Abstract
Background
Pancreatic cancer is one of the most lethal malignant tumors which remains a rampant killer across the globe. Lack of early diagnosis and toxic drugs have failed to improve the survival rate of pancreatic cancer patients, thus new agents that are safe, available and effective are urgently needed.
Objective
The study aimed to investigate the efficacy of Agarwood essential oils in the inhibition of metastasis and induction of apoptosis in the pancreatic cell line (MIA PaCa-2).
Methods
Essential oils of Aquilaria crassna were obtained by hydrodistillation. Chemical characterization was analyzed using FTIR and GCMS. The effects of essential oils against three steps of metastases have been investigated, including cell proliferation, migration and clonogenicity. Hoechst and rhodamine assays confirmed the mechanism of pancreatic cancer cell death.
Results
The results showed that essential oils exhibited potent cytotoxic activity against MIA PaCa-2 cells with an IC50 (11 ± 2.18 μg/ml). Cell migration was effectively inhibited at (10 μg/ml). Moreover, at a sub-toxic dose (5 μg/mL), essential oils obstructed the colony formation properties of MIA PaCa-2 significantly. The mechanism of cell death was determined due to the induction of nuclear condensation and disruption of mitochondrial membrane potential in the cells. Interestingly, several active components were existed in the chemical profile of the essential oils extract such as β-Caryophyllene, 1-Phenanthrenecarboxylic acid, azulene, naphthalene and Cyclodecene.
Conclusion
The present study elucidated for the first time the anti-pancreatic cancer properties of A. crassna essential oils, It can be concluded that the anticancer effects of the extract could be due to the synergistic effect of the biologically active phytoconstituents present in the essential oils.
1 Introduction
Cancer is the general name for a heterogeneous group of more than 100 diseases which arises from dysregulation of the normal cellular mechanism, characterized by alterations in expression of multiple genes, leading to local tissue invasion and eventually metastasis.Citation1 Pancreatic cancer is one of the most aggressive malignant solid tumors which remains the fourth leading cause of cancer-related death with an overall 5-year survival rate of less than 5%. Recently, about 227,000 cases of deaths were reported annually worldwide.Citation2,Citation3 Several risk factors have been implicated in this malignant disease including smoking, alcohol, diet, age and family history.Citation4 However, the most critical hurdle in the pancreatic cancer therapy is metastatic nature of the disease, where the malignant cells detach from the primary tumor location and grow in distant organs.Citation5 Clinical studies have estimated that only 10% of mortality is caused by the primary tumors, whereas metastasis alone contributes about 90% of cancer deaths.Citation6 Plants have been utilized for medicinal purposes since the early stage of civilizations. According to a botanical survey approximately there are 350,000 plants species that have been identified and recorded globally out of which, about 35,000–70,000 plants have been used for medicinal purposes and wonderingly, just 0.5% of it has been chemically studied and pharmacologically validated so far.Citation7 The World Health Organization (WHO) has estimated that 80% of population in the developing countries relies on traditional herbal medicine for the primary health care needs. In more recent times, phytochemicals such as paclitaxel, vincristine, and camptothecin have become major and significant sources for chemotherapy in cancer treatment protocols, which provided the most successful, alternative and complementary anticancer regimenCitation8.
Aquilaria crassna (Thymelaeaceae) is an evergreen tree. It is an important traditional medicinal plant, used to treat various infectious ailments including inflammatory diseases. It has also been used by Arabs and Japanese to treat digestive and sedative disorders.Citation9,Citation10 Extracts of A. crassna have revealed significant antioxidant and cytotoxic properties.Citation11 Moreover, different parts of A. crassna have been studied and explored dynamic biological effects such as antioxidant, anti-ischemic, antifungal, and antibacterial effects.Citation12–Citation15 To the best of our knowledge, there is no report on essential oils (EOs) extracted from Agarwood used against human pancreatic cancer. In the present study anti-metastatic properties of EOs on highly invasive and metastatic pancreatic cancer cell line (MIA PaCa-2) was evaluated. In addition, chemical profile of EOs of A. crassna was studied to identify the active principles present in the plant.
2 Materials and methods
2.1 Plants material and extraction of essential oils
Fresh sample of A. crassna stem bark was obtained from a local farm in Kajang, Selangor, Malaysia, in the year 2013. In addition, flowers and leaves with twigs were collected for taxonomical authentication and deposited at the School of Biological Sciences, USM (Ref. No. USM/122083). The bark of A. crassna was cleaned thoroughly, cut into small slices and ground mechanically. Ground material of the stem bark (500 g) was macerated with distilled water (5 L) at room temperature (25 ± 2 °C) for 1 week. Finally, hydrodistillation was employed on the extract for 48 h at boiling temperature of water. Essential oils (EOs) were obtained by Clevenger-type apparatus. The EOs were collected as pale-yellow liquid with 12.6 g yield.
2.2 Characterization techniques
2.2.1 FT-IR
The extracted contents were collected as thick yellowish to orange colored fluid so a thin layer method was used to collect FT-IR patterns. According to this method the contents were layered between thallium bromide disks. The disks were then exposed to IR radiations to collect the transmittance patternsCitation16.
2.2.2 Gas chromatography–mass (GC–MS) spectral analysis of the extract
Quantitative chemical analysis of A. crassna stem bark was carried out using GC–MS with an aim to identify the active compounds that might be responsible for the anti-pancreatic effect. The assay conditions were as follows: HP-5MS capillary column (30 m × 0.25 mm ID × 0.25 μm, film thickness); held at 70 °C for 2 min, raised to 285 °C at a rate of 20 °C/min and held for 20 min; 285 °C for MSD transfer line heater; carrier helium at a flow rate of 1.2 mL/min; 2:1 split ratio. 1 μL solution of SF-1 in chloroform (10 mg/mL) was injected automatically. Scan parameter low mass: 35 and higher mass: 550. The constituents were identified by comparison with standards using NIST 02. A total ion chromatogram (TIC) was used to compute the percentage of the identified constitutes.
2.3 Chemicals and reagents
Dulbecco’s Modified Eagle’s medium (DMEM), trypsin, phosphate buffer saline (PBS) and heat inactivated fetal bovine serum (HIFBS) were purchased from Gibco, UK. Standard drug fluorouracil (5FU), 3-(4,5-dimethylthiazol-2-yl)-2-5-diphenyltetrazolium bromide (MTT) reagent, crystal violet, rhodamine 123, and Hoechst 33258 were purchased from Sigma–Aldrich, U.S.A.
2.4 Cell lines and culture conditions
Human pancreatic carcinoma cell line (MIA PaCa-2 ATCC® CRMCRL1420™) was purchased from ATCC (Rockville, MD, U.S.A.). Cell line was grown at 37 °C and 5% CO2 in sterile DMEM medium with 10% fetal bovine serum and 2.5% horse serum. Then, the cell was supplemented by 4 mM l-glutamine and penicillin–streptomycin.
2.5 Cell viability assay
Viability of the (MIA PaCa-2) was determined by MMT assay according to the method previously described.Citation17 Cells were seeded at 1.5 × 104 cells in each well of 96-well plate in 100 μL of fresh culture medium and were allowed to attach for overnight. For screening, the cells (70–80% confluency) were treated with the truffle extracts serial concentration (3.1–200 μg/mL) to get the IC50. After 48 h of the treatment the medium was aspirated and the cells were exposed to MTT solution prepared at 5 μg/mL in sterile PBS was added to each well at 10% v/v in the respective medium and was incubated at 37 °C in 5% CO2 for 3 h. The water insoluble formazan salts was solubilized with 200 μL DSMO/well. Absorbance was measured by infinite® Pro200 TECAN Group Ltd., (Switzerland) at primary wavelength of 570 nm and reference wavelength of 620 nm. Each plate contained the samples, negative control and blank. DMSO (1% v/v) was used as a negative control. 5-fluorouracil was used as standard reference control. The assay was performed in triplicate and the results were presented the mean ± SD.
2.6 Cell migration assay
The effect of essential oils extract on the migration of MIA PaCa-2 cells was studied by the wound healing assay as previously described.Citation18 MIA PaCa-2 cells were seeded separately in 6-well plate and incubated for 48 h to achieve almost 100% confluent monolayer. A straight scratch was created using a 200 μl micropipette tip. The cells were washed with PBS and incubated in serum-reduced medium (2% FBS) containing Eos extract (5 and 10 μg), control cell received 0.1% DMSO. The wound was photographed at zero, 12 and 24 h. The distance of cell-free area was measured using Leica Quin software, and the results are presented as average of percentage of inhibition of migration in comparison with the negative control (±SD, n = 6).% inhibition of cell migration = [1 − (the width at the indicated times/the width at zero time)] × 100
2.7 Colony formation assay
The effect of essential oils extract on MIA PaCa-2 cells was investigated by colony formation assay.Citation19 Briefly, the cells (500 cells/ml) were separately seeded in 6-well plate and incubated for 12 h. Subsequently, the cells were treated for 48 h with Eos extracts (5, 10 and 20 μg/ml), or 5-fluorouracil (10 μg) or 0.1% DMSO. The cells were maintained until sufficiently large colonies (⩾50 cells) were produced for 10 days. The colonies were fixed, stained with 0.2% crystal violet and counted under stereomicroscope. Percentage of plating efficiency (PE%) was calculated. The results are presented as the mean ± SD (n = 3).
2.8 Determination of nuclear condensation and mitochondrial membrane potential
MIA PaCa-2 cells (0.5 × 106 cells/mL) were cultured in 24-well plates. After cell attachment, then the cells were received new medium with or without Eos extracts (5 and 10 μg/mL). The cells were washed twice with PBS, fixed with 4% paraformaldehyde for 30 min, washed with PBS, and stained simultaneously (30 min) with Hoechst 33258 at 10 μg/mL and rhodamine 123 at 5 μg/mL. Then, the cells were washed twice and observed under fluorescent microscopy. For chromatin condensation analysis, cells with bright condensed or fragmented nuclei were considered apoptotic; the images were acquired after 6 and 12 h.
The loss of mitochondrial membrane potential was indicated by the appearance of more brightly-stained cells; the images were captured after 6 and 12 h. The apoptotic cells were counted in 4 randomly selected fields per well. DMSO (0.1%) and 5-fluorouracil (10 μg) were used as negative and positive controls, respectively. The apoptotic index was calculated as the percentage of apoptotic cells compared to the total number of cells, and was presented as a mean ± SD. (n = 10).
2.9 Statistical analysis
Statistical difference between the treatments and the control was evaluated by one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test. Differences were considered significant at p < 0.05, and p < 0.01.
3 Results
3.1 Characterization of Agarwood essential oils using FT-IR and GC–MS
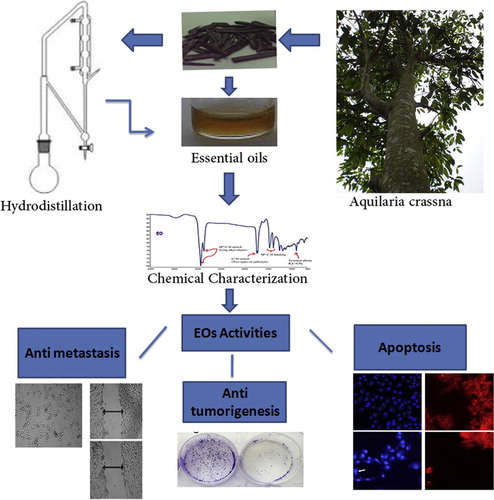
Hydrodistillation of Agarwood gave a pale yellow oil with a strong pleasant aromatic odor (yield of 2.52%.). The chemical characteristic of the investigated essential oils is presented in , where the FT-IR spectrum depicted two sharp (twin) vibrational bands at 1711 and 1725 cm−1 indicating the presence of at least two types of carbonyls. Furthermore, two strong vibrational bands at 2857 and 2924 cm−1 assigned the presence of alkyl chains. This is also evident from the appearance of another set of two peaks at 1377 and 1455 cm−1 for Csp3–H bending vibrations (alkyl chain). Also, a weak vibrational band at 886 cm−1 indicated the presence of alkene groups. Additionally, a broad vibrational band at 1588 (for C=C) and 3466 cm−1 (for –OH) indicated the existence of phenol group. Moreover, the Eos of Agarwood was subjected to GC–MS analysis to quantify the major chemical constituents and molecular weight, respectively. The GC–MS data such as retention time, % area peak, molecular formula and molecular weight obtained for the major constituents are given in . Detailed characteristics of the peaks identified in the GC–MS were presented in the . The quantitative analysis of essential oils showed that the major dominant peak corresponds to β-Caryophyllene (8.111%), Octamethyl (7.103%), 2-Naphthalenemethanol (6.193%), alpha.-Caryophyllene (4.755%), benzenedicarboxylic acid (4.642%), azulene (3.925%), naphthalene (2.694%) and cyclodecene (2.583%).
Figure 1 FT-IR spectral features of the extract A. crassna stem bark depict the major chemical groups as the functional components of the bioactive phytoconstituents.
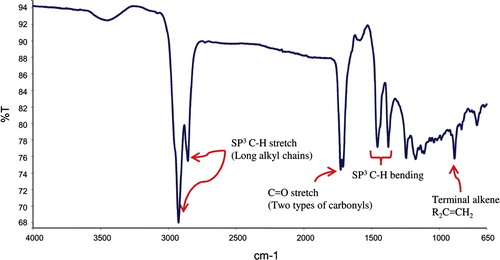
Figure 2 Gas chormatographic analysis of the essential oils of Aquilaria crassna on chromatogram of GC–MS. The pie charts depict the relative chemical compositions of sub-fractions. The details of the peaks are given in the .
Table 1 GC–MS quantitative estimation of essential oils phytochemicals.
3.2 Effect of EOs on the cell viability
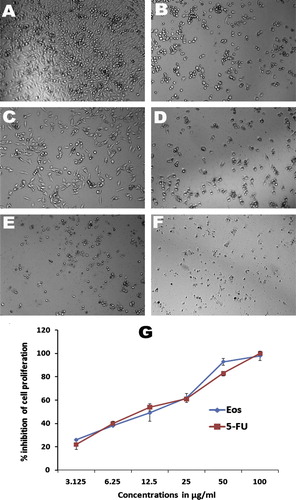
The MTT cell proliferation assay was used to confirm the effect of Eos on MIA PaCa-2 cell viability. As shown in , Eos inhibited the proliferation of MIA PaCa-2 in a dose dependent manner. Although moderate effect on cell viability was detected at lower concentrations (3.12 and 6.25 μg/mL), EOs at higher concentrations were potentially decreased the cell viability with a median inhibitory concentration IC50 of 11 ± 2.18 μg/mL. 5-FLU, on the other hand, a standard therapeutic drug for pancreatic cancer, was used as a positive control, and showed significant toxicity toward MIA PaCa-2 cells with an IC50 of 6.5 ± 1.4 μg/mL.
Figure 3 Effect of essential oils on cellular morphology of human pancreatic cancer. Photomicrographic images of pancreatic cancer cell lines were taken under an inverted phase-contrast microscope at 200× magnification using a digital camera at 48 h after treatment with essential oils. (A) Cells treated with vehicle (0.1% DMSO). The cells treated with 0.1% DMSO (Vehicle) revealed an aggressive proliferation. (B) Cells treated with essential oils at 6.25 μg/ml. clear inhibition at sub-toxic dose. (C) Cell treated with essential oils at 12.5 μg/ml. (D) Cell treated with essential oils at 25 μg/ml. (E) Cell treated with essential oils at 50 μg/ml. (F) Cell treated with standard drug, 5-fluorouracil at 10 μg/ml. (G) Dose-dependent anti-proliferative effect of essential oils and 5-fluorouracil on pancreatic cancer cell line was assessed by MTT-assay (values are represented as mean ± SD, n = 3).
3.3 Eos inhibited cell migration
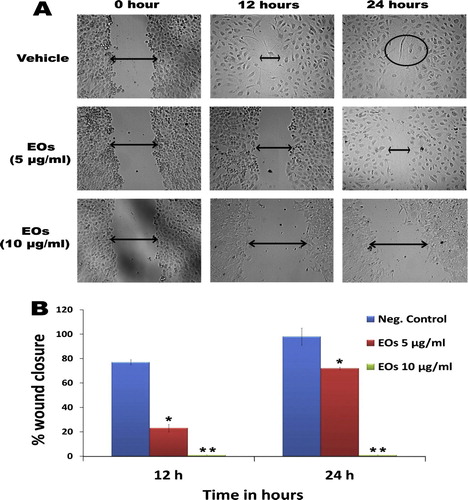
The effect of EOs on the migration of MIA PaCa-2 cells was determined by the wound healing assay. The results are presented as percentage inhibition of migrating cells with Eos relative to untreated cells as shown in A. The percentage of the wound closure after 24 h was 92.6 ± 4.5% in the untreated cells, whereas Eos displayed dose and time dependent, at subcytotoxic concentrations 5 μg/mL the percentage of wound closure calculated after 12 h was 21.1 ± 3 (P < 0.05). The value of Eos at 10 μg/mL was significantly inhibited the motility of MIA PaCa-2 cells (P < 0.01) B.
Figure 4 (A) Anti-migratory effect of essential oils against pancreatic cancer cell line. Interestingly, the extract displayed pronounced effect even at its subtoxic concentration (5 μg/ml), the essential oils caused dislodgement of monolayer of MIA PaCa-2cells, which can be visualized under microscope. The photomicrograph., taken under an inverted phase-contrast microscope at 200× magnification using a digital camera at 12 and 14 h after treatment with essential oils. (B) Graphical representation of the time and dose and time-dependent inhibitory effect essential oils on migration of MIA PaCa-2cells (Values are in mean ± SD, n = 6, ∗ = P < 0.1, ∗∗ = P < 0.005).
3.4 EOs inhibited MIA PaCa-2 colony formation
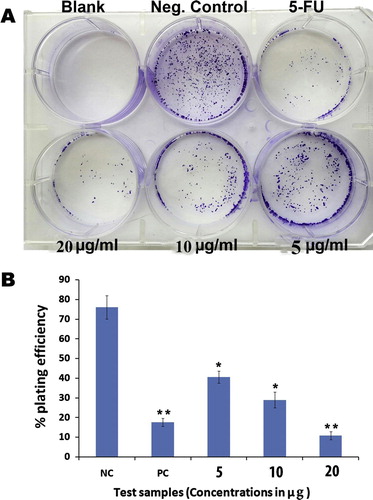
MIA PaCa-2 cells were treated with essential oil extracts after 48 h in the concentration ranging from (5–20 μg/ml) and the results showed significant dose dependent inhibition of the pancreatic cancer cells as depicted in . Percentage of plating efficiency (PE) in negative control group was 76 ± 2% which was significantly decreased to 41 ± 3%, 28.6 ± 2% and 10 ± 4% with Eos treatment at 5, 10 and 20 μg/ml respectively A. The graphical representation B illustrates the quantitative estimation of the EOs activity against the clonogenicity of pancreatic cancer cells. The results of colony formation assay suggested that Eos can significantly obstructed colonization of MIA PaCa-2 cells.
Figure 5 (A) Effect of essential oils on survival of MIA PaCa-2cells colonies in colony formation assay. The picture clearly depicts the strong anti-clonogenic effect of essential oils on colonies of the cancer cells. (B) The graphical representation illustrates the percentage of plating efficiencies after the treatment of the cells with essential oils at 5, 10 and 20 μg/ml in comparison with negative control and the standard reference drug, 5-flourouracil. The results were presented as mean ± SD, n = 3 (∗ = p < 0.05, ∗∗ = p < 0.01).
3.5 EOs induced nuclear morphological changes of MIA PaCa-2 cells
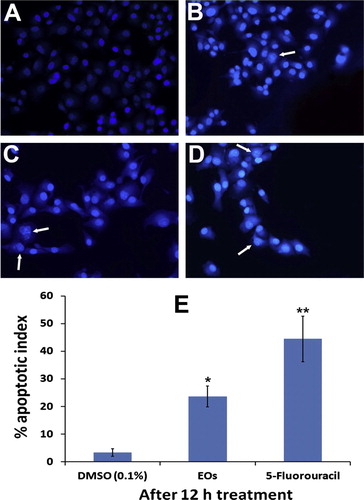
The capability of EOs extract on nuclear morphology of MIA PaCa-2 cells was assessed by staining the nucleus with Hoechst 33258 stain. A illustrates that the cells treated with 0.1 DMSO showed distinct morphological characteristics with prominent nuclei and aggressive growth, whereas the signs of early and late apoptosis were noticed in nuclei of the treated cells with EOS in a time dependent manner. EOs at 10 μg/mL caused nuclei condensation (arrows) at 6 h treatment B, whereas the typical apoptotic morphology was observed after 12 h with clear signs of nuclear shrinkage and crescent-shaped structure (arrows) indicates the morphological features of apoptosis C. These results were compared with the standard drug, 5-fluorouracil D. The apoptotic index of the negative control was 1.9 ± 0.2% which was increased to 22.6 ± 4 with EOs 10 μg/mL after 12 h E.
Figure 6 The photomicrographs depict the images of MIA PaCa-2cells with Hoechst 33258 stain, under an inverted phase-contrast microscope at 200× magnification using a digital camera at 6 and 12 h after treatment with essential oils. (A) The cells treated with 0.1% DMSO (Vehicle) revealed an intact cell membrane with prompt and evenly distributed nucleus in cytosol. (B) Cells treated with 10 μg/ml of essential oils after 6 h. The cells displayed early stage apoptotic such as membrane blebbing and chromatin condensation. (C) Cells treated with 10 μg/ml of essential oils after 12 h. The arrows indicate the late staged apoptotic, signs such as of nuclear dissolution including crescent shaped apoptotic nuclei. (D) Cells treated with standard reference, 5-flourouracil (10 μg/ml) also displayed significant induction of apoptosis in the cells. (E) Graphical representation of percentage of apoptotic indices MIA PaCa-2cells. The apoptotic index was expressed as a percentage of the ratio of number of apoptotic cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), ∗ represents p < 0.05 and ∗∗ represents p < 0.01.
3.6 Eos induced loss of the mitochondrial membrane potential in MIA PaCa-2 cells
The effect of Eos on the mitochondrial membrane potential of MIA PaCa-2 cells was studied by staining the cells with rhodamine 123 in order to measure the mitochondrial function in the treated cells. The result of this study showed a strong intensity of fluorescence in the untreated cells, indicates the aggressive growth and proliferation of the cells A, whereas the fluorescent signal decreased in the cells treated with EOs in a time dependent manner B and C. On the other hand, 5-fluorouracil exhibited remarkable reduction for the fluorescent signal D.
Figure 7 The photomicrographs depict the images of HCT 116 cells with Rhodamin 123 stain, taken under an inverted phase-contrast microscope at 200× magnification using a digital camera at 6 and 12 h after treatment with essential oils. (A) Cells treated with vehicle (0.1% DMSO). (B) Cells treated with essential oils 10 μg/ml after 6 h. (C) Cells treated with essential oils 10 μg/ml after 12 h. (D) Cells treated with standard reference, 5-flourouracil. (E) Graphical representation of percentage of apoptotic indices. The apoptotic index was expressed as a percentage of the ratio of number of unstained cells to the total number of cell in 10 different microscopic fields. Values are presented as mean ± SD (n = 10), ∗ represents p < 0.05 and ∗∗ represents p < 0.01.
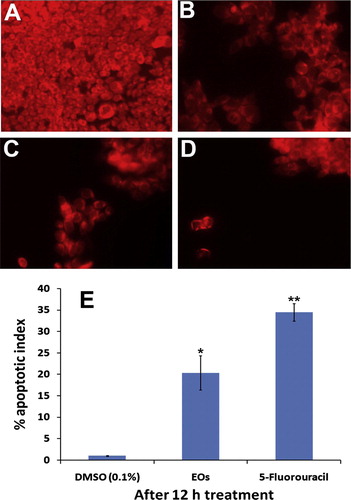
The apoptotic index estimated for EOs treatment on MIA PaCa-2 cells after 12 h treatment was 21 ± 5.4 E.
4 Discussion
Pancreatic cancer is one of the most aggressive and devastating disease, which was characterized by rapid invasion and metastasis. Since the poor diagnosis and lack of chemotherapy response have failed to improve the survival rate of pancreatic cancer patients, thus new agents derived from plants which are safe, affordable and effective have attracted attention of researchers worldwide. However, essential oils are very interesting complex mixtures of volatile compounds, formed by aromatic plants as a secondary metabolites and characterized by a strong odors with a wide range of monoterpenes, sesquiterpenes and aromatic constituents which reported to have a potent anticancer activities against several cancers such as thymol, γ-terpinene from (Carum copticum), Eugenol and β-caryophyllene from (Commiphora gileadensis), α-thujone and terpenyl acetate from (Anisomeles malabarica) and carvacrol from (Santalum album).Citation20–Citation23 In this study, Eos exhibited a strong dose dependent antiproliferative activity, with an IC50 value 11 ± 2.18 μg/ml, and this result may be attributable to the collective phytochemical components, such as phenolic and aromatic contents particularly, β-Caryophyllene, Octamethyl, 2-Naphthalenemethanol, benzenedicarboxylic acid, azulene, and cyclodecene.
The mechanism of cell death due to the cytotoxic effect of the EOs was investigated by apoptosis as revealed in the Hoechst and rhodamine staining. The early and late stages of apoptotic process were observed in the cells treated with EOs in a time dependent manner, whereas untreated cells displayed prominent nuclei and intact cell membrane without significant changes in cellular structures. Morphologically, it is well established that the apoptosis is characterized by shrinkage of the cell, membrane blebbing, cell membrane disruption, condensation of nuclear chromatin, DNA fragmentation and formation of apoptotic bodies.Citation24 Moreover, a loss of mitochondrial membrane potential is a hallmark sign of apoptosis.Citation25 In order to obtain a deeper insight into the apoptotic effect of EOs the mitochondrial membrane potential was used by staining the cells with lipophilic cationic dye rhodamine 123. When the mitochondrial membrane potential decreases, uptake of the lipophilic cationic dye by the cells also decreases and eventually the florescent signal declines exponentially. In the present study, the MIA PaCa-2 cells treated with Eos appeared brighter than the controls with 0.1% DMSO, which indicates that the Eos extracts induced the apoptosis by activation of both DNA and mitochondrial pathways.
Pancreatic cancer is one of the most lethal form of cancers, which was characterized by high rates of local recurrence.Citation26 Because of late diagnosis and lack of chemotherapy response, the 5-year survival rate for pancreatic cancer has still less than 5%. Currently, only gemcitabine and fluorouracil have been used consistently to improve the survival of pancreatic patients with marginal survival advantage.Citation27 More recently, several attempts have been made to find new agents derived from plants which are safe, available and effective against pancreatic cancer. Curcumin has been shown to enhance the antitumor activity of gemcitabine and to suppress NFκB activation with potent capability to control invasion, migration and induce apoptosis.Citation28 Another compound is boswellic acid, obtained from medicinal plant Boswellia serrata, have been found to inhibited the proliferation with K-Ras and p53 mutations, suppress the growth and metastasis of human pancreatic tumors in vitro and in vivo.Citation29 However, the inhibition of multi-step process of metastasis including cell proliferation, migration, invasion and colony formation is the hallmark of cancer treatment. Herein, we evaluated the effect of EOs against three key steps of metastasis. It is postulated that the proliferation and migration of tumor cell plays a dominant role at the initiation of metastatic cascade by increasing the motility of malignant cells, which detach from the primary site and invade the body’s circulation. Once the tumor cells circulated in distant organs, formation of new vascular network will take place (angiogenesis) to supply the oxygen and nutrients, allowing these cells to survive and colonize distant metastatic location. EOs significantly inhibited proliferation and migration of pancreatic cancer cells. More importantly, colonization of cancer cell was clearly obstructed by dose-dependent inhibitory effect of EOs. The significant suppression of numbers of colonies occurred after 7 days due to cytotoxic activity of EOs.
5 Conclusion
In conclusion, this is the first evidence for the effect of Agarwood essential oil on apoptosis and in vitro metastasis of pancreatic cancer cells. These findings suggest that the EOs exhibited significant antimetastatic activities based on the strong inhibitory effect in proliferation, migration and colony formation. Thus, further investigation on the active principles of the extract could probably lead to the discovery of promising chemotherapeutic agents against pancreatic cancer.
Conflict of interest
We have no conflict of interest to declare.
Acknowledgments
Saad S. Dahham would like to acknowledge Universiti Sains Malaysia for the USM Fellowship.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 29 July 2015
References
- R.W.RuddonCancer biology2007Oxford University Press
- A.VincentJ.HermanR.SchulickR.H.HrubanM.GogginsPancreatic cancerThe Lancet3782011607620
- O.P.ZakharovaG.G.KarmazanovskyV.I.EgorovPancreatic adenocarcinoma: outstanding problemsWorld J Gastrointest Surg42012104
- M.ZavoralP.MinarikovaF.ZavadaC.SalekM.MinarikMolecular biology of pancreatic cancerWorld J Gastroenterol: WJG1720112897
- S.KelegP.BüchlerR.LudwigM.W.BüchlerH.FriessInvasion and metastasis in pancreatic cancerMol Cancer2200314
- C.L.ChafferR.A.WeinbergA perspective on cancer cell metastasisScience331201115591564
- A.K.ShasanyA.K.ShuklaS.P.KhanujaMedicinal and aromatic plantsTechnical crops2007Springer175196
- Z.D.NassarA.F.AishaF.S.R.Al SuedeA.S.Abdul MajidA.M.S.Abdul MajidIn vitro antimetastatic activity of koetjapic acid against breast cancer cellsBiol Pharm Bull352012503508
- H.OkugawaR.UedaK.MatsumotoK.KawanishiA.KatoEffects of agarwood extracts on the central nervous system in micePlanta Med5919933236
- H.OkugawaR.UedaK.MatsumotoK.KawanishiA.KatoEffect of jinkoh-eremol and agarospirol from agarwood on the central nervous system in micePlanta Med62199626
- A.M.S.A.MajidS.S.DahhamM.B.K.AhamedS.M.SaghirF.S.AlsuedeM.A.IqbalBioactive essential oils from Aquilaria crassna for cancer prevention and treatmentGlob J Adv Pure Appl Sci20144
- H.HideakiAgarwood (Aquilaria crassna) extracts decrease high-protein high-fat diet-induced intestinal putrefaction toxins in micePharm Anal Acta2012
- P.JermsriS.KumphuneEthylacetate extract of Aquilaria crassna preserve actin cytoskeleton on stimulated ischemia induced cardiac cell deathJ Med Plants Res6201240574062
- E.NovriyantiE.SantosaW.SyafiiM.TurjamanI.R.SitepuAnti fungal activity of wood extract of Aquilaria crassna Pierre ex Lecomte against agarwood-inducing fungi, Fusarium solaniIndonesian J Forest Res72010155165
- S.KamonwannasitP.KumkraiN.NantapongS.KupittayanantN.ChudapongseAntioxidant and antibacterial activities of the extract of Aquilaria crassna leavesPlanta Med772011PM15
- M.A.IqbalR.A.HaqueM.B.K.AhamedA.A.MajidS.S.Al-RawiSynthesis and anticancer activity of para-xylyl linked bis-benzimidazolium salts and respective Ag (I) N-heterocyclic carbene complexesMed Chem Res22201324552466
- M.B.K.AhamedA.F.AishaZ.D.NassarCat’s whiskers tea (Orthosiphon stamineus) extract inhibits growth of colon tumor in nude mice and angiogenesis in endothelial cells via suppressing VEGFR phosphorylationNutr Cancer6420128999
- O.S.A.Al-SalahiC.Kit-LamA.M.S.A.MajidAnti-angiogenic quassinoid-rich fraction from Eurycoma longifolia modulates endothelial cell functionMicrovasc Res9020133039
- H.M.BaharethaZ.D.NassarA.F.AishaProapoptotic and antimetastatic properties of supercritical CO2 extract of Nigella sativa Linn. against breast cancer cellsJ Med Food16201311211130
- Q.-H.YinF.-X.YanX.-Y.ZuAnti-proliferative and pro-apoptotic effect of carvacrol on human hepatocellular carcinoma cell line HepG-2Cytotechnology6420124351
- C.P.PreethyR.PadmapriyaV.S.PeriasamyAntiproliferative property of n-hexane and chloroform extracts of Anisomeles malabarica (L). R. Br. in HPV16-positive human cervical cancer cellsJ Pharmacol Pharmacother3201226
- J.R.PatilG.JayaprakashaK.C.MurthyS.E.TichyM.B.ChettiB.S.PatilApoptosis-mediated proliferation inhibition of human colon cancer cells by volatile principles of Citrus aurantifoliaFood Chem114200913511358
- A.BommareddyB.RuleA.L.VanWertS.SanthaC.Dwivediα-Santalol, a derivative of sandalwood oil, induces apoptosis in human prostate cancer cells by causing caspase-3 activationPhytomedicine192012804811
- R.J.BoldP.M.TermuhlenD.J.McConkeyApoptosis, cancer and cancer therapySurg Oncol6November 1997133142
- S.A.SusinH.K.LorenzoN.ZamzamiMolecular characterization of mitochondrial apoptosis-inducing factorNature3971999441446
- A.L.MihaljevicC.W.MichalskiH.FriessJ.KleeffMolecular mechanism of pancreatic cancer—understanding proliferation, invasion, and metastasisLangenbeck’s Arch Surg39542010295308
- V.BhardwajA.BhushanJ.C.LaiS.M.TadinadaFailure of pancreatic cancer chemotherapy: consequences of drug resistance mechanisms2012INTECH Open Access Publisher
- A.B.KunnumakkaraP.DiagaradjaneS.GuhaCurcumin sensitizes human colorectal cancer Xenografts in nude mice to γ-radiation by targeting nuclear factor-κB–regulated gene productsClin Cancer Res14200821282136
- B.ParkS.PrasadV.YadavB.SungB.B.AggarwalBoswellic acid suppresses growth and metastasis of human pancreatic tumors in an orthotopic nude mouse model through modulation of multiple targetsPLoS One6201126943