Abstract
Background
Dietary glycotoxins and androgen excess have been independently associated with a negative influence on the kidney. There are no data concerning the additive effects of these two factors on the kidney function and structure, in females. The present study aims to investigate the effect of dietary glycotoxins and androgen excess on the kidneys of an androgenized female rat model.
Methods
The study involved 80 female Wistar rats divided into 3 groups. The animals from group A were androgenized at 4 weeks of age (n = 30), rats of group B were androgenized at 12–20 weeks of age (n = 20) and group C consisted of non-androgenized animals (n = 30). All groups were further randomly assigned, either to a high-Advanced Glycation End product diet (HA diet) or low-AGE diet (LA diet), for 3 months.
Results
The rats fed with HA diet had significantly higher serum creatinine levels (p ⩽ 0.0002), when compared with those fed with LA diet. The androgenized group fed with HA diet exhibited higher levels of serum AGE (p = 0.0005), creatinine levels (p < 0.0001) and C-reactive protein (CRP) levels (p ⩽ 0.002), when compared with the non-androgenized group fed with HA diet. AGE immunoreactivity was higher on the renal tubules of the androgenized animals fed with HA diet, when compared with the animals fed with LA diet, but did not significantly differ among the two groups.
Conclusions
The above mentioned data suggest that dietary glycotoxins, in combination with increased androgen exposure, exert a more profound negative impact on the kidney of an androgenized female rat model that mimics the metabolic characteristics of polycystic ovary syndrome.
Keywords:
1 Introduction
Advanced glycation end products (AGEs) are a heterogeneous group of compounds, formed from nonenzymatic reaction of reducing sugars with proteins, lipids and nucleic acids.Citation1 They may induce many structural and vascular changes in several tissues because of insoluble cross-link formation, induction of oxidative stress (OS) and subsequent cell activation.Citation2
Systemic AGEs are derived via either endogenous formation or exogenous food ingestion. Endogenous AGEs are spontaneously produced in human tissues and in circulation as part of normal metabolism and accumulate during normal ageing. The process is accelerated in conditions of hyperglycaemia and enhanced OS and has been implicated in the progression of age-related diseases such as diabetes mellitus, atherosclerosis, chronic renal failure and Alzheimer’s disease.Citation3
Exogenous derived AGEs are absorbed from sources such as tobaccoCitation4 and Westernized diets, since thermally processed common foods have high glycotoxin content.Citation5 Evidence of the last decade, generated from data in humans and experimental animals, supports the contribution of exogenous food-ingested AGEs to elevated serum levels and increased tissue deposition.Citation6,Citation7
Excessive AGEs deposition may contribute to tissue injury by at least two mechanisms. The first is the receptor-independent modification of the protein structure, leading to the cross-linking of matrix proteinsCitation8, the decreased catalytic activity of enzymesCitation9, the occurrence of epitopes with new immunological propertiesCitation10, or the decreased clearance of lipoproteins.Citation11 DNA damage may be caused via induction of strand breaks, punctual mutations, the occurrence of a basic sites, or depurination.Citation12 Secondly, AGEs interact directly with their specific receptors, of which the receptor for AGEs (RAGE) is of pathogenic importance.Citation13
Expression of RAGE is rapidly enhanced in tissues where AGEs accumulate.Citation14 The AGE–RAGE interaction mediates pro-atherogenic, inflammatory and immune responses via activation of nuclear factor-κB (NF-κB). This activation leads to increased expression/synthesis of cytokines, chemokines, growth factors, adhesion molecules and reactive oxygen species (ROS) production.Citation15,Citation13
The predominant removal of AGEs takes place in the kidney. The important role of the kidneys in the metabolism and excretion of endogenous and dietary AGEs has been demonstrated in animals and humans with severe renal disease.Citation16–Citation18 The filtered AGEs are partially degraded in the tubular system of the kidney, while the rest are excreted with urine.Citation19 The proximal tubule has been identified as the site of catabolism of AGE proteins and peptides both in vivo and in vitroCitation20, but almost all renal structures (basement membranes, mesangial and endothelial cells, podocytes and tubules) are susceptible to AGE accumulation.Citation11,Citation21 AGEs are independently related to a decrease in the glomerular filtration rate (GFR)Citation22, making them a possible class of uremic toxins.Citation23
Besides AGEs, it is already known that testosterone may play a crucial role in the acceleration of the tubular apoptotic process and the progression of chronic kidney disease in males. Testosterone is known to be profibrotic, leading to mesangial expansion and renal dysfunction.Citation24
Moreover, the positive correlation between AGE and androgens has been demonstrated in young normoglycemic women with polycystic ovary syndrome (PCOS), which presented with high AGE levels compared to a group of ovulatory normoandrogenic women. Interestingly, in this study the correlation between AGE proteins and testosterone levels remained high controlling for body mass index (BMI), insulin levels and area under curve of glucose (AUCGLU), implying an interaction between AGE proteins and hyperandrogenemia.Citation25
Considering the above mentioned data, the aim of the present study was to analyse the effect of dietary glycotoxins and androgen excess on the kidneys of an androgenized female rat model, which simulates the reproductive and metabolic environment of women with hyperandrogenemic syndromes, such as PCOS.
2 Materials and methods
2.1 Study population
The study involved 80 female Wistar rats, which were divided into 3 main groups: Group A consisted of 30 rats, aged 4 weeks, which at 4 weeks of age were androgenized by subcutaneous implantation of 90-d continuous release pellets containing 7.5 mg dihydrotestosterone (DHT), leading to a daily exposure of 83 μg DHT. The above mentioned process previously presented by Manneras et al.Citation26 This experimental model was selected to mimic both the reproductive and metabolic characteristics of PCOS women, who have ∼1.7-fold increase in DHT levels, than those of normal women.Citation27,Citation28 Group A was subsequently randomly divided into two subgroups based on food content; Subgroup A1 (n = 15, baseline body weight = 99.7 ± 2.7 g) was fed commercial chow with low AGE content, for 3 months, while subgroup A2 (n = 15, baseline body weight = 94.7 ± 2.6 g) was fed commercial chow with high content in AGE, for 3 months.
Group B included 20 older rats, aged 12–20 weeks, which were also androgenized with the same way as previously described. Similarly, a further random division into two subgroups, based on the criteria of AGE content, followed androgenization. Subgroup B1 (n = 10, baseline body weight = 190.5 ± 5.5 g) was fed commercial chow with low in AGE content for 3 months, while subgroup B2 (n = 10, baseline body weight = 184.0 ± 8.2 g) was exposed to a HA diet, for 3 months.
Finally, group C included 30 nonandrogenized animals, which at 12–20 weeks of age, were equally subdivided into two groups. Subgroup C1 (n = 15, baseline body weight = 197.0 ± 4.3 g) followed a LA diet for 3 months, while subgroup C2 (n = 15, baseline body weight = 199.7 ± 4.2 g) was fed commercial chow with high in AGE content for 3 months.Citation7
The animals were housed four to five per cage under controlled conditions (21–22 °C, 55–65% humidity, 12-h light/12-h dark cycle) and were given pelleted food and water ad libitum at ELPEN (Experimental Research Centre, Athens, Greece). Animal care and experimental procedures conformed to the “Guide for the Care and Use of Laboratory Animals” (Department of Health, Education and Welfare, Athens, Greece). This study was approved by the Institutional Animal Care and Use Committee.
Body weight was monitored weekly. The study was concluded after 3 months, and rats were sacrificed, after a 12 h fasting. Initially, the animals were subjected to anaesthesia with ether, allowing blood sample collection after puncture of the tail vein. The whole blood was collected in a covered test tube and allowed to clot at room temperature for 30 min. Then, the clot was removed by centrifugation at 2500 rpm for 15 min, in a refrigerated centrifuge. Following centrifugation the serum was immediately transferred into 0.5 ml aliquots, transported and stored at −80 °C.Citation29
The parameters measured from rat serum were: AGE (U/mL) levels in order to study the effect of dietary glycotoxins on the circulating AGE levelsCitation7,Citation16,Citation18, creatinine (mg/dL) and CRP (μg/mL) levels in order to study the effect of dietary glycotoxins on the kidney function and the possible provocation of inflammatory process.Citation13,Citation24,Citation42 In addition, testosterone (ng/mL) levels were measured in order to study the effect of dietary glycotoxins on the hormonal status and particularly on testosterone levels, since testosterone has been implicated in kidney damage.Citation40,Citation41 Finally, insulin (μU/mL) and glucose (mg/dL) levels were measured in order to investigate the effect of dietary glycotoxins on the metabolism.Citation7
Subsequently, the animals were sacrificed with administration of 20 mg/mL xylazine hydrochloride (dose 100 mg/kg) and 100 mg/mL ketamine hydrochloride (dose 10 mg/kg), at a dose rate 0.2 ml/100 g body weight, by intraperitoneal injection, allowing kidney tissue retrieval. The kidney was removed immediately and placed in 10% formalin in phosphate-buffered saline (PBS), pH 7.4, for 18 h before paraffin embedding.Citation30
2.2 Diets
The diets used were derived from a single standard rat chow (AIN-93G) purchased from Bioserve (Frenchtown, NJ, USA), consisting of 18% protein, 58% carbohydrate, 7.5% fat, and 3.73 kcal/g. Regular AIN-93G chow is normally prepared by heating at 190 °C for 30 min. Analysis of this preparation was performed as previously described.Citation7
The HA diet contained 76.0 ± 15.3 mg CML/100 g sample (or 436.9 ± 88.1 mg CML/100 g protein), 205.32 ± 22.25 mg fructoselysine/100 g sample (or 1.179.98 ± 127.90 mg fructoselysine/100 g protein) and 52.68 ± 5.71 mg furosine/100 g sample (or 302.78 ± 32.82 mg furosine/100 g protein) and was considered as a HA diet.
The same rodent mix was also prepared without heating. This preparation was of equivalent macro- and micronutrient and energy content but contained 1.3 ± 0.4 mg CML/100 g sample (or 7.7 ± 2.2 mg CML/100 g protein), 104.58 ± 3.08 mg fructoselysine/100 g sample (or 601.01 ± 17.7 mg fructoselysine/100 g protein) and 26.83 ± 0.79 mg furosine/100 g sample (or 154.22 ± 4.54 mg furosine/100 g protein) and was considered an LA diet.
2.3 Biochemical and hormonal assays
Testosterone was measured by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (Calbiotech, CA, USA). Insulin was also quantified using ELISAs purchased by Biovendor Laboratory Medicine (Brno, Czech Republic) and Neogen Corporation (Lexington, KY, USA), respectively. Serum AGE levels (U/mL) were measured by CML-specific competitive ELISA, as described previously. Biochemical assays (serum creatinine, glucose and CRP levels) were performed in the chemical analysis system ADVIA 1200 (Siemens, Healthcare Diagnostics, NY, USA), using commercially available kits (Bayer AG, Athens, Greece).
2.4 Immunohistochemical analysis
Paraffin-embedded sections of formalin-fixed kidney tissue were deparaffinized by xylene and dehydrated in graded ethanol. Sections were treated in 3% hydrogen peroxide in phosphate buffered saline (PBS) for 15 min and then rinsed in PBS. To increase the immunoreactivity of AGEs, the sections were placed in 500 mL of 0.01 mol/L citric acid-buffered solution (pH 7.0) and microwaved at 500 W for 5 min. After thorough washing, the sections were incubated with normal rabbit serum for 20 min at room temperature to avoid nonspecific binding of the antibodies. The sections were then incubated overnight at 4 °C with the anti-AGE monoclonal antibody 6D12 (0.25 mg/Ml stock, dilution 1:50; Research Diagnostics, Concord, MA, USA) in PBS containing 1% bovine serum albumin. Immunoreactivity was detected by the streptavidin–biotin–peroxidase method according to the manufacturer’s protocol. The final reaction product was visualized with 3,3′-diaminobenzidine tetrahydrochloride (LSAB detection kit; Dako, Carpentaria, CA, USA). Lung tissue sections from diabetic rats were used as positive controls for AGE antibody. Negative controls (for example, kidney tissue in which the primary antibody was substituted with nonimmune mouse or goat serum) were also stained in each run. The percentage of positive cells was estimated using light microscopy. The evaluation of the immunostained slides was performed blindly and independently by two observers. AGE immunoreactivity was expressed in terms of H-score, i.e. the percentage of positive cells multiplied by the intensity of staining.Citation31
2.5 Statistical methods
Descriptive statistics were calculated for the examined parameters (body weight at baseline, body weight at 3 months, AGEs, serum testosterone, insulin, CRP, serum glucose, creatinine, AGEs H-scores in the glomeruli, proximal and distal convoluted tubules, collecting ducts); values are presented as mean ± standard error of the mean (SE).
Given the deviation from normality, verified by the Shapiro–Wilk test, non-parametric statistical tests were performed. Specifically, the overall heterogeneity between the six study groups was evaluated by the Kruskal–Wallis test. Seven meaningful pairwise comparisons were a priori constructed in the study design, namely: A1 vs. A2, B1 vs. B2, C1 vs. C2 (to assess the high vs. low AGEs comparison), as well as A1 vs. B1, A2 vs. B2 (aiming to evaluate the effect of age at androgenization), B1 vs. C1, B2 vs. C2 groups (to examine the effects of androgenization per se); all pairwise comparisons were assessed by the Mann–Whitney–Wilcoxon test for independent samples. The level of statistical significance regarding the seven pairwise comparisons was set at 0.05/7 = 0.007 (Bonferroni correction for multiple comparisons) as appropriate, whereas the level of statistical significance regarding the overall heterogeneity remained at the 0.05 level, given that multiple comparisons did not pertain to the latter overall notion. Finally, the intercorrelations between study variables were examined by means of the Spearman’s rank correlation coefficient. Statistical analysis was performed with STATA/SE version 13 (Stata Corp., College Station, TX, USA).
3 Results
3.1 Comparisons evaluating the effect of AGEs
The descriptive statistics regarding body weight, measured serum parameters and AGEs immunostaining are presented in . Significant between-groups heterogeneity was noted concerning the majority of examined parameters, except for serum glucose.
Table 1 Serum parameters and AGEs IHC expression in the six studied groups. Values are presented as mean ± S.E. Bold cells denote statistically significant differences. §Derived from Mann–Whitney–Wilcoxon test for independent samples (level of statistical significance: 0.007, due to the Bonferroni correction); †derived from Kruskal–Wallis test (level of statistical significance: 0.05).
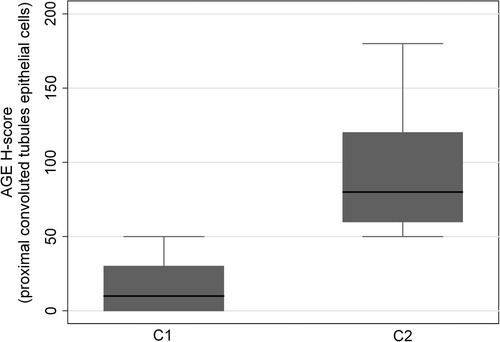
Overall, AGE immunoreactivity was mainly confined to the tubules, the glomerular tufts being positive on only 11 cases. Since the expression of AGEs in the epithelial cells was similar in the distal tubules and collecting ducts, this was analysed as a single parameter.
Compared with group A1, group A2 exhibited higher AGEs levels (as expected, p < 0.0001), lower body weight at 3 months (p = 0.0001), higher serum testosterone (p = 0.0004), higher serum insulin (p = 0.0001), CRP (p < 0.0001) and creatinine (p < 0.0001) ( and ). AGE immunoreactivity on the proximal and distal convoluted tubules was increased in A2 animals () but did not reach statistical significance among the two groups (p = 0.1578, p = 0.1483 respectively).
Figure 1 Effect of HA-diet and androgen exposure on creatinine levels. The prepubertal androgenized rats (A2) and adult androgenized rats (B2) on a HA-diet, exhibit higher serum creatinine levels, than the respective groups of rats on a LA-diet (A1 and B1). The non-androgenized group does not present any statistically significant difference (C1 and C2).
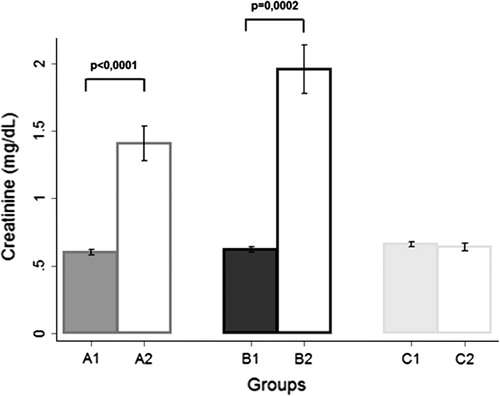
Figure 2 Effect of androgen exposure on CRP levels. The adult androgenized animals (B1 and B2) exhibited higher CRP levels when they were compared with the age-matched adult non-androgenized animals (C1 and C2), either fed with HA- or LA-diet.
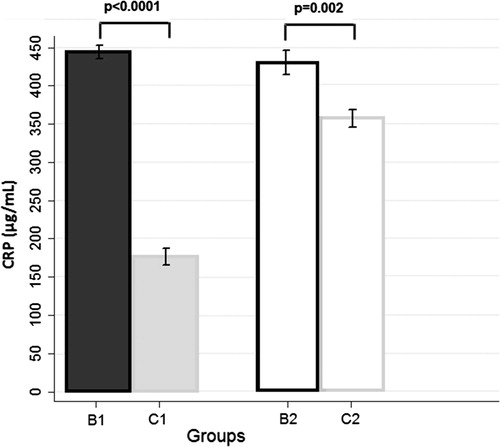
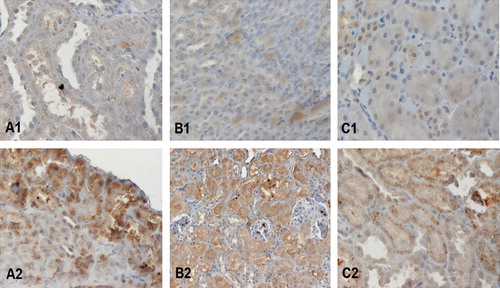
Figure 3 Expression of AGEs in epithelial cells of proximal convoluted tubules. The group of rats fed with HA-diet [(A2) represents the prepubertal androgenized rats, (B2) the androgenized rats and (C2) the adult non-androgenized rats, fed with HA-diet] [exhibit higher AGE immunoreactivity than the respective animals fed with LA-diet [(A1) represents the prepubertal androgenized rats, (B1) the adult androgenized rats and (C1) the adult non-androgenized rats, fed with LA-diet]. Magnification ×100.
Compared with group B1, group B2 presented with higher AGEs levels (p = 0.0002), higher serum testosterone levels (p = 0.0002) and creatinine (p = 0.0002) ( and ). B2 animals exhibited higher but not statistically significant AGE immunoexpression in the glomerular tuft and collecting ducts or distal convoluted tubules () (p = 0.9395 and p = 0.3118 respectively).
Regarding the group C2 vs. C1 comparison, higher AGEs levels were observed in the serum (p < 0.0001) as well as in the proximal convoluted tubules (p = 0.055, and ) followed by elevated serum testosterone (p < 0.0001), insulin (p < 0.0001) and CRP (p < 0.0001) in C2 group compared to C1 ( and ).
3.2 Comparisons pertaining to the effect of age at androgenization and androgenization per se
presents the results of the a priori designed comparisons between the study groups pertaining to the effects of age at androgenization (left panels) and to the effect of androgenization per se (right panels).
Table 2 Detailed list of the a priori designed comparisons between the study groups pertaining to the effects of age at androgenization or androgenization per se. Bold cells denote statistically significant differences. §Derived from Mann–Whitney–Wilcoxon test for independent samples (Level of statistical significance: <0.007 due to the Bonferroni correction).
With respect to the effects of age at androgenization, Groups A1 and A2 presented with significantly lower body weight at baseline than groups B1 and B2, respectively (p < 0.0001 for both comparisons), but no differences in body weight were noted at three months. Lower CRP (p < 0.0001) levels were noted in group A1 vs. group B1, but this pattern was not reproducible upon the A2 vs. B2 comparison.
With respect to the effect of androgenization per se, groups C1 and C2 presented with significantly lower weight at three months than groups B1 and B2 (p = 0.0008 and p = 0.005, respectively), although the respective differences were not significant at baseline. Group B2 exhibited higher levels of serum AGEs (p = 0.0005), testosterone (p = 0.002), and creatinine (p < 0.0001) than group C2, whereas these differences were not observed in the C1 vs. B1 comparison. CRP levels in groups B1/B2 were consistently higher than the respective levels in groups C1/C2 (p < 0.0001 and p = 0.002). No consistent differences were noted regarding AGEs immunohistochemical indices.
3.3 Intercorrelations between study parameters
presents the results pertaining to the intercorrelations between study parameters. A network of strong associations was noted, implicating higher serum testosterone, creatinine, CRP, AGEs in the serum, as well as AGEs immunostaining in tubules, which were all closely associated with each other in the study population. Nevertheless, this table should be deemed explorative in view of the numerous correlations tested simultaneously and cross-sectionally, a fact that per se does not allow the establishment of causality.
Table 3 Intercorrelations between study parameters. Spearman’s rho values with respective p-values in brackets are presented. Correlations with p-values < 0.05 have been highlighted in bold.
4 Discussion
It has been previously demonstrated that AGEs are metabolized and removed by the kidney,Citation19,Citation28,Citation32, but the kidney is also a site for accumulation of AGEs and AGE-related damage.Citation33 Therefore, the kidney is affected by AGEs, and declining renal function entails an increase in serum AGE levels, amplifying the damage from them.Citation13 Furthermore, dietary intake of glycotoxins contributes to a substantial portion of circulating AGE levelCitation18, and dietary restriction of glycotoxins has been shown to reduce serum AGE levels in patients with renal failure.Citation34
In agreement with the above mentioned data, the present study demonstrated that a diet high in AGE content may negatively affect the renal biochemistry and morphology. In more details, the animal groups fed with a HA diet had significantly higher serum creatinine levels, when they were compared with those fed with a LA diet. The effect of HA diet on the kidney was also shown through AGE immunohistochemistry. Higher AGE expression was observed in the epithelial cells of proximal and distal convoluted tubules of androgenized animals without however reaching statistical significance, while in the nonandrogenized group significant AGE immunoreactivity was observed in the proximal tubules of high AGE fed animals.
The kidney is known to play an important role in the metabolism of AGEs. The present data are in agreement with previous studies suggesting that almost all renal structures are susceptible to the accumulation of AGEs including basement membranes, mesangial and endothelial cells, podocytes and tubules.Citation35,Citation36 Vlassara et al., demonstrated that chronic administration of in vitro-prepared protein-AGEs to otherwise healthy rats could lead to advanced pathological changes in renal glomerular structure and function.Citation37 Moreover, the proximal tubule has been identified as the site of catabolism of AGE proteins and peptides both in vivo and in vitro.Citation20 In addition, as described by Gu et al., AGE-modified bovine serum albumin (BSA) induced upregulation of monocyte chemoattractant protein-1 (MCP-1) expression in podocytes through activation of RAGE and generation of intracellular ROS.Citation38 In human renal biopsies, AGE-accumulation is primarily found in renal basement membranes in diabetic and non-diabetic nephropathy, and its accumulation involves upregulation of RAGE in podocytes.Citation39 All the above information, offers confirmatory evidence for a cause and effect relationship between long-term AGE accumulation and renal pathology.
Interestingly, the only group that did not present any statistically significant difference in the serum creatinine levels was the one that was not subjected to the androgenization process (). This finding may suggest that the interplay of the high dietary intake of AGEs with the high androgen exposure may contribute to a more profound kidney injury. Trying to clarify this hypothesis, we extended our data analysis in order to evaluate the effect of androgenization on renal biochemistry and structure. Therefore, the adult androgenized group fed with HA diet exhibited higher levels of serum AGE and creatinine levels, when compared with the non-androgenized group fed with HA diet, while in the respective groups fed with LA diet there were no statistically significant differences. These data imply the cumulative aggravating effect of exogenously derived AGEs and androgen excess on kidney function.
Indeed, it has been shown that the sexual dimorphism is deeply reflected on renal morphology and physiology, most likely due to the actions of gonadal steroids and to the endocrine/paracrine pathways of the kidney. Testosterone is known to be profibrotic since it stimulates extracellular matrix proteins (EMP) deposition in glomerular mesangial cells (GMCs), leading to mesangial expansion and renal dysfunction.Citation40
Moreover, Pawluczyk et al. have shown that male rat mesangial cells express higher baseline fibronectin mRNA, tumor necrosis factor-α (TNF-α) and interleukin-1β levels than female rat mesangial cells, indicating the potential proinflammatory and profibrotic actions of testosterone in the kidney.Citation24 Additionally, androgens have been shown to increase proapoptotic signalling.Citation41 Furthermore, glomerular sclerosis is known to occur faster and more intensely in males than in females.Citation40 Taken together, these data indicate that testosterone may play a crucial role in the acceleration of the tubular apoptotic process and the progression of chronic kidney disease in males.
Another interesting finding of the present study is that the adult androgenized animals exhibited higher CRP levels, when they were compared with the age matched adult nonandrogenized animals. Moreover, the CRP levels were positively correlated with serum creatinine and AGE levels and with AGE deposition in the kidney. These data imply, that either androgen excess per se, or via its deteriorating effect on renal function and structure, creates an inflammatory environment for the kidney. Consistent with this view are older studies that have described chronic kidney disease as a “micro-inflammatory state”Citation42, with a high prevalence of acute phase inflammation and OS, both of which are associated with a high rate of cardiovascular morbidity and mortality.Citation43–Citation45 On the other hand, the proinflammatory and profibrotic actions of testosterone on the kidney have been indicated by in vitro studies, which have demonstrated that basal tumor necrosis factor-α (TNF-α) and interleukin-1β levels are higher in male mesangial cells compared to female mesangial cells. This sexual dimorphism in mesangial cells may play a role in the faster progression of glomerulosclerosis leading to end-stage renal disease in males.Citation41
This study has several limitations. Although, the sample size of the analysed groups was satisfactory, some differences did not reach statistical significance. Additionally, determination of serum creatinine level consists of a simple and reliable method for the control of renal function. Nevertheless, more accurate methods, such as creatinine clearance or glomerular filtration ratio, should be estimated. Finally, the androgenized animals exhibited higher body weights when compared with the non-androgenized age matched animals. It would be useful to analyse the body composition by dual-emission X-ray absorptiometry (DEXA), in order to determine the body fat and lean body mass.
In conclusion, while the studies upon male subjects, concerning the androgen effect on the kidney, vary, females with hyperandrogenemic syndromes (such as PCOS), lack evidence on the effect of hyperandrogenemia on the female kidney. This is the first study to our knowledge, which analysed the possible synergistic effects of exogenously derived glycotoxins and androgen excess on renal biochemistry and morphology. The study included a female rat model that exerts the metabolic and hormonal characteristics of women with hyperandrogenemia. In summary, the present study suggests that dietary glycotoxins, in combination with increased androgen exposure, exert a more profound negative impact on the kidney of an androgenized female rat model that mimics female hyperandrogenemic syndromes, such as PCOS. Moreover, dietary glycotoxins and androgen excess induce an inflammatory environment for the kidney, which could further aggravate its structure and function. However, further studies are necessary to elucidate the mechanisms via which androgens augment the detrimental effect of exogenously derived AGEs on the female kidney.
Conflict of interest
The authors stated that there are no conflict of interests regarding the publication of this article.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 8 August 2015
References
- T.NiwaMass spectrometry for the study of protein glycation in diseaseMass Spectrom Rev252006713723
- R.D.SembaE.J.NicklettL.FerrucciDoes accumulation of advanced glycation end products contribute to the aging phenotype?J Gerontol A Biol Sci Med Sci652010963975
- S.Y.GohM.E.CooperClinical review: the role of advanced glycation end products in progression and complications of diabetesJ Clin Endocrinol Metab93200811431152
- C.CeramiH.FoundsI.NichollT.MitsuhashiD.GiordanoS.VanpattenTobacco smoke is a source of toxic reactive glycation productsProc Natl Acad Sci USA9419971391513920
- T.GoldbergW.CaiM.PeppaV.DardaineB.S.BaligaJ.UribarriAdvanced glycoxidation end products in commonly consumed foodsJ Am Diet Assoc104200412871291
- A.M.de AssisD.K.RiegerA.LongoniC.BattuS.RaymundiR.F.da RochaHigh fat and highly thermolyzed fat diets promote insulin resistance and increase DNA damage in ratsExp Biol Med (Maywood)234200912961304
- E.Diamanti-KandarakisC.PiperiP.KorkolopoulouE.KandarakiG.LevidouA.PapaloisAccumulation of dietary glycotoxins in the reproductive system of normal female ratsJ Mol Med85200714131420
- N.C.AveryA.J.BaileyThe effects of the Maillard reaction on the physical properties and cell interactions of collagenPathol Biol (Paris)542006387395
- K.ŠebekovαR.SchinzelH.LingA.SimmG.XiangM.GekleAdvanced glycated albumin impairs protein degradation in the kidney proximal tubular cell line LLC-PK1Cell Mol Biol44199810511060
- G.LubeckA.PollakReduced susceptibility of non-enzymatically glycosylated glomerular basement membrane to proteinasesRenal Physiol3198048
- R.BucalaZ.MakitaG.VegaS.GrundyT.KoschinskyA.CeramiModification of low-density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiencyProc Natl Acad Sci USA91199494419445
- H.StopperN.SchuppA.KlassenK.SebekovaA.HeidlandGenomic damage in chronic renal failure – potential therapeutic interventionsJ Ren Nutr1520058186
- J.M.BohlenderS.FrankeG.SteinG.WolfAdvanced glycation end products and the kidneyAm J Physiol Renal Physiol2892005F645F659
- D.SuzukiM.ToyodaN.YamamotoM.MiyauchiM.KatohM.KimuraRelationship between the expression of advanced glycation end-products (AGE) and the receptor for AGE (RAGE) mRNA in diabetic nephropathyIntern Med452006435441
- A.BierhausD.M.SternP.P.NawrothRAGE in inflammation: a new therapeutic target?Curr Opin Investig Drugs72006985991
- H.VlassaraAdvanced glycation in health and disease. Role of modern environmentAnn NY Acad Sci10432005452460
- T.KoschinskyC.J.HeT.MitsuhashiR.BucalaC.LiuC.BuentingOrally absorbed reactive glycation products (glycotoxins): an environmental risk factor in diabetic nephropathyProc Natl Acad Sci USA94199764746479
- J.UribarriM.PeppaW.CaiT.GoldbergM.LuS.BaligaDietary glycotoxins correlate with circulating advanced glycation end product levels in renal failure patientsAm J Kidney Dis422003532538
- A.GugliucciM.BendayanRenal fate of circulating advanced glycation end products (AGEs): evidence for absorption and catabolism of AGE-peptides by renal proximal tubular cellsDiabetologia391996149160
- A.SaitoT.TakedaK.SatoH.HamaA.TanumaR.KasedaSignificance of proximal tubular metabolism of advanced glycation end products in kidney diseasesAnn NY Acad Sci10432005637643
- L.J.JensenJ.OstergaardA.FlyvbjergAGE–RAGE and AGE cross-link interaction: important players in the pathogenesis of diabetic kidney diseaseHorm Metab Res37suppl 120052634
- R.D.SembaL.FerrucciJ.C.FinkK.SunJ.BeckM.DalalAdvanced glycation end products and their circulating receptors and level of kidney function in older community-dwelling womenAm J Kidney Dis5320095158
- P.StenvinkelJ.J.CarreroJ.AxelssonB.LindholmO.HeimburgerZ.MassyEmerging biomarkers for evaluating cardiovascular risk in the chronic kidney disease patient: how do new pieces fit into the uremic puzzle?Clin J Am Soc Nephrol32008505521
- I.Z.PawluczykE.K.TanK.P.HarrisRat mesangial cells exhibit sex-specific profibrotic and proinflammatory phenotypesNephrol Dial Transplant24200917531758
- E.Diamanti-KandarakisC.PiperiA.KalofoutisG.CreatsasIncreased levels of serum advanced glycation end-products in women with polycystic ovary syndromeClin Endocrinol (Oxf)6220053743
- L.ManneråsS.CajanderA.HolmängZ.SeleskovicT.LystigM.LönnA new rat model exhibiting both ovarian and metabolic characteristics of polycystic ovary syndromeEndocrinology148200737813791
- M.FassnachtN.SchlenzS.B.SchneiderS.A.WudyB.AllolioW.ArltBeyond adrenal and ovarian androgen generation: increased peripheral 5α-reductase activity in women with polycystic ovary syndromeJ Clin Endocrinol Metab88200327602766
- M.E.SilfenM.R.DenburgA.M.ManiboR.A.LoboR.JaffeM.FerinEarly endocrine, metabolic, and sonographic characteristics of polycystic ovary syndrome (PCOS): comparison between nonobese and obese adolescentsJ Clin Endocrinol Metab88200346824688
- J.B.HenryClinical Diagnosis and Management by Laboratory Methodsvol. 11979W.B Saunders CompanyPhiladelphia
- S.WolfensohnM.LloydHandbook of Laboratory Animal Management and Welfare1994Oxford University PressOxford
- P.J.van DiestP.van DamS.C.Henzen-LogmansE.BernsM.E.van der BurgJ.GreenA scoring system for immunohistochemical staining: consensus report of the task force for basic research of the EORTC-GCCG. European Organization for Research and Treatment of Cancer-Gynaecological Cancer Cooperative GroupJ Clin Pathol501997801804
- T.MiyataY.UedaK.HorieM.NangakuS.TanakaC.van Ypersele de StrihouRenal catabolism of AGEs: the fate of pentosidineKidney Int531998416422
- R.SchinzelG.MünchA.HeidlandK.SebekovaAdvanced glycation end products in end-stage renal disease and their removalNephron872001295303
- A.BostomHomocysteine: “expensive creatinine” or important modifiable risk factor for arteriosclerotic outcomes in renal transplant recipients?J Am Soc Nephrol112000149151
- E.D.SchleicherE.WagnerA.G.NerlichIncreased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and agingJ Clin Invest991997457468
- K.HorieT.MiyataK.MaedaS.MiyataS.SugiyamaH.SakaiImmunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathyJ Clin Invest100199729953004
- H.VlassaraL.J.StrikerS.TeichbergH.FuhY.M.LiM.SteffesAdvanced glycation end products induce glomerular sclerosis and albuminuria in normal ratsProc Natl Acad Sci USA9119941170411708
- L.GuS.HagiwaraQ.FanM.TanimotoM.KobataM.YamashitaRole of receptor for advanced glycation end-products and signaling events in advanced glycation end-product-induced monocyte chemoattractant protein-1 expression in differentiated mouse podocytesNephrol Dial Transplant212006299313
- N.TanjiG.S.MarkowitzC.FuT.KislingerA.TaguchiM.PischetsriederExpression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal diseaseJ Am Soc Nephrol11200016561666
- J.R.LombetS.G.AdlerP.S.AndersonC.C.NastD.R.OlsenR.J.GlassockSex vulnerability in the subtotal nephrectomy model of glomerulosclerosis in the ratJ Lab Clin Med11419896674
- P.D.MetcalfeJ.A.LeslieM.T.CampbellD.R.MeldrumK.L.HileK.K.MeldrumTestosterone exacerbates obstructive renal injury by stimulating TNF-alpha production and increasing proapoptotic and profibrotic signalingAm J Physiol Endocrinol Metab2942008E435E443
- G.A.KaysenThe microinflammatory state in uremia: causes and potential consequencesJ Am Soc Nephrol12200115491557
- J.HimmelfarbP.StenvinkelT.A.IkizlerR.M.HakimThe elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremiaKidney Int62200215241538
- M.AriciJ.WallsEnd-stage renal disease, atherosclerosis, and cardiovascular mortality: Is C-reactive protein the missing link?Kidney Int592001407414
- P.StenvinkelInflammatory and atherosclerotic interactions in the depleted uremic patientBlood Purif1020015361