?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Calcium is one of the most important signalling ions in cell biology performing numerous functions with high specificity. A calcium wave triggers life at fertilization but also can cause cell death. The means by which this single ion can be both highly specific and universal is believed to lie in its spatiotemporal dynamics mediated by ion channels, pumps, receptors and calcium buffers. During oocyte maturation the calcium signalling machinery undergoes differentiation which results in distinctly different calcium release patterns on all organizational scales from puffs to waves. The calcium concentration patterns required during different stages of oocyte maturation are still not completely known. Also the mechanisms involved in calcium dynamics in oocyte cell are still not well understood. In view of above a two dimensional model has been proposed to study calcium dynamics in an oocyte cell. The parameters such as buffers, ryanodine receptor and voltage gated calcium channel are incorporated in the model. Based on the biophysical conditions the initial and boundary conditions have been framed. The model is transformed into variational form and Ritz finite element method has been employed to obtain the solution. A program has been developed in MATLAB 7.10 for the entire problem and executed to obtain numerical results. The numerical results have been used to study the effect of buffers, RyR and VGCC on calcium distribution in oocyte. The results indicate that buffers can significantly decrease the calcium concentration and RyR & VGCC can significantly raise the calcium concentration level in the oocyte cell in order to initiate, sustain and terminate specific activities in the cell. The information generated from the model can be useful to biomedical scientists for clinical and biomedical applications.
1 Introduction
Ca2+ is a second messenger that mediates a plethora of cellular function ranging from neurotransmitter release to fertilization. Specially Ca2+ signalling is encoded in the spatial, temporal and amplitude features of cytoplasmic Ca2+ dynamics.Citation1 That is in the same cell Ca2+ signals of disparate duration, amplitude or frequency result in different cellular response. For example localized Ca2+ release through ryanodine receptor in vascular smooth muscle leads to vasodilation.Citation2 Whereas global sustained Ca2+ signals lead to vasoconstriction.Citation3 Ca2+ signals achieve this specificity by differentially activating Ca2+ dependent efforts based on their frequency, location, duration and amplitude. At fertilization, vertebrate eggs undergo a major transition from gametogenesis with dramatic cellular alteration referred to collectively as egg activation. Ca2+ is the universal signal for egg activation in all sexually reproducing species studied to date from plants to humans.Citation4,Citation6 The fertilization induced Ca2+ signal has specific spatial and temporal dynamics which is essential to activate the egg and initiate embryonic development.Citation4,Citation5 This specialized Ca2+ signal takes the form of a single or multiple Ca2+ transients depending on the species.Citation4 Changes in the concentration of cytosolic free calcium have been found to be responsible for the initiation and regulation of a variety of cellular functions including cellular proliferation, secretion, metabolic, adjustments and changes in gene expression.Citation7,Citation8 The spatiotemporal patterns of [Ca2+]c as a result of agonist stimulation are as diverse as the roles of Ca2+ play in different cells. The temporal pattern of [Ca2+]c observed in a variety of cells includes oscillations or repetitive spiking.Citation7,Citation9–Citation11 Some cells, most notably Xenopus oocyte also exhibit interesting spatial patterns of [Ca2+]c including propagating waves and target and spiral patterns.Citation12 Ca2+ waves have also been observed in myocytes, astrocytesCitation13 hepatocytesCitation10 and airways epithelial cells.Citation14 The dynamics of Ca2+ is very important in cellular physiology because Ca2+ regulates their activity and interactions.Citation15 Ca2+ waves are dependent on the diffusion of Ca2+ ions both within and possibly between the cells: modulating Ca2+ ion diffusion may predictably alter the spatial and temporal character of the Ca2+ wave. Zeng and co-workersCitation25 developed a mathematical model of simulation of spontaneous Ca2+ oscillations in astrocytes mediated by voltage gated calcium channels (VGCC). A good number of attempts have been made by scientists on study of calcium distribution in neurons cells, astrocyte cells, but very few attempts are reported in the literature on modelling of calcium distribution in oocytes. No attempt is reported in the literature for modelling calcium distribution in oocytes in the presence of VGCC. In view of above a mathematical model has been developed to study effect of buffers and ryanodine receptor over Ca2+ profile in oocytes in the presence of VGCC. The model has been developed for a two dimensional unsteady state case. The finite element methodCitation17 is employed to solve the problem. A computer program has been developed in MATLAB 7.10 for the whole problem and executed on Intel(R) Core™ i3 CPU, 4.00 GB RAM, 2.40 GHz processor.
2 Mathematical model and solution
Calcium kinetics in Oocytes is governed by a set of reaction–diffusion equations which can be framed assuming the following bimolecular reaction between Ca2+ and buffer speciesCitation18,Citation19(1)
(1) where [
], [
] and [
] represent the cytosolic
concentration, free buffer concentration and calcium bound buffer concentration respectively and ‘j’ is an index over buffer species,
and
are on and off rates for jth buffer respectively. Using Fickian diffusion, the buffer reaction diffusion system in two dimensions is expressed asCitation20,Citation30,Citation32,Citation33
(2)
(2)
(3)
(3)
(4)
(4) where reaction term
is given by
(5)
(5)
are diffusion coefficients of free calcium, free buffer and
bound buffer respectively. Where
is net influx of
from the voltage gated calcium channel,
is net influx of
from the ryanodine receptor which is assumed to be within the cell i.e., at the centre
and
is net influx of
from the source and
is the standard Dirac delta function placed at the Ca2+ source. Let
be the total buffer concentration of jth buffer and the diffusion coefficient of buffer is not affected by the binding of calcium i.e.,
Then Eq. (Equation5
(5)
(5) ) can be written asCitation21
(6)
(6)
It is assumed that the buffer concentration is present in excess inside the cytosol so that the concentration of free buffer is constant in space and time, i.e. . Under this assumption Eq. (Equation3
(3)
(3) ) is approximated byCitation19
(7)
(7) where
is the background buffer concentration. Thus for single mobile buffer species Eq. (Equation2
(2)
(2) ) can be written asCitation18,Citation19
(8)
(8) where
is the diffusion coefficient of free calcium,
is the source amplitude due to the calcium channel.
is the flux due to VGCC and this has been modelled using the Goldman–Hodgkin–Kartz (GHK) current equation.Citation20,Citation22 We assume a single point source of
,
at
, there are no sources for buffers and buffer concentration is in equilibrium with
far from the source and GHK equation as
(9)
(9) where [Ca2+]i and [Ca2+]o are the intracellular and extracellular calcium concentration respectively.
is the permeability of calcium ion,
is the valency of calcium ion.
is the Faradays constant.
is the membrane potential.
is gas constant and
is absolute temperature. Eq. (Equation9
(9)
(9) ) is converted into molar/s by using the following equation
(10)
(10)
The negative sign in Eq. (Equation10(10)
(10) ) is taken due to the inward current by convection. GHK current equation gives the current density as a function of voltage. The GHK equation is derived from the constant field which assumes that the electric field in the membrane is constant and thus ions move in the membrane in the same way as in free solution.
is flux due to ryanodine receptor given asCitation26,Citation31
(11)
(11) Combining Eqs. (Equation8
(8)
(8) )–(Equation11
(11)
(11) ) we get proposed mathematical model as given below
(12)
(12)
The point source of calcium is assumed at and as we move away from the source, the calcium concentration achieves its background value i.e.,
. Thus the initial and boundary conditions for the above problem areCitation30,Citation32,Citation33
Initial Condition:(13)
(13) Boundary Conditions:
(14)
(14)
(15)
(15)
Here is the background calcium concentration,
represents the rate of calcium efflux from the cytosol into extracellular space.
represents the flux due to
and incorporated on the boundary tends to the background concentration of 0.1
as
but the domain taken by us is not infinite one. Here we are taking the distance required for
to attain background concentration as
for Oocyte along x-axis and y-axis.Citation30,Citation32,Citation33 Our problem is to solve Eq. (Equation12
(12)
(12) ) coupled with Eqs. (Equation13
(13)
(13) )–(Equation15
(15)
(15) ). For our convenience we are writing ‘u’ in lieu of [Ca2+]. From (Equation12
(12)
(12) ) we get
(16)
(16)
(17)
(17)
Applying finite element method on Eq. (Equation16(16)
(16) ) we can get variational form as
(18)
(18)
Here we have used ‘u’ in lieu of [Ca2+] for our convenience and e = 1, 2, 3…162. Also for
and
for rest of elements. The following linear shape function for calcium concentration within each element has been taken asCitation17
(19)
(19)
(20)
(20) where
(21)
(21) and
(22)
(22)
Substituting nodal conditions in Eq. (Equation21(21)
(21) ), we get
(23)
(23) where
(24)
(24)
From the Eq. (Equation23(23)
(23) ), we have
(25)
(25) where
(26)
(26)
Substituting from Eq. (Equation25
(25)
(25) ) in (Equation20
(20)
(20) ), we get
(27)
(27)
The expression (Equation18(18)
(18) ) can be written as
(28)
(28) where
(29)
(29)
(30)
(30)
(31)
(31)
(32)
(32)
(33)
(33)
Now we extremize the integral w.r.t. each nodal calcium concentration
as given below
(34)
(34) and
(35)
(35) where
(36)
(36)
(37)
(37)
This leads to a following system of linear differential equations.(38)
(38) Here
A and B are the system matrices and C is the system vector. The Crank–Nicolson method is used to solve the system of differential Eq. (Equation38
(38)
(38) ). A computer program has been developed in MATLAB 7.10 for the entire problem and executed on Intel(R) Core™ i3 CPU, 4.00 GB RAM, 2.40 GHz processor. The numerical values of biophysical parameters used in the model are stated in .Citation16,Citation23,Citation24,Citation31
Table 1 Values of biophysical parameters.Citation16,Citation23,Citation24,Citation31
3 Results and discussion
In this part, we have shown the numerical results for calcium profile against different biophysical parameters which have been obtained using values of parameters given in unless stated along with figures.
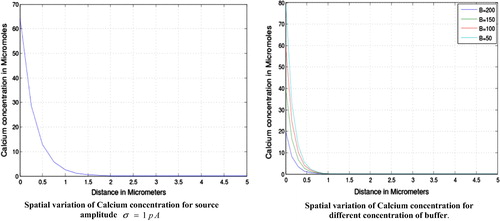
From the effect of source amplitude and the buffers is clearly visible. We see that the calcium concentration is higher near the source and decreases slowly as we move away from the source. The concentration is higher from and then decreases slowly up to
and finally tends to its initial value of
for the source amplitude. The figure further shows that the calcium concentration is higher for lower value of buffer this is because the higher concentration of buffer binds much calcium and makes the system to attain the steady state earlier. The calcium concentration is higher from
and then onwards decreases gradually to attain the initial value.
Figure 1 Spatial variation of calcium concentration for source amplitude and for different concentration of buffer.
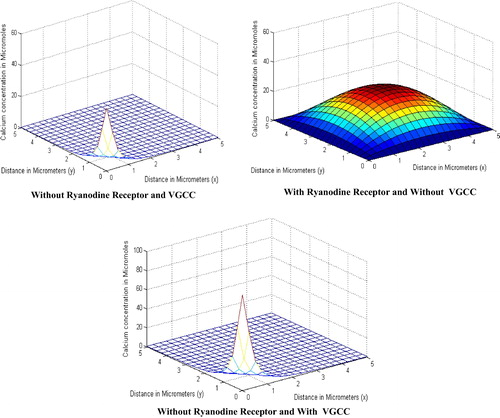
shows the effect of parameters i.e., the calcium concentration with and without these parameters. The figure shows that the calcium concentration is lower in the absence of parameters and very much higher in the presence of them. This is clearly visible from the figure because without these parameters there is less calcium to diffuse as these parameters contribute nothing because of their absence, but the calcium concentration is higher in the presence of these parameters as they release the calcium and contribute in the diffusion so as to make the concentration high. The figure shows the Ca2+ concentration is low in the absence of VGCC & RyR which is and increases when RyR is involved and reaches up to
. Further the Ca2+ concentration reaches the higher level of
when VGCC is present.
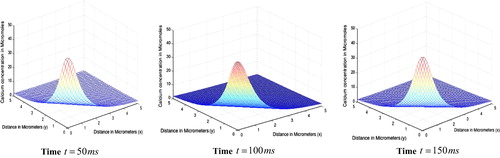
gives the spatial variation of calcium concentration for different time periods. We observe that at the peak calcium concentration is
respectively. We observe that the peak Ca2+ concentration at source increases with time. Also the spread of Ca2+ increases with time.
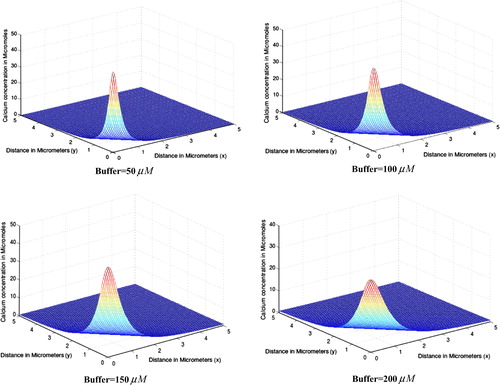
shows the steady state effect of increasing buffer concentration of EGTA buffer on calcium distribution in oocytes for source amplitude . In all the four cases different equilibrium concentrations
of EGTA buffer is taken to be
respectively. From the figure it is evident that as buffer concentration increases, the peak cytosolic calcium concentration at source decreases from
for
to
for
. Our results suggest that if buffer concentration increases too much then it either balkanizes Ca2+ signal or makes it less effective. In our calculations we also observed that buffer concentration alters the time required to achieve the steady state. Here we further observe that as buffer concentration increases, the area of dispersion of cytosolic calcium concentration decreases. The results obtained in this study are in a close agreement with the experimental studiesCitation27,Citation28 and the results obtained by Panday and Pardasani.Citation29 and Jha et al.Citation30.
4 Conclusion
The two-dimensional finite element model has been proposed for an unsteady state case and employed to study effect of VGCC, RyR and buffers on calcium concentration distribution in oocytes. The results provide us information about spatio–temporal relationship of calcium concentration with buffers, RyR and VGCC in oocyte cell. It is concluded from results that VGCC and RyR can significantly raise the level of calcium concentration in the cell in response to the requirements for initiation, sustenance of the specific activities of the cell. Also the buffers are capable of lowering down the calcium concentration in the cell when the calcium concentration becomes high in the cell for a specific activity. The cell exhibits a beautiful coordination of RyR, VGCC and buffers in order to regulate the calcium concentration in the oocyte cell. The finite element method is quite flexible and versatile in the present situation as it has been possible to incorporate the important parameters such as VGCC, RyR and buffers in the model. Such models can be developed further to study the relationship among various parameters under normal and abnormal conditions to generate the information which can be of great use to biomedical scientists for developing protocols for diagnosis and treatment of diseases related to reproduction.
Conflict of interest
The authors declare that there is no any kind of conflict of interest.
Acknowledgement
The authors are highly thankful to University Grants Commission (UGC), New Delhi, India for providing financial support to carry out this work.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 20 March 2015
References
- M.J.BerridgeM.D.BootmanH.L.RoderickCalcium signalling dynamics homeostasis and remodellingNat Rev Mol Cell Biol42003517529
- M.T.NelsonH.ChengM.RubartRelaxation of arterial smooth muscle by calcium sparksScience2701995633637
- M.A.HillH.ZouS.J.PotocnikG.A.M.J.DavisInvited review: arteriolar smooth muscle mechanotransduction: Ca2+ signalling pathways underlying myogenic reactivityJ Appl Physiol912001973988
- S.A.StrickerComparative biology of calcium signalling during fertilization and egg activation in animalsDev Biol2112000157176
- S.T.HomaJ.CarrollK.SwannThe role of calcium in mammalian oocyte maturation and egg activationHum Reprod8199312741281
- A.F.AntoineL.E.FaureS.CordeiroC.DumasM.RougierJ.A.FeijiA calcium influx is triggered and propagated in the zygote as a wave front during invitro fertilization of flowering plantsProc Natl Acad Sci USA9720011064310648
- M.J.BrridgeInositol triphosphate and calcium signallingNature (London)3611993315325
- T.N.DevisWhat’s new with calciumCell711992557564
- P.H.CobboldK.S.R.CuthbertsonCalcium oscillations: phenomena, mechanisms and significanceSemin Cell Biol11990311321
- T.MeyerL.StryerCalcium spikingAnnu Rev Biophy Chem201991153174
- Y.TsunodaOscillatory calcium signalling and its cellular functionNew Biol31991317
- J.D.LechleiterD.E.ClaphamMolecular mechanisms of intracellular calcium excitability in Xenopus oocyteCell691992283294
- A.CharlesJ.E.MerrillE.R.DirksenM.J.SandersonIntercellular signalling in gilial cells: calcium waves and oscillations in response to mechanical stimulation and glutamateNeuron61991983992
- M.J.SandersonA.C.CharlesE.R.DirksenMechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cellsCell Regul11990585596
- M.J.BrridgeElementary and global aspects of calcium signallingJ Physiol41997291306
- B.K.JhaN.AdlakhaM.N.MehtaFinite element model to study calcium diffusion in astrocytesIJPAM782012945955
- S.S.RaoFinite element method in engineering2004Elsevier Science and Technology
- G.D.SmithAnalytical steady state solution to the rapid buffering approximation near an open Ca2+ channelBiophys J71199630643072
- G.D.SmithL.DaiR.M.MiuraA.ShermanAsymptotic analysis of buffered calcium diffusion near a point sourceSIAM J Appl Math61200018161838
- E.NeherConcentration profile of intracellular Ca2+ in the presence of cells presumed to be glia in cerebral cortex of catJ Neurophysiol361973855868
- S.TewariA variational-ritz approach to study cytosolic calcium diffusion in neuron cells for a one dimensional unsteady state caseGAMS J Math Math Biosci22009110
- JKeenerJSneydMathematical physiology81998SpringerBerlin5356
- S.PandayK.R.PardasaniFinite element model to study effect of advection diffusion and Na+/Ca2+ exchanger on Ca2+ distribution in oocytesJ Med Imaging Health Inf32013374379
- P.A.NaikK.R.PardasaniOne dimensional finite element model to study calcium distribution in oocytes in presence of VGCC, RyR and buffersJ Med Imaging Health Inf532015471476
- S.ZengB.LiS.ZengS.ChenSimulation of spontaneous Ca2+ oscillations in astrocytes mediated by voltage gated calcium channelsBiophys J97200924292437
- AmritaTripathiN.AdlakhaFinite element model to study calcium diffusion in a neuron cell involving JRyR,JSercaandJLeakJ Appl Math Inf312013695709
- S.L.DarganI.ParkerBuffer kinetics shape the spatiotemporal patterns of IP3-evoked Ca2+ signalsJ Physiol5532003775788
- P.H.BackxP.P.De TommbeJ.H.Ven DeenB.J.MulderH.E.Ter KeursA model of propagating calcium-induced calcium release mediated by calcium diffusionJ Gen Physiol931989963977
- S.PandayK.R.PardasaniFinite element model to study the mechanics of calcium regulation in oocytesJ Mech Med Biol1420141450022
- B.K.JhaN.AdlakhaM.N.MehtaTwo dimensional finite element model to study calcium distribution in astrocytes in presence of VGCC and excess bufferInt J Model, Simul Sci Comput420131250030
- P.A.NaikK.R.PardasaniOne dimensional finite element method approach to study effect of ryanodine receptor and serca pump on calcium distribution in oocytesJ Multiscale Model522013113
- S.TewariK.R.PardasaniFinite element model to study two dimensional unsteady state cytosolic calcium diffusion in presence of excessIAENG J Appl Math403201015
- S.TewariK.R.PardasaniFinite element model to study two dimensional unsteady state cytosolic calcium diffusionJ Appl Math Inf291–22011427442