacterial strains.
2 Material and methods
All reagents and solvents used in this study were of reagent grade purity and were used without further purification. All apparatus used were freshly autoclaved and were free from any micro-organisms. Experiments were performed in triplicate and the results reported were the average of triplicates. The isolated bacterial stains, obtained from the department of zoology, University of the Punjab, were identified as E. coli, Salmonella and Ps. aeruginosa bacterial strains, and were incubated in different flask mediums by using the standard conditions of laminar flow chamber.
The bacterial cultures were poured into falcon tubes and centrifuged at 16,000 rpm in freezing conditions for 10 min to convert them into supernatant and pellets. The pellets were dissolved in autoclaved solvent solution. The suspensions were checked for their optical density and diluted enough to give the solvents of 0.14 optical densities which referred to approximately 1.00 × 108 cfu/mL of each stain. The bacteria were not multiplying during the study. Half of each aliquot was taken into a separate sterile tube and was heated at 70 °C for 40 min in a water bath. 1.00 mL sample from all killed suspensions of bacterial stains was placed in sterile glass tubes to confirm their sterility and cell morphology. No liability was found in any of these controls and the morphology was similar to that of the original stains from fresh culture.
Each sample was evaluated after 30 min, 1.0 h and 4.0 h of incubation interval with 99mTc-fluoroquinolones or 99mTc− (blank) at room temperature. Experiments for each labelled fluoroquinolones were conducted on three separate days, so that 12 samples from each group were evaluated. The 99mTc-fluoroquinolone was constituted on experimental days.Citation16
2.1 Pertechnetate kit formation
Each of ciprofloxacin, ofloxacin and enorfloxacin kit contained the following constituents: 3.00 mg of fluoroquinolones, 3.00 mg of cysteine monohydrochloride, 2.00 mg of ascorbic acid, 20.00 mg of NaCl dissolved in 800 μL distilled water, mixed on stirring and followed by addition of 200.00 μg of SnCl2·2H2O from a solution containing 100.00 mg stannous tartrate in 0.4 mL conc. HCl by gentle heating and diluted up to 10 mL with distilled water. pH was adjusted to 4.5 by using 0.1 N HCl/0.1 N NaOH solutions. Stability of pH value was monitored for 15 min during the stirring process. After lyophilization, the freeze dried kits were stored at 4 °C.
2.2 Radio-labelling of kit
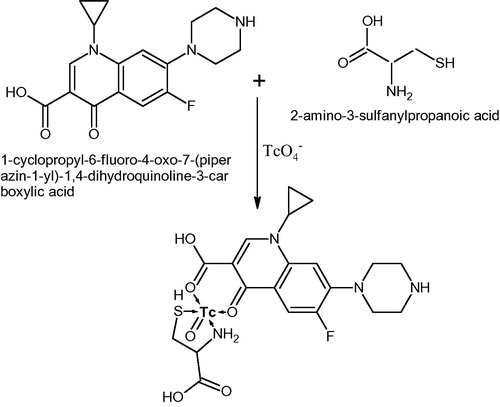
For radio-labelling of the freeze dried kits, sodium per technetate (300 MBQ) was drawn up from a freshly eluted in-house developed ‘Sterile PAKGEN 99Mo/99mTc generator’Citation16 (PINSTECH, Islamabad-Pakistan), and put into the vials containing ciprofloxacin, ofloxacin and enorfloxacin kits by using insulin syringes. The vials were shaken for 30 s, incubated at room temperature for 10–15 min, and volume was brought to 1.00 mL by using saline solution (0.9% NaCl). Quality control of the radio-labelled kits was performed with the help of paper chromatography in acetone and ITLC (instant thin layer chromatography). Reaction mechanisms of 99mTc with studied antibiotics are reported in –. The labelling efficiency of fluoroquinolones with 99mTc is reported in .
Table 1 Labelling efficiency of 99mTc with fluoroquinolones.
2.3 Radiopharmaceutical incubation and measurement
Culture reviving and heat killing of bacterial stains are repeated as follows; 1.00 mL of each sample was withdrawn in a sterile glass pipette and placed in a polypropylene micro-centrifugation tube (VWR international). 50 μL 99mTc-fluoroquinolones along with 50 μL 99mTc (blank) were placed in each sample of live or killed bacterial stains. At the spent of time, each sample tube was spun on 14,000 rpm (Eppendorf Minispin Plus Microcentrifuge) at 4 °C for 5 min. The supernatant was transferred via micro pipette (Redi-IPS Fisher brand) to a new sterile glass tube. The pellet was re-suspended with 1.00 mL sterile saline solution and centrifuged. The re-suspended supernatant was added to initial supernatant. This pellet was then transferred via pipette into a new sterile glass test tube. This pipette tip was saved, as the bacteria within it could not be completely evacuated. Radioactivity in the pipette tip was considered to be the part of bacterial pellet. Transfer of the pellet to a new glass test tube was necessary due to some binding of 99mTc-quinolones to the micro-centrifugation tube, which was then preserved and considered the part of supernatant after being washed with 1.00 mL of sterile saline solution.
The radioactivity in the pellet, tips supernatant and micro-centrifugation tubes was counted separately for 5 s in a sodium iodide well counter equipped with dedicated nuclear medicine software. The percentage of total activity in the pellet was calculated as follows:
(1)
(1)
The results thus obtained for each labelled antibiotics for live and killed microbes and for blank solution after 30 min, 1 h and 4 h are reported in .
Table 2 Activity of free 99mTc−1 and labelled fluoroquinolones on live and killed bacteria after different time intervals.
3 Results and discussion
The results obtained are discussed under the following headings.
3.1 Labelling efficiency of fluoroquinolones with 99mTc
In this study, three fluoroquinolones were labelled with 99mTc and their activity was checked against three bacterial stains. The radiochemical purity was checked with the help of paper chromatography in acetone and instant thin layer chromatography (ITLC) in saline. For all the radio labelled antibiotics, the labelling efficiency was more than 95%, which shows promising chemical purity for the present study. Labelling efficiency of three antibiotics () indicates that minute amount of free TcO4−1 (pertechnetate) and prominent amount of colloid formation results during complex formation. All the three quinolones showed similar behaviour for both paper and ITLC. The colloidal remains at the origin, while free TcO4−1 moved along the solvent front. The labelling of the fluoroquinolones was determined by the formula:
(2)
(2)
3.2 Binding assay of labelled fluoroquinolones with bacteria
The stains of bacteria used in this research were commonly infection causing pathogens. The overnight culture of bacteria’s was incubated and approximately, 1.0 × 108 cfu/mL was conformed by optical density. shows negligible binding efficiency of free 99mTc with bacterial stains at three different intervals, as compared to binding efficiencies of 99mTc-flouroquinolones.
3.3 Activity of 99mTc-ciprofloxacin in live and killed bacteria
99mTc-ciprofloxacin showed an un-expected behaviour of accumulation in both live and killed bacteria. The activity of 99mTc-ciprofloxacin in live E. coli after 30 min, 1.0 h and 4.0 h was 37, 27 and 34%, respectively, with an average of 33%, while in killed E. coli its activity was 46, 39 and 40% with an average of 42%, which is about 10% more than that of live E. coli. The activity of 99mTc-ciprofloxacin in live Salmonella after 30 min, 1.0 h and 4.0 h was 22, 34 and 29%, respectively, with an average of 28%. In killed salmonella, its activity was 43, 37 and 47%, respectively, with an average of 42%, it is also 14% more than live Salmonella. The activity of 99mTc-ciprofloxacin in Ps. aeruginosa after 30 min, 1.0 h and 4.0 h was 46, 42 and 37%, respectively, with an average of 42%, while in killed Ps. aeruginosa, its activity was 37, 39 and 37%, respectively, with an average of 38%, which is 4% less than the live Ps. aeruginosa. We may say that 99mTc-ciprofloxacin binds with both live and killed bacteria under the study. Our finding contradicts with the other workersCitation9,Citation10, who state that fluoroquinolones are thought not to bind to dead as well as in non-microbial inflammatory processes. The behaviour of 99mTc-ciprofloxacin indicates that it not only participates in binding with gyrase but also with other parts of the pathogens as well.
3.4 Activity of 99mTc-ofloxacin in live and killed bacteria
The behaviour of 99mTc-ofloxacin was different from that of 99mTc-ciprofloxacin. The activity of 99mTc-ofloxacin in live E. coli, Salmonella and Ps. aeruginosa was higher than those of killed ones. The activity of 99mTc-ofloxacin in live E. coli after 30 min, 1.0 h and 4.0 h was 34, 41 and 41%, respectively with an average of 39%, while in killed E. coli its activity was 5, 19 and 13% with an average of 9%, which is 30% less than that of live E. coli. Similarly the activity of 99mTc-ofloxacin in live Salmonella was 46, 49 and 46% after 30 min, 1.0 h and 4.0 h respectively, with an average of 47%. However, its activity in killed Salmonella was 6, 10, and 17% with an average of 11%. This value (36%) is less than live cell activity. The activity of 99mTc-ofloxacin in Ps. aeruginosa live cells was 10, 31 and 46% with an average of 29% after 30 min, 1.0 h and 4.0 h respectively. Its activity in dead cells was 8, 31 and 46% with an average of 28%. This figure is also less than 1% to that of live cell activity. As stated above, this difference in the activities of 99mTc-ofloxacin in all three types of pathogens and the activity of 99mTc-ofloxacin is almost two to three folds more in live cells than those of killed ones. There is much more possibility of binding of 99mTc-ofloxacin to gyrase enzyme and our finding supports the findings of other workers.Citation9,Citation10
3.5 Activity of 99mTc-enorfloxacin in live and killed bacteria
The activity of 99mTc-enorfloxacin after 30 min, 1.0 h and 4.0 h in live E. coli was 45, 48 and 44%, respectively, with an average of 46%, while its activity in killed E. coli was 44, 36 and 13%, respectively, with an average of 31%. This activity indicates a decreasing behaviour with time. The activity of 99mTc-enorfloxacin in Salmonella was 34, 47 and 38% with an average of 40% after 30 min, 1.0 h and 4.0 h respectively. The activity of 99mTc-enorfloxacin in killed Salmonella was 49, 27 and 19%, respectively, and it again showed a decreasing activity trend with time, with an average of 32%. The activity of 99mTc-enorfloxacin in live Ps. aeruginosa was 21, 16 and 14% after 30 min, 1.0 h and 4.0 h respectively, with an average of 17%. The activity of 99mTc-enorfloxacin in Ps. aeruginosa was about 50% less than that of other live pathogens. The activity of 99mTc-enorfloxacin in dead Ps. aeruginosa after 30 min, 1.0 h and 4.0 h was 29, 35 and 26% with an average of 30%. Overall, in case of 99mTc-enorfloxacin, the activity in dead cells was once again prominent. Therefore, we may conclude that it is not necessary that all fluoroquinolones do not act with dead pathogens as they bind with gyrase enzyme as well as with other microbial stuffs.Citation11–Citation14
The relative high activities of 99mTc-ciprofloxacin in both live and dead pathogens suggests that it may be used for imaging all three types of microbes, while 99mTc-ofloxacin may be used for imaging live E. coli and Salmonella and to lesser extent live Ps. aeruginosa (29% activity). 99mTc-enorfloxacin showed excellent activity in live and dead cells except live Ps. aeruginosa with activity of only 17%. 99mTc-enorfloxacin activity in both E. coli and Salmonella live and a dead cell was moderate. In-vitro studies of these complexes indicate that they may be used for imaging specific infection.
4 Conclusion
Our study indicates that on an average, 99mTc-ciprofloxacin showed 33% and 42% activity in live and killed E. coli, 28% and 43% activity in live and killed Salmonella and 42% and 38% activity in live and killed Ps. aeruginosa. The accumulation of 99mTc-ciprofloxacin was higher in dead cells of E. coli and Salmonella, and live Ps. aeruginosa. The accumulation of 99mTc-ofloxacin in live and dead E. coli was 39% and 12% respectively, while in Salmonella it was 47% and 11% in live and dead pathogens. The activity of 99mTc-ofloxacin in Ps. aeruginosa live and killed was 29% and 28% respectively. These results indicate that 99mTc-ofloxacin accumulates more than three times in live cells. 99mTc-enorfloxacin activity in both E. coli and Salmonella live and dead cells was moderate except for live Ps. aeruginosa where its activity was found to be only 17%. 99mTc-ofloxacin may be used for imaging all three live pathogens, while 99mTc-ofloxacin and 99mTc-enorfloxacin may be used for imaging both live and dead pathogenic infections. All these labelled compounds showed reasonable activity even after 4 h. All these properties indicate that these compounds may be tested for imaging infections.
Conflict of interest
There is no conflict of interest between the authors.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 28 October 2014
References
- O.C.BoermanH.RennenW.J.OyenF.H.CorstensRadiopharmaceuticals to image infection and inflammationSemin Nucl Med312001286295
- J.P.LavenderJ.LoweJ.R.BarkerGallium 67 citrate scanning in neoplastic and inflammatory lesionsBr J Radiol441971361366
- Y.ItoS.OkuyamaT.AwanoDiagnostic evaluation of Ga-67 scanning of lung cancer and other diseasesRadiology1011971355362
- M.F.TsanMechanism of gallium-67 accumulation in inflammatory lesionJ Nucl Med2619858892
- J.G.McAfeeM.L.ThakurSurvey of radioactive agents for the vitro labeling of phagocytes, I Soluble agents. II. ParticlesJ Nucl Med171976480492
- M.L.ThakurJ.P.LavenderR.N.ArnotD.J.SilvesterA.W.SegalIndium-111-labeled autologous leukocytes in manJ Nucl Med18197710141021
- M.L.ThakurR.E.ColemanM.J.WelchIndium-111-labeled leukocytes for the localization of abscesses: preparation, analysis, tissues distribution, and comparison with gallium-67 citrate in dogsJ Lab Clin Med891977217228
- R.H.RubinL.S.YoungW.P.HansenM.NedelmanR.WilkinsonM.J.NellesSpecific and nonspecific imaging of localized Fisher immunotype 1 Pseudomonas aeruginosa infection with radiolabeled monoclonal antibodyJ Nucl Med291988651656
- S.VinjamuriA.V.HallK.K.SolankiJ.BomanjiQ.SirajE.O’ShaughnessyComparison of 99mTc infection imaging with radio labelled white-cell imaging in the evaluation of bacterial infectionLancet3471996233235
- A.BenitezM.RocaJ.MartinLabelling of antibiotics for infection diagnosisQ J Nucl Med Mol Imaging502006147152
- F.GemmelN.DumareyC.J.PalestroRadionuclide imaging of spinal infectionEur J Nucl Med Mol Imaging33200612261237
- A.K.SinghJ.VermaA.BhatnagarA.AliTc-99-labelled sparofloxacin: a specific infection imaging agentWorld J Nucl Med22003103109
- K.AlexanderW.T.DrostJ.S.MattoonJ.J.KowalskiJ.A.FunkA.C.CrabtreeBinding of ciprofloxacin labelled with technetium Tc 99m versus 99mTc-pertechnetate to a live and killed equine isolate of Escherichia coliCan J Vet Res692005272277
- P.KyprianidouC.TsoukalasA.ChiotellisFirst example of well-characterized Re and 99mTc tricarbonyl complexes of ciprofloxacin and norfloxacin in the development of infection-specific imaging agentsInorg Chem Acta3702011142236
- S.A.R.NaqviM.M.IshfaqZ.A.KhanS.A.NagraI.H.BukhariA.I.Hussain99mTc labelled levofloxacin as an infection imaging agent: a novel method for labelling levofloxacin using cysteine·HCl as co-ligand and in vivo studyTurk J Chem362012267277
- A.MushtaqS.PervezS.HussainJ.A.MirzaM.M.KhanM.AsifEvaluation of Pakgen 99mTc generators loaded with indigenous fission 99MoRadiochim Acta1002012793801