Abstract
Aim and objective
The aim of the study was the evaluation of purified HLT from B. pertussis vaccine strain 134 by employing indigenous technology and examining the immuno-biochemical aspects of the purified protein.
Materials and methods
Shaker cultivation of B. pertussis strain 134, sterility, opacity confirmation, TCA precipitation of cellular proteins, G50 purification subsequent DEAE purification, purity analysis, specific activity of HLT, and immune response analysis.
Results
The shaker cultivated B. pertussis strain 134 passed its quality attributes such as sterility, opacity and purity. During TCA precipitation the B. pertussis desired proteins were precipitated and confirmed. The indigenous bed height was optimized and recovery was also calculated in G50 purification. The fractions were analyzed for OD, the total protein concentration and the results were 0.074–0.214, and the total protein content was found between 12.33 μg/ml and 35.67 μg/ml. Subsequent DEAE purification of selected G50 fractions was done and the fractions 9 and 14 had higher OD values of 0.675 and 0.397. Furthermore the DEAE purified samples were structurally analyzed through SDS PAGE and it was found that HLT in the single polypeptide band was around 140 kDa. The qualitative immune response analysis of DEAE purified selected fractions pool showed positive immune response in ODD assay, and in the case of guinea pig antisera it led to the development of diagnostic kits such as ELISA and other vital techniques. Here, in case of guinea pig experiment, the hemorrhagic necrosis analysis showed the necrosis on the skin at the injection site.
Conclusion
The B. pertussis HLT could be purified through two phase with G50 and DEAE, cost effective techniques, the G50 purification has reduced the bioburden problems during DEAE purification and at the same time the quality of the product was high.
1 Introduction
Pertussis, also known as whooping cough, is a highly infectious and communicable disease caused by a gram-negative bacterium Bordetella pertussis. Being an important cause of death in infants and adolescents worldwide, it still continues to be a public health concern, even in countries with high vaccine coverage. Estimates from WHO suggest that, there are 16 million cases reported about 195,000 deaths per year, 95% of these cases are prevalent mostly in the developing nations.Citation1
The disease is characterized by the intense paroxysmal coughing, which terminates in an inspiratory “whoop sound”.Citation2 Whole cell pertussis vaccine (Wcpv) is a suspension of killed B. pertussis organisms and has been used worldwide for nearly 90 years for the universal immunization of children. To control whooping cough disease, it is used in combination with diphtheria and tetanus vaccines, commonly known as DTP vaccine. B. pertussis organism produces a wide range of biologically active components called virulence factors, which contributes to the ability of the organism to cause the disease. These virulence factors are Pertussis Toxin (PT), Adenylate Cyclase Toxin (ACT), Filamentous Haemagglutinin (FHA), Lipopolysaccharide (LPS), Pertactin (PRN) (69 kDa), Agglutinogens and Heat Labile Toxin (HLT).
Each of the virulence factors has its own role in immunogenicity and the biological property of the final pertussis vaccine component. The Heat Labile Toxin (HLT) is one among the virulence factors of B. pertussis, which may play an important role in Bordetellosis, since this toxin is one of only two toxins known to be produced by all virulent Bordetella species. Bordet and GengouCitation3 who were the first to describe the action of this toxin, noted that B. pertussis cells were dermonecrotizing and lethal, when injected into animals.Citation4,Citation5 The HLT is a cytoplasmic protein present in all Bordetella species. The activity of the toxin is detoxified by heat inactivation at 56 °C for 30 min during Wcpv preparation. Therefore, the aim of the study was the evaluation of purified HLT from B. pertussis vaccine strain 134 by employing indigenous technology and examining the immuno-biochemical aspects of the purified protein.
2 Materials and methods
2.1 Strain of B. pertussis
B. pertussis vaccine strain 134 (Rijks institute, Bilthoven, Holland) was used in this study. The strain was maintained in lyophilized state at 4 °C and is an important strain used for the production of routine Wcpv.
2.2 Bordet-Gengou (BG) medium
Bordet-Gengou (BG) Medium was prepared as per CruikshankCitation6 with the significant modifications as prescribed by WHO.Citation7
2.3 B2 culture medium
The B2 culture medium was prepared with following compositions: Bacto casamino acid (BCA) 1800 g, l-glutamic acid 1500 g, NaCl 750 g, KH2PO4 150 g, MgSO4 30 g, CaCl2 3 g, FeSO4 3.74 g, CuSO4 0.15 g, Glutathione 3.05 g, yeast extract 1500 g, and soluble starch 450 g. Starch solution was prepared by dissolving starch in cold water. The suspension was then added to 20 l of hot distilled water and steamed in autoclave at 118 °C for 20 min separately. The remaining chemicals were dissolved in serial order in 50 l of warmed distilled water in separate vessel. l-glutamic acid solution was prepared by dissolving in 50% NaOH to get amorphous solution in warm distilled water. BCA was dissolved in 10 l of distilled water and yeast extract was added to this solution. Finally l-glutamic acid solution and other chemicals were added, made up to 300 l and mixed properly, then it was transferred to the fermentor and the medium was sterilized at 121 °C for 30 min.Citation8
2.4 Sterility media
Nutrient agar medium, Soyabean Caesin Digest Medium (SCDM) and Alternate Thio glycolate medium (ATGM) were used to study the purity and sterility of the culture at the appropriate stage to ensure its purity and safety.
2.5 Preparation of seed culture
One ampoule freeze dried working seed stock (134 strain) was opened under sterile environment and resuspended in 2 ml of sterile B2 medium. The suspension was then inoculated in BG slope and incubated at 35 °C ± 1 °C for 72 h, and the purity was ensured by Gram staining. Furthermore, the culture was scraped at aseptic conditions and inoculated into a 1 l flask containing 400 ml of B2 medium. The flasks were loaded on seed shaker for 24-h at 35 °C ± 1 °C. Further flasks were loaded on shakers (140 ± 10 RPM) and sterile filters were applied over the surface of the medium (3–5 Lpm). Cultivation was allowed to continue for 30–36 h until the maximum yield of organism was achieved. Samples were collected and checked for purity, opacity and pH. The pH should range from 7.8 to 8.2.
2.6 Purity and sterility test
The purity and morphology were studied by Gram staining method. The purity of seed samples during fermentor culture was also checked by taking 1 ml of seed sample and inoculating it into 3-nutrient agar slopes. The other three slopes were kept as control without adding any sample and both control and test samples were incubated at 35 °C ± 1 °C for 24 h and observed for sterility. Samples (1 ml) of vaccines are inoculated into 4 bottles (100 ml each) of thioglycolate medium and Soya bean Caesin Digestive medium. Four bottles of Thioglycolate medium were incubated at 35 °C and other 4 bottles were incubated at 20–22 °C for 14 days.
2.7 Cell mass determination (opacity test)
0.5 ml of the test vaccine was taken in the opacity tube and diluted the sample with normal saline until the opacity was identical with 5th International reference preparation of opacity (5th IRP) when compared by eye under uniform background, taking the dilution factor into account, and opacity was calculated.Citation9
2.8 Purification of B. pertussis proteins
100 ml of B. pertussis was clarified through centrifugation for 10 min at 6000 rpm in a cooling condition. The pellet was collected and subsequently precipitated with 5% of TCA for 15 min at the ambient temperature (+18 to 22 °C). The supernatant was taken and analyzed for total protein concentration and OD absorbance tests.
2.9 Preparation of column chromatography for gel filtration and processing of B. pertussis
A 1.5 cm diameter thickened (Borosil), two-sided open glass tube was taken for Sephadex 50 (G50) (Pharmacia, Uppsala, Sweden) matrix (). The bottom side was connected with silicon tubing, and the lower bed was made with Teflon coated perforated neoprene O rings. The surface was covered with muslin cloth and cellulose acetate hydrophilic membranes (0.45 and 0.22 μm). Prior to usage, it was checked for leakage. Subsequently 10 g of G50 matrix was weighed and dissolved in buffer A (100 mM NaCl in 15 mM PBS) and the slurry was degassed and loaded with the help of glass rod to the column, while pouring the outlet was blocked at starting time and then later it was opened with controller.
The bed height was optimized at various heights with different bed volumes. Furthermore, the B. pertussis, TCA precipitated, centrifuged supernatant was diluted with 1:10 with buffer A. Around 10 ml of the material was loaded to the G50 column. Prior to loading the G50 column was equilibrated with 300 ml of buffer A for equilibrations. Once this was achieved, the run was started. 2 ml volume of 15 fractions was eluted, labeled and stored at +4 °C until further use. The condition of the run must be +4 °C because the pertussis proteins are heat labile. Three consecutive runs were conducted for each G50 experiment.
2.10 Purification of HLT
G50 purified fractions were analyzed for its qualities through optical absorbance, and total protein SDS. The selected fractions were pooled and estimated for the total proteins. It was loaded to the DEAE matrix in the column of 150 mm × 1000 mm, with bed height and bed volume of 45 mm and 60 ml, respectively. The purification procedure was conducted at 4 °C, the column was equilibrated with 100 mM NaCl in 15 mM PBS pH 7.5, and the proteins were eluted with the elution buffers (buffer B-15 mM PBS in 300 mM NaCl and buffer C-15 mM PBS in 600 mM NaCl) and were eluted at a flow rate of 75 ml/h. The eluted fractions (2 ml) were assayed for absorbance at 280 nm and for ischemia-inducing activity.
2.11 Analysis of purified protein
The SDS–PAGE was analyzed as per Laemmli et al.Citation10 with significant modifications in a vertical electrophoresis Unit (Bio-Rad Laboratory USA). Samples were loaded in the wells and the gel was run at 180 V. After the tracking dye reached the bottom, the gel was removed gently and silver stained. The gel was submerged in fixative and left overnight (50% methanol, 12% Glacial Acetic Acid, 37 μl formaldehyde). It was then impregnated in Sodium thiosulphate – Na2S2O3·5H2O (0.02 gm%). Finally, the gel was treated with the developer (6% Na2CO3, 40 μl of 0.02 gm% Sodium Thiosulphate – Na2S2O3·5H2O, 10 μl of formaldehyde). Once the gel was stained, it was stored in storing solution (50% methanol, 12% Glacial Acetic Acid).
2.12 Total protein analysis
The total protein was calculated by Lowry’s method.Citation11
2.13 Qualitative estimation of proteins by absorbance
The absorbances of the protein solution were measured and the A260/A280 values for the protein samples were calculated and compared with the standard values.Citation12
2.14 Measurement of ischemia-inducing activity of HLT
DEAE purified selected fraction samples were qualitatively checked (100 μl) for an ischemic inducing activity in guinea pigs (Hartley female guinea pigs’ weight range between 300 and 350 g), and the samples were further diluted with PBS pH 7.2, and administrated to guinea pigs intracutaneously. The activity was observed after 24 h.
2.15 Analysis of immune response
Animals were immunized through intramuscular route: the DEAE purified pooled fractions with Fraund’s complete adjuvant. As an adjuvant on primary dose the booster on the 7th day was observed. On 14th day venous blood ranging from 3 to 5 ml was collected from the Guinea pigs. The collected blood was kept at room temperature until coagulation was noticed. This was followed by incubation at 35 °C for 30 min, and storage at 4 °C for 24 h. The serum was removed by centrifugation and 0.01% thiomersal was added as preservative.
2.16 Ouchterlony’s double immunodiffusion (ODD)
Guinea pigs were immunized subcutaneously with purified protein for 1 week intervals. The blood samples were collected and the serums were separated in sterile condition. The serum samples were taken for ODD assay.Citation13 Four wells, 3 mm in diameter and 1 cm in distance from each other, were made on the agarose plate. The gel was allowed to solidify at room temperature and the wells were punched using a gel punch, resulting in corresponding pairs of wells. Next, 15 μl of antiserum and B. pertussis (134) was added into each well and was left standing for 3 days in a humid atmosphere at 37 °C. The precipitation line was observed using a scattered light illuminator.
3 Results and discussion
The B. pertussis vaccine seed strain 134 was subcultured aseptically on BG medium. After the seed growth it was consequently propagated in shaker culture employed with B2 broth medium and the sterility of the culture medium was ensured. Before analysis with chromatography technique, the B. pertussis culture was precipitated with TCA. Prior to this, culture was clarified by centrifugation at 6000 rpm for one hour in cooling conditions (to avoid protein loss). Subsequently, the supernatants and pellets of precipitated TCA were stored and OD was taken for (A) culture neat, (B) 1:10 diluted sample of the culture, (C) crude TCA supernatants and (D) TCA pellets and were evaluated for OD at 280 nm and the values are found to be 1.245, 0.160, 1.635 and 0.028 respectively.
The crude and TCA supernatants showed higher OD values which represent the amount of protein present (). This was also reflected in the total protein quantification (207.5 and 272.5 μg respectively). These proteins may also contain several virulence factors’ proteins of B. pertussis such as PT, FHA, Fimbriae, PRN, ACT, HLT and endotoxins.
Table 1 Total protein analysis.
When the column of 4 cm (6 ml of bed volume of Sephadex G50) was used, the purification peak analysis of pertussis proteins and the level of impurities were found to be in symmetry which indicated the maximal recovery of bacterial proteins and the removal of maximal quantity of impurities. At that same time the OD values of all the different heights of the matrix was found to be 1.402, 1.802, 1.902 and 2.102 respectively, the total protein concentrations of the columns were 233.6, 300.3, 317 and 350 μg/ml. In this experiment, the 4 cm height peak was symmetry and the total protein was also higher when comparing with others ().
Table 2 Bed height analysis for the optimal recovery of pertussis protein.
3.1 G50 purification
The TCA precipitated B. pertussis cell supernatant was loaded to the 4 cm bed height of the G50 column, and prior to loading the cell extract was mixed with loading buffer (buffer A). The ratio of the crude and buffer was 1:1; this was loaded and the prime purification was done by the principle of specific gravity of 20 ml of the material and was subjected to 2 ml of fraction volume. A total of 15 fractions were collected by additional loading of buffer A. All the fractions were analyzed at 280 nm: OD, total protein content by Lowry’s method, and the OD values ranged between 0.074 and 0.214, and the total protein content range between 12.33 μg/ml and 35.67 μg/ml. Out of 15 fractions, 5 fractions were selected based on the protein concentration as well as the OD values for further DEAE purification.
3.2 DEAE purification
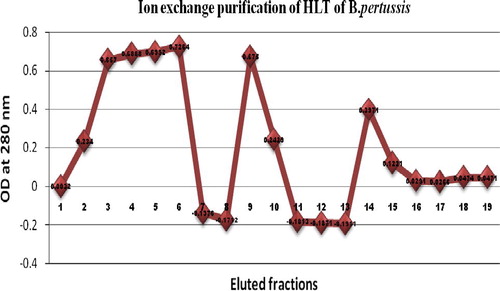
G50 purified selected fractions (5 numbers) were further subjected to DEAE purification. The fractions were pooled and diluted with buffer A (equal volume) and loaded to 5 cm bed height DEAE (10 g/ml bed volume) column. A total of 19 fractions were eluted and the absorbance was analyzed.
The fractions 9 and 14 had the higher OD values of 0.675 and 0.397 respectively when compared with other fractions. The fraction 9 was eluted by the 300 mM of NaCl Strength buffer. In case of fraction 14, it was eluted with 600 mM NaCl strength. All the fractions were additionally focused on total protein content by Lowry method. The values tabulated were () 112.5 μg/ml and 66.1 μg/ml of total protein content of fractions 9 and 14 respectively.
3.3 Analysis of purified protein
DEAE purified HLT proteins from culture were subjected to SDS–PAGE analysis which gives a clear molecular mass of 140 kDa (). Similarly, the crude fraction showed different molecular bands ranging from 40 kDa to 205 kDa. This result indicates that apart from HLT (140 kDa), the pertussis vaccine also contained other virulence factors with different molecular weight. Although some contamination was observed on SDS gels at low molecular weight, it is likely that this resulted in part from nicking of the intact toxin molecule by proteolytic enzymes. Molecular size gave variable results, probably because of extraordinary biological activity of the HLT. HLT is reported to be serologically related, and molecular weights of 102,000Citation14 and 190,000Citation15 for the HLT of Bordetella bronchiseptica have been reported by different investigators.
3.4 ODD assay
The antiserum raised against purified HLT in guinea pigs showed that in ODD assay, serum obtained was tested for reactivity against specific antigens by immunodiffusion. Immune sera were tested against DEAE purified HLT applied to the wells (1, 2 and 4). Wells 1, 2 and 4 contained 10 μl of culture centrifuged pellet, pooled DEAE purified fractions (fractions 9 and 14) and TCA precipitated culture. Well 3 contained PBS, the center well contained immune serum after diffusion, and the gels were fixed and stained with coomassie blue (). The qualitative immunogenicity shown in this test was of superior quality compared to all three samples, especially the DEAE pooled materials diffusion line is thicker and the other samples are showing positive.
This result indicated that the guinea pig is an ideal laboratory animal for the preparation of antiserum against HLT.
3.5 Ischemic inducing activity
Ischemia-inducing activity of DEAE purified HLT from B. pertussis strain 134 Samples (100 μl) of an HLT preparation which had been diluted logarithmically in phosphate-buffered saline, pH 7.2. These were injected intracutaneously into the shaved back of female Hartley guinea-pigs weighed 300–350 g and showed colored lesions, ranging from light reddish (erythema) to purple (hemorrhagic necrosis) after 24 h (). The minimum quantity of protein producing an ischemic lesion 10 mm in diameter, is defined as the minimum ischemia-inducing dose (MID).Citation16 The similar studies by Kurokawa et al. (1969), in their experiments showed that the HLT preparations which induced hemorrhagic lesions in guinea pigs produced ischemic lesions in rabbits either with or without haemorrhage.Citation17 Thus, rabbits appeared to be less susceptible than guinea pigs to the hemorrhagic action of crude HLT preparations.
Nakase and Endoh (1985) in their study reported that, the dermonecrotic toxin activity of B. pertussis inhibits a Na+-k+-ATPase in vitro assay and causes contraction of the smooth muscle surrounding peripheral blood vessels, resulting in vasoconstriction and increased perfusion pressure in perfused lungs from guinea pigs.Citation14,Citation18
4 Conclusion
Pertussis vaccine has been used for nearly 9 decades worldwide for the universal immunization of children to control whooping cough disease. The vaccine is usually administered in the National Childhood Program as a combined vaccine DTwP or DTaP. B. pertussis organism produces several virulence factors such as PT, ACT, and FHA, including endotoxin and Heat Labile Toxin (HLT). Each virulence factor has their own role of immunogenicity and biological property in the final WCpV vaccine. So far there has been little systematic and quantitative study of HLT by different strains of B. pertussis under different culture conditions. The mechanism action of HLT is unknown and possible involvement of HLT in pathogenesis of pertussis is also unclear. In this study we purified the HLT through two stages such as G50, subsequently with DEAE technique as indigenously developed. The HLT was assessed through SDS–PAGE and showed molecular weight 140 kDa as a single polypeptide. Furthermore, the immunogenicity was also analyzed. ODD revealed its high-quality and the guinea pigs sera were obtained against DEAE. Purified pooled fractions were used as diagnostic purposes viz., antibodies and development of ELISA kit. In addition, toxicity assessment in guinea pig study found that HLT induced hemorrhagic lesions at the injection site around 10 mm thickness. The crude purified toxin produced whole range of hemorrhagic and ischemic inducing activity in the guinea pig experiment found that the HLT has an intracellular location. It is necessary to disrupt the activity of toxin in the course of vaccine preparation. Hence, HLT should be detoxified by heat inactivation at 56 °C for 30 min, which is the most prepared method during Wcpv production. From this study, it is concluded that the toxoid HLT plays a significant role in the pathogenic activity in laboratory animals. Therefore, the careful evaluation of each toxin during the development of new and safer vaccines is important. In this attempt, we purified the HLT using indigenous technique, to evaluate a suitable vaccine which is capable of eliciting protective immune responses after passing standard quality and safety tests. The implementation of these assays will contribute to the enhanced and safer activity of future vaccines.
Conflict of interest
None declared.
Acknowledgments
We would like to thank Dr. B. Sundran and Dr. K.N. Venkataramana, Assistant Directors PII, Coonoor for their grateful guidance, and constant support, help to carry out this work. We extend our thanks to Mr. R. Vijayakumar, Department of Biochemistry, North Eastern Hill University, Shillong, India for his assistance in internal reviewing of this manuscript, Mr. Balaganesh Kuruba, Illinois Institute of Technology, Chicago, USA and Mrs. Swetha Salla, Alabama A&M University, Alabama, USA for proof reading of this manuscript.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 23 June 2015
References
- Centers for Disease Control and Prevention (CDC). Pertussis – United States, 1997–2000. MMWR. Morbidity and mortality weekly report, vol. 51; 2002. p. 73–6.
- D.P.GreenbergC.-H.W.von KonigU.HeiningerHealth burden of pertussis in infants and childrenPediatr Infect Dis J242005S39S43 <http://journals.lww.com>
- J.BordetO.GengouL’endotoxine coquelucheuAnn Inst Pasteur (Paris)231909415419
- C.R.Gentry-WeeksB.T.CooksonW.E.GoldmanR.B.RimlerS.B.PorterR.CurtissDermonecrotic toxin and tracheal cytotoxin, putative virulence factors of Bordetella aviumInfection Immun56198816981707 <http://iai.asm.org>
- W.E.GoldmanTracheal cytotoxin of Bordetella pertussisA.C.WardlawR.PartonPathogenesis and immunity in pertussis1988John Wiley and SonsChichester (UK)231246
- Cruickshank R, Duguid JP, Swain RHA. Medical microbiology. A guide to the laboratory diagnosis and control of infection. Medical microbiology. A Guide to the Laboratory Diagnosis and Control of Infection; 1965.
- World Health OrganizationManual for the production and control of vaccines: pertussis vaccineExpanded programme on immunization1977World Health OrganizationCity <http://www.who.int>
- K.C.ShivanandappaK.R.ManiS.JagannathanR.VijayakumarAssessment of Bordetella pertussis strain 509 cell mass yield in baffle and vortex mode of agitation during large scale industrial fermentor cultivationJ Bacteriol Parasitol6201516 http://doi:10.4172/2155-9597.1000214
- F.T.PerkinsF.W.SheffieldA.S.OutschoornD.A.HemsleyAn international collaborative study on the measurement of the opacity of bacterial suspensionsJ Biol Stand11973110 http://doi:10.1016/0092-1157(73)90025-5 <http://www.sciencedirect.com>
- U.K.LaemmliCleavage of structural proteins during the assembly of the head of bacteriophage T4Nature2271970680685
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem1931951265275
- E.LayneSpectrophotometric and turbidimetric methods for measuring proteinsMethods in enzymologyvol. 31957Academic Press447454 <http://www.sciencedirect.com>
- O.OuchterlonyDiffusion-in-gel methods for immunological analysisProg Allergy51958178
- Y.NakaseM.EndohBordetella heat-labile toxin: further purification, characterization and mode of actionDev Biol Stand61198593102
- K.KumeT.NakaiY.SamejimaC.SugimotoProperties of dermonecrotic toxin prepared from sonic extracts Bordetella bronchisepticaInfect Immun521986370377 <http://iai.asm.org/content/52/2/370.abstract>
- M.EndohM.NagaiD.L.BurnsC.R.ManclarkY.NakaseDevelopment of a cell culture assay for Bordetella parapertussis heat-labile toxinBiologicals181990309313 http://doi:10.1016/1045-1056(90)90035-X <http://www.sciencedirect.com>
- M.KurokawaS.IshidaS.AsakawaAttempts at analysis of toxicity of pertussis vaccine. II. Quantitative determination of the heat-labile toxin by skin reactionJpn J Med Sci Biol221969293307 <http://europepmc.org>
- M.EndohM.AmitaniY.NakasePurification and characterization of heat-labile toxin from Bordetella bronchisepticaMicrobiol Immunol301986659673 http://doi:10.1111/j.1348-0421.1986.tb02992.x