Abstract
Onychomycosis is the most common nail disorder, accounting for up to 50% of all nail problems and about 30% of cutaneous fungal infections. Treatment of onychomycosis is expensive. It requires long-term therapy with an oral antifungal medication with potential side effects. Therefore, a proper diagnosis of infection is needed. Aim of the study: This study aimed to compare real time PCR using novel primers targeting the pan-dermatophyte-specific sequence of the chitin synthase 1 gene (CHS1) with nested PCR targeting the same gene, KOH microscopy, direct microscopy in relation to culture for diagnosis of clinically suspected onychomycosis.
Subjects and methods: This study was conducted during the period from April 2013 through May 2014. Eighty patients attending Outpatient Dermatology and Andrology clinic at Benha University Hospital were included in this study. They were 30 females and 50 males with suspected clinical diagnosis of onychomycosis. Their ages ranged from 22 to 77 years. Thirty eight of them were living in rural areas, while the other 42 came from urban areas. Nail scrapings were collected and examined by the following: direct KOH microscopic examination, culture, nested PCR using double sets of primers and finally real time PCR. Results: As regards direct microscopy by KOH examination, 66 (82.50%) cases were positive, while 14 (17.5%) were negative. Culture was positive only in 38 (47.5%) of nail samples revealing different fungi. Dermatophytes were isolated from 30 (37.5%) cases; most of them were Trichophyton mentagrophytes, and in 8 cases the only isolated non-dermatophytic organism was Aspergillus Niger spp. (10.00%). Nested PCR was positive in 52 (65.00%) of nail samples while real time PCR was positive in 58 (72.5%) of nail samples. Conclusion: Real-time PCR followed by melting-point analysis, gives a diagnostic tool that has a higher sensitivity (93.3%) and is faster than nested PCR (73.3%) and other conventional methods.
Abbreviations:
1 Introduction
Onychomycosis is a fungal infection of the toenails or fingernails. It is the most common nail disorder and is present in 2–13% of general population increasing up to 48% by 70 years of age. Onychomycosis can cause discomfort and disfigurement and may produce physical and occupational limitations, as well as reducing quality of life.Citation1 There are three groups of fungi associated with onychomycosis: dermatophytes as members of the genera Trichophyton, non-dermatophytes such as Acremonium spp., Alternaria spp. and Aspergillus spp. and yeasts as Candida albicans which is the principal pathogen responsible for candidal onychomycosis. Candida species other than C. albicans such as C. krusei, C. parapsilosis, C. glabrata, C. guilliermondii, C. tropicalis and Blastoschizomyces capitatus (B. capitatus) have also been found to cause onychomycosis.Citation2,Citation3
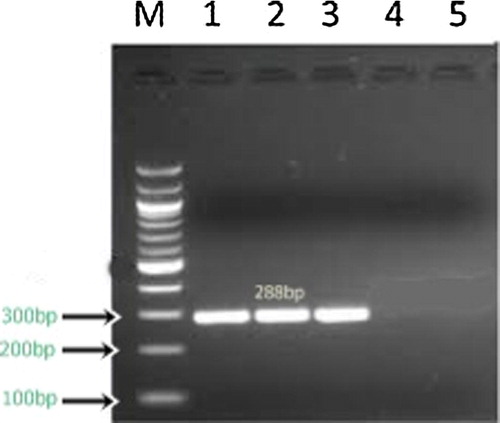
Figure 2 Results of nested PCR of clinical specimens from patients with onychomycosis. Lane M 100-bp DNA ladder (molecular mass markers); lane 1,2,3, nested-PCR-positive specimens (288 bp); lane 4,5 nested-PCR-negative specimens.
Dermatophytes account for 90% of toenail and 50% of fingernail onychomycosis.Citation4 The term “dermatophytosis” describes infections by members of the genera Microsporum, Trichophyton, and Epidermophyton. The species that most often cause onychomycosis are Trichophyton rubrum (T. rubrum), Trichophyton mentagrophytes (T. mentagrophytes), and Epidermophyton floccosum (E. floccosum): The first two species are much more often implicated than E. floccosum.Citation5
Predisposing factors for onychomycosis include increasing age, male gender, trauma, immunosuppression, diabetes mellitus, poor peripheral circulation, smoking and tinea pedis. In addition for fingernails’ persistent exposure to water, the use of artificial nails, and trauma induced by pushing back the cuticles and aggressive manicuring may also be predisposing factors.Citation6
Treatment of onychomycosis is expensive. It requires long-term therapy with an oral antifungal medication with potential side effects. Therefore, a proper diagnosis of infection is needed.Citation7
The laboratory diagnosis of onychomycosis routinely involves direct microscopic examination of clinical specimen followed by in vitro culture techniques. Microscopic identification of fungal elements directly from clinical specimen is a rapid diagnostic method but it lacks specificity and sensitivity, with false negative results in up to 15% cases, furthermore although rapid and economical, it does not provide genus or species identification and hence does not differentiate between dermatophytes and other molds. In vitro culture is a specific diagnostic test but it is a slow technique, and may take up to 8 weeks to give results.Citation8
The advent of molecular technology has enabled the development of techniques like polymerase chain reaction (PCR), which is a highly sensitive and specific test and can be used for diagnosis of various microorganisms including fungal pathogens.Citation9
PCR for detection of dermatophytes has been widely employed targeting the nontranscribed spacer (NTS) regions, metalloprotease gene.Citation10 Chitin synthase (CHS) gene,Citation11 tubulin gene, promoter region within ribosomal intergenic spacer, transcription elongation factor 1, actin gene and calmodulin gene.Citation12
2 Aim of the work
This study aimed to compare realtime PCR using novel primers targeting the pan-dermatophyte-specific sequence of the chitin synthase 1 gene (CHS1) with nested PCR targeting the same gene, KOH microscopy in relation to culture for diagnosis of clinically suspected onychomycosis.
3 Subjects and methods
This study was carried out at Microbiology and Immunology Department of Benha faculty of Medicine and Dermatology and Andrology Department, at Benha University Hospital, in the period from April 2013 through May 2014. A written informed consent (in Arabic language) was obtained from the patients before participation. Eighty patients attending Outpatient Dermatology and Andrology Clinic were enrolled in this study with clinically suggestive symptoms and signs of onychomycosis (discoloration, thickening, subungual keratosis, onycholysis, longitudinal and transverse grooves, and dystrophic nail). Each patient was subjected to the following sections:
3.1. Full medical history taking (personal, present and past history).
3.1.1. Personal history: Including sex, age, residence, and occupation.
3.1.1.2. Complaint.
3.1.1.3. Present history: Including the onset, course and duration of the lesion and associated nail changes.
3.1.1.4. Past history: History of fungal infection, previous trauma, previous treatment, diabetes mellitus, associated medical or immunological diseases and/or peripheral vascular diseases and peripheral neuropathy.
3.1.2. General examination (for associated conditions and lesions predisposing to and suspecting fungal infection as diabetic foot, peripheral vascular disease and concomitant fungal infections e.g. tinea pedis).
3.1.3. Local examination of the nail, mycological investigation and molecular detection of fungal DNA by PCR.
Forty six patients had one or more affected finger, and thirty four patients had one or more affected toes. Clinical types were distal, distolateral, proximal, proximal and distolateral and total dystrophic.
3.2 Mycological investigation
3.2.1 Samples
The suspected nails were cleaned with 70% alcohol to remove contaminants, Scrapings were taken with a sterile scalpel blade and collected in a clean paper, and the collected specimens were sent to the Mycology Unit, Medical Microbiology and Immunology Department at Faculty of Medicine, Benha University.
3.2.2 Methods
The collected specimens were divided into three portions. The first portion of the specimens was examined microscopically using 20% KOH. The second portion was cultured on two sets of media: SDA containing chloramphenicol (0.5%) with/without cycloheximide (0.5%). DNA extraction was performed on the third portion of specimen.
3.2.2.1 Direct microscopic examinationCitation13
The sample to be examined was placed on a clean glass slide. A drop of 20% KOH reagent was added and mixed with the sample. The softened nail material was examined under both low (10×) and high (40×) power fields of the microscope for the presence of fungal elements. The details regarding the hyphae, spores, budding cells and pseudo-hyphae were noted.
3.2.2.2 CultureCitation13
All samples were cultured irrespective of the negative or positive direct microscopic examination results. Samples were inoculated on the two sets of media (SDA with/without actidione). Inoculated media incubated aerobically at room temperature for 4 weeks. Growth inspection was done daily for the first week and then twice weekly for the next 3 weeks. Negative cultures were discarded after 30 days. Identification of the obtained growth was done by
3.2.2.2.1 Macroscopic examination of the culture
The identification of isolates from SDA was done by observing the morphological characteristic of the colony including size, shape, consistency, margins, color of the colony both in recto and verso sides, type of the growth whether fluffy, cottony or creamy and the presence or absence of diffusible pigments.
3.2.2.2.2 Microscopic Lactophenol Cotton Blue (LPCB) stained preparation
It was used for microscopic examination of fungal structures. This was done by tease mount which is the most common technique used for rapid mounting of fungi for microscopic examination, and by slide culture which is the best method for preserving and observing the actual structure of a fungus. It is not a rapid technique, but it is useful for microscopic studying of fine fungal structures.
3.3 Molecular detection of fungal DNA by PCR
3.3.1 DNA extraction
DNA extraction was done using Gene JET Genomic (Thermos Scientific, Germany) DNA Purification Kit. Nail samples were digested with Proteinase K in lysis solution. RNA was removed by treating the samples with RNase A. The lysate was then mixed with ethanol and loaded on the purification column where the DNA bound to the silica membrane. Impurities were effectively removed by washing the column with the prepared wash buffers. Genomic DNA was then eluted under low ionic strength conditions with the elution buffer. Extracted DNA from nail samples was stored at −20 °C till processing. For each of the samples, two sets of PCR were performed.
3.3.2 Nested conventional PCRCitation14
It was performed by using primers targeting dermatophyte-specific chitin synthase 1 gene (CHS1), whose sequences were as follows 1S (5′-CAT CGA GTA CAT GTG CTC GC-3′; nucleotides [nt] 70 to 89) (Bioneer) and 1R (5′-CTC GAG GTC AAA AGC ACG CC-3′; nt 485 to 504) (Bioneer). Nested PCR was performed by reamplification of the first PCR product using a novel set of primers, whose sequences were as follows: JF2 (5′-GCA AAG AAG CCT GGA AGA AG-3′; nt 111 to 130) (Bioneer) and JR2 (5′-GGA GAC CAT CTG TGA GAG TTG-3′; nt 378 to 398) (Bioneer).
The PCR reaction was performed in a 50 μl reaction volume containing 25 μl Taq PCR Master Mix (Maxima Hot Start Green PCR Master Mix, Fermentas), 2.5 μl primer, 10 μl template DNA and 10 μl nuclease free water. The conditions of the reaction were as follows: One cycle of initial denaturation at 95 °C for 5 min., 40 amplification cycles of denaturation at 95 °C for 30 s, annealing at 56 °C for 30 s and extension at 72 °C for 1 min., followed by one final extension cycle at 72 °C for 15 min. Ten microliters of the PCR product were used as a template for the second (nested) PCR, using the same PCR conditions, except that the annealing temperature was 52 °C. The specific band size of the PCR product was (288 bp).
3.3.3 Real time PCRCitation15
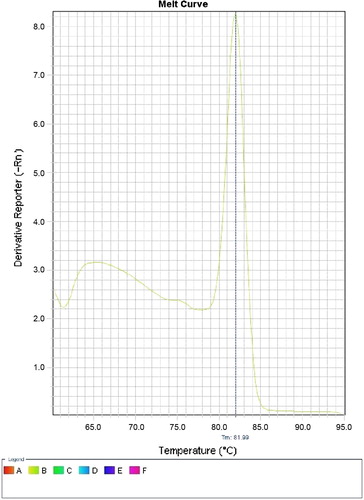
The real time PCR was performed using the same primers targeting pan dermatophyte-specific chitin synthase 1 gene (CHS1). The PCR mixture contains 10 μl Super Real Pre Mix Plus (SYBR Green) TIANGEN Biotech (Beijing), 2 μl of Rox dye, 0.6 μl of each primer, and 2 μl of template DNA. To achieve a reaction volume of 20 μl, 4.8 μl of nuclease-free water was added. The real time PCR instrument used was ABI7900HT (Applied Biosystems, Foster City, CA, USA) with the following conditions: an initial denaturation at 95 °C for 2 min., followed by 35 amplification cycles of 95 °C for 3 s and 60 °C for 30 s. Negative control (2 μl nuclease free water) was included in the run. Finally melting curve analysis was performed. The principle is when double-stranded DNA bound with SYBRR Green I dye is heated, a sudden decrease in fluorescence is detected when the melting point (Tm) is reached, due to dissociation of the DNA strands and subsequent release of the dye. The fluorescence is plotted against temperature. The PCR product of pan dermatophyte gene melts at 81.9 °C ().
4 Statistical analysis
Mean ± SD and range were used to describe the quantitative data and proportion was used to describe the qualitative data of the studied population. The test of proportion (z test) was used to compare the results of the direct KOH microscopy, the nested PCR and RT-PCR with the results of the reference test (fungal culture) and a P-value <0.05 was considered statistically significant. The sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated for the different diagnostic tests against the reference test and Receiver Operating Characteristic (ROC) curve analysis was carried out. All statistical analyses were carried out in STATA/SE version 11.0 for Windows.
5 Results
The results of the study are represented in the following – and –. The age of the study population ranged between 22 and 77. The study population comprised 50 males and 30 females. Urban participants represented 52.5% of the study population while rural participants represented 47.5% ().
Figure 1 (a) Slide culture of T. mentagrophytes. (b) Culture of T. mentagrophytes on SDA (microscopic). (c) Slide culture of T. verrucosum. (d) Culture of T. verrucosum on SDA. (e) Slide culture of T. rubrum. (f) Culture of T. rubrum on SDA.
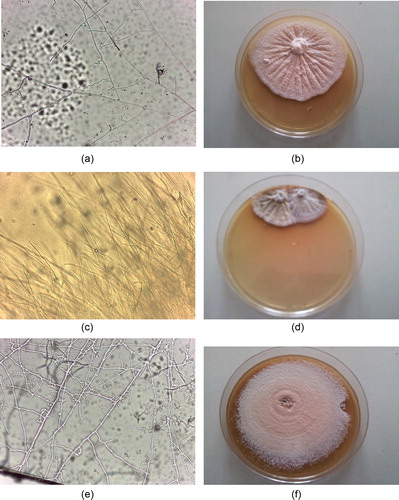
Figure 4 Receiver operating characteristic (ROC) curve for direct KOH microscopy, nested PCR and RT-PCR against the gold standard test (fungal culture) for the diagnosis of onychomycosis (N = 80). KOH microscopy: n = 66 positive and n = 14 negative; Area Under the Curve (AUC) = 0.48, P < 0.001 Nested PCR: n = 52 positive and n = 28 negative; AUC = 0.57, P < 0.001. RT-PCR: n = 58 positive and n = 22 negative; AUC = 0.67, P < 0.001.
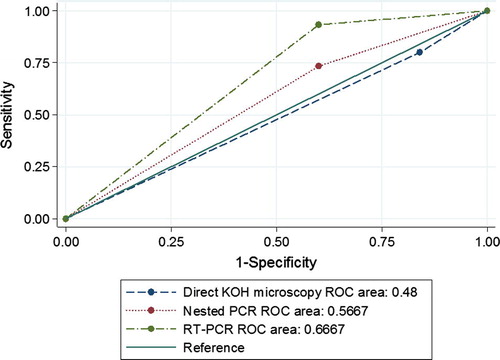
Table 1 Socio-demographic characteristics of the study population.
Table 5 Analysis for results of direct KOH microscopy, nested PCR and RT-PCR against the reference test (fungal culture).
The most common associated clinical condition in suspected patients with onychomycosis was anemia which represented 35% of study population while the least common one was trauma which represented 12.5% of patients ().
Table 2 Patients with suspected cases of onychomycosis.
The most common clinical types of onychomycosis in fingers & toes were Total dystrophic & proximal types respectively while the least common clinical type in fingers and toes was Proximal & distolateral for both ().
Table 3 Clinical features of patients with suspected cases of onychomycosis.
Of the 80 patients with clinically suspected cases of onychomycosis, 82.5% (66/80) were positive for fungal elements by KOH microscopy. Dermatophytes were detected in 65% and 72.5% of the cases by nested PCR and real time PCR respectively and isolated in 37.5% (30/80) of the cases by culture. Non-dermatophytic molds were isolated in 10% (8/80) (, and ).
Table 4 Comparison of different methods used for diagnosis of onychomycosis among the study group.
The isolated dermatophytes were T. mentagrophytes detected in 14 cases, followed by T. rubrum detected in 8 cases and lastly T. verrucosum and T. schoenleinii detected in 4 cases for each. The only isolated non-dermatophyte was Aspergillus Niger in 8 cases.
The results of direct KOH microscopy, nested PCR and Real Time PCR (RT-PCR) were compared to these of fungal culture in and regarding sensitivity, specificity, PPV and NPV, and direct KOH microscopy shows the lowest sensitivity and specificity 80%, 16%, respectively, while RT-PCR shows the highest sensitivity and specificity 93.3%, 40%, respectively.
Real time PCR shows the highest sensitivity (93.3%) while nested PCR shows the least sensitivity (73.3%). Real time PCR is more accurate in diagnosis of onychomycosis as its area under the curve (AUC) was 0.67 compared with that of nested PCR and direct KOH microscopy they were 0.58, 0.48 respectively, and this is shown in and .
6 Discussion
Onychomycosis is a major problem in dermatology because it is so widespread. The prevalence has increased since World War I and today it is considered a disease of civilization, characterized by extreme chronicity and resistance to therapy.Citation16
In our study, occurrence of onychomycosis was confined between 22 and 77 year. This is in accordance with that reported by Garg et al.Citation17 and Bokhari et al.Citation18 The increased prevalence of nail lesions by fungi in adults can be explained as a consequence of nail trauma and slow nail growth.Citation19
Our study included more male patients (62.5%) affected by onychomycosis than females (37.5%). This is in line with Ghannoum et al.Citation20 and Heikkila and StubbCitation21 who detected that male subjects were found to have twice or three times as likely onychomycosis as female subjects respectively, which they attributed to the suggestion that ’’men exercise more” and so more liable to trauma. This higher incidence was observed in other studies conducted by Ching et al.Citation22 and Neupane et al.Citation23
On the other hand Brilhante et al.Citation24 and Bonifaz et al.Citation25 founded that male-to-female ratio was 1:1.6 and 1:3 respectively, probably because females see the doctor more often or for cosmetic reasons. Also Sais et al.Citation26 found that females were more affected, suggesting that trauma from wearing women’s fashionable shoes might account for the difference or maceration from wet work and washing dishes. However Roberts, 1992Citation27 found the incidence to be the same between sexes.
Our study showed that patients with anemia represent 35% of study population while those with DM, peripheral vascular disease and trauma accounted for 27.5%, 20% and 12.5% respectively and patients with associated nail disease accounted for 47.5% of study population. This is in agreement with Dogra et al., 2002Citation28 who stated that the prevalence of onychomycosis has been shown to be significantly higher in diabetic patients than normal individuals. Increasing age, male gender, concurrent intake of immunosuppressive agents and peripheral vascular disease have been shown as independent risk factors for the development of onychomycosis.
Total dystrophic type of onychomycosis was the most common clinical type in our study followed by Proximal and Distal/lateral Subungual Onychomycosis (DLSO) types which is comparable to the findings of Agarwalla et al.Citation29; Wang and Ching.Citation30
It is essential that good laboratory methods are available for rapid and precise identification of the dermatophytes involved in onychomycosis, in order to apply appropriate treatment and preventive measures. The conventional methods of fungal detection have their own drawbacks; for example KOH microscopy has low specificity and fungal culture is associated with low sensitivity and takes long time. Thus newer fungal diagnostic methods are need of the hour for accurate diagnosis and identification of the etiological agent.Citation31
In the present study, the number of positive samples for fungi was 66 (82.50%) samples by 20% KOH microscopic examination which was comparable with that of Pontes et al.Citation32 (68.4%).
The number of positive samples for fungi by culture on SDA was 38 (47.50%) samples which is near to that detected by Brilhante et al.Citation24 (36%). However, the percentage of positive samples for fungi by culture found in Brazil by Lopes et al.Citation33 (56.6%) and Pontes et al.Citation32 (66.5%), was higher; this may be due to selection and higher prevalence of mycotic infection from the start in such hot humid climates.
The KOH positive/culture negative results may be due to the presence of artifacts which may yield false positive results.
Dermatophytes were detected in 30 (37.50%) samples of fungal cultures and they were of T. mentagrophytes, T. rubrum, T. verrucosum and T. schoenleinii species. NDMs represent the second common isolated organisms constitute 10% (8 samples). Aspergillus species detected in all these 8 samples of positive cultures represent the only isolated NDMs. Similarly, dermatophytes were reported as principal pathogen followed by non-dermatophytic molds in studies from Mexico and Malaysia in patients of onychomycosis.Citation34,Citation35
Dermatophytes were detected in 65% and 72.5% of the cases by nested PCR and real time PCR respectively and isolated in 37.5% (30/80) of the cases by culture. Possible explanation for culture negative/PCR positive results is that the nail sample may contain only dead nonviable organisms or maybe sometimes insufficient sample for culture was collected or lastly the most important reason which is the lack of sensitivity of the culture based detection of dermatophytes, confirming previously published results.
In our study, mycological culture was chosen as the reference method to assess the performance of each test. The sensitivity, specificity, accuracy, PPV and NPV were assessed. Real time PCR, nested PCR and direct microscopy have 93.3% 73.33% and 80% sensitivity respectively. The specificity was 40%, 40% and 16% respectively. This is in harmony with Winter et al.Citation36 who calculated the diagnostic sensitivity of the PCR assay as 79.0%. Also Bergman et al.Citation15 and Garg et al.Citation37 reported that real time PCR results show higher sensitivity than nested PCR and direct microscopy respectively.
7 Conclusion
Real-time PCR followed by melting-point analysis, gives us a diagnostic tool that has a higher sensitivity and is significantly faster than nested PCR and other conventional methods for diagnosis of onychomycosis.
Conflict of interest
The authors declare that there are no conflicts of interest.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 29 August 2015
References
- K.K.LillyR.L.KoshnickJ.P.GrillZ.M.KhalilD.B.NelsonE.M.WarshawCost-effectiveness of diagnostic tests for toenail onychomycosis: a repeated measure, single-blinded, cross-sectional evaluation of 7 diagnostic testsJ Am Acad Dermatol552006620626
- G.MorenoR.ArenasOther fungi causing onychomycosisClin Dermatol2822010160163
- R.KaurB.KashyapP.BhallaOnychomycosis – epidemiology, diagnosis and managementIndian J Med Microbiol2622008108116
- B.E.ElewskiOnychomycosis: pathogenesis, diagnosis and managementClin Microbiol Rev111998415429
- R.C.SummerbellEpidemiology and ecology of onychomycosisDermatology194119973236
- A.K.GuptaE.A.CooperUpdate in antifungal therapy of dermatophytosisMycopathologia1665–62008353367
- R.BaranR.HayE.HankekeOnychomychosis: the current approach to diagnosis and therapy1999Martin Dunitz Ltd. PublishersUK
- J.C.GentlesLaboratory investigations of dermatophyte infections of nailsSabouraudia91971149152
- M.MonodO.BontemsC.ZauggFast and reliable PCR/sequencing/RFLP assay for identification of fungi in onychomycosisJ Med Microbiol5520061211
- O.JoussonB.LechenneO.BantonnesS.CapacciaB.MignonJ.BarblanMultiplication of an ancestral gene encoding secreted fungalysin preceded species differentiation in the dermatophytes, Trichophyton and MicrosporumMicrobiology1502004301310
- R.Y.KanoN.T.WatariS.WatanabesH.TakahashiH.TsujimotoA.HosegawaMolecular analysis of chitin synthase gene sequences of Trichpohyton mentagrophytes complex and T. rubrumCurr Microbiol371998236239
- A.K.GuptaY.KohliR.C.SummerbellmExploratory study of single copy genes and ribosomal intergenic spacers for distinction of dermatophytesStudies Mycol4720028796
- D.H.LaroneMedically important fungi: a guide to identification4th ed.2002ASM PressWashington, D.C 409 pp
- J.GargR.TilakS.SinghA.K.GulatiA.GargP.PrakashEvaluation of pan-dermatophyte nested pcr in diagnosis of onychomycosisJ Clin Microbiol4510200734433445
- A.BergmanD.HeimerN.KondoriH.EnrothFast and specific dermatophyte detection by automated DNA extraction and real-time PCR clinical microbiology and infectionClin Microbiol Infect192013205211
- C.MüggeU.F.HausteinP.NenoffCausative agents of onychomycosis-a retrospective studyJ Dtsch Dermatol Ges42006218228
- A.GargV.VenkateshM.SinghK.P.PathakG.P.KaushalS.K.AgrawalOnychomycosis in central India: a clinicoetiologic correlationInt’l J Dermatol432004498502
- M.A.BokhariI.HussainM.JahangirT.S.HaroonS.AmanK.KhurshidOnychomycosis in Lahore, PakistanInt J Dermatol381999591595
- D.S.LooOnychomycosis in the elderly: drug treatment optionsDrugs Aging242007293302
- M.A.GhannoumR.A.HajjehR.ScherN.KonnikovA.K.GuptaG.F.Ponce-De-LeonA large-scale North American study of fungal isolates from nailsJ Am Acad Dermatol432000641
- H.HeikkilaS.StubbThe prevalence of onychomycosis in FinlandBr J Dermatol1331995699
- C.C.ChingS.H.WangM.C.ChouThe causative pathogens of onychomycosis in southern TaiwanMycoses482005413420
- S.NeupaneD.B.PokhrelB.M.PokhrelOnychomycosis: a clinico-epidemiological studyNepal Med Coll J11220099295
- R.S.BrilhanteR.A.CordeiroD.J.MedranoM.F.RochaA.J.MonteiroC.S.CavalcanteOnychomycosis in Ceará (Northeast Brazil): epidemiological and laboratory aspectsMem Inst Oswaldo Cruz10022005131135
- A.BonifazP.Cruz-AguilarR.M.PonceOnychomycosis by molds. Report of 78 casesEur J Dermatol17120077072
- G.SaisA.JucglaJ.PeyriPrevalence of dermatophyte onychomycosis in Spain: a crosssectional studyBr J Dermatol13251995758761
- D.T.RobertsPrevalence of dermatophyte onychomycosis in the United Kingdom: results of an omnibus surveyBr J Dermatol1263919922327
- S.DograB.KumarA.BhansaliA.ChakrabartyEpidemiology of onychomycosis in patients with diabetes mellitus in IndiaInt J Dermatol41200264765110.1046/j.1365-4362
- A.AgarwallaS.AgrawalB.KhanalmOnychomycosis in Eastern NepalNepal Med Coll J82006215219
- S.H.WangC.C.ChingOnychomycosis in TaiwanInt’l J Clin Prac592005906911
- Y.ShirakiN.SodaN.HiroseM.HirumaScreening examination and management of dermatophytosis by Trichophyton tonsurans in the Judo Club of a UniversityNippon Ishinkin Gakkai Zasshi4512004; 712
- Z.B.PontesE.O.LimaN.M.OliveiraJ.P.Dos SantosA.L.RamosM.F.CarvalhoOnychomycosis in João Pessoa city, BrazilRev Argent Microbiol3420029599
- J.O.LopesS.H.AlvesC.R.MariL.T.OliveiraL.M.BrumJ.B.WestphalenA ten year survey of onychomycosis in the central region of the Rio Grande do Sul, BrazilRev Inst Med Trop São Paulo411999147149
- E.Vásquez-del MercadoR.ArenasOnychomycosis among children. A retrospective study of 233 Mexican casesGac Med Mex1442008710
- K.P.NgT.S.Soo HooS.L.NikL.S.AngDermatophytes isolated from patients in University Hospital, Kuala Lumpur, MalaysiaMycopathologia1552002203206
- I.WinterS.UhrlaC.KrügerJ.HerrmannG.BezoldA.WinterMolecular biological detection of dermatophytes in clinical samples when onychomycosis or tinea pedis is suspected. A prospective study comparing conventional dermatomycological diagnostics and polymerase chain reactionHautarzt6442013283289
- J.GargR.TilakS.SinghA.K.GulatiA.GargP.PrakashG detection of dermatophytes from skin and hairBMC Res Notes2200960