Abstract
The paper emphasizes on the synthesis of Palladium(II) iodide complexes containing based ligands. The new compounds of general formulae [Pd(L)4]I2 where L = Thiourea (Tu), Methylthiourea (Metu), Dimethylthiourea (Dmtu), Tetramethylthiourea (Tmtu), Imidazolidine-2-thione (Imt), Mercaptopyridine (Mpy), Mercaptopyrimidine (Mpm), and Thionicotinamide (Tna) were prepared simply by reacting K2[PdCl4] with the corresponding thioamides in 1:2 M ratio and then with 2 equivalents Potassium iodide. The complexes were characterized by elemental analysis and spectroscopic techniques (IR, 1H and 13C NMR). All the synthesized complexes were screened for antibacterial activity and some of compounds have shown good activities against both gram positive and gram negative bacteria. POM analyses reveal that the compounds are only slightly toxic and present a potential for antibacterial activity. Moreover, they have 16–23% drug score which is an important parameter for the compound possessing the drug properties.
1 Introduction
The coordination and structural chemistry of Palladium based complexes with donor atoms, especially sulfur containing ligands have been extensively studied.Citation1–Citation5 Palladium(II) complexes with heterocyclic thiones and thioureas as ligands showing a square-planar geometry have promising bio-relevant activities, particularly Mercaptopyrimidine, 6-Mercaptopurines, Imidazolidine-2-thione, and Diazinane-2-thione and their complexes with Palladium(II) are known to have antitumor activities.Citation6–Citation16 In view of great potential, several complexes of thioamides with Au(I), Ag(I), Cu(I) Zn(II) Mo(II), Cd(II) Pd(II) and Hg(II) have been widely studied.Citation17–Citation36
The treatment of infectious diseases still remains an important and challenging problem because of a combination of factors including emerging infectious diseases and the increasing number of multi-drug resistant microbial pathogens. In spite of a large number of antibiotics and chemotherapeutics available for medical use, at the same time the emergence of old and new antibiotic resistance created in the last decades revealed a substantial medical need for new classes of antimicrobial agents. There is a real perceived need for the discovery of new compounds endowed with antimicrobial activity, possibly acting through mechanism of action, which is distinct from those of well-known classes of antimicrobial agents to which many clinically relevant pathogens are now resistant. Due to the outbreak of infectious diseases caused by different pathogenic bacteria and the development of antibiotic resistance, researchers are searching for new antibacterial agents.
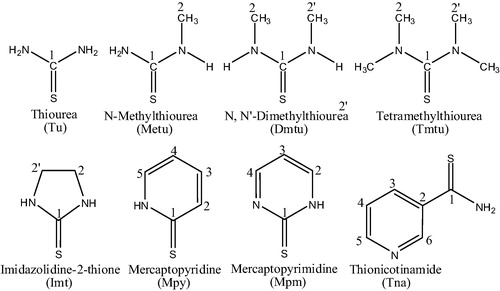
In continuation of our interest to further study the structural chemistry and biological properties of Palladium–sulfur interaction, in this paper, we describe the coordination of Palladium(II) iodide with a number of heterocyclic thiones as well as thioureas and their characterization by IR, 1H and 13C NMR spectroscopic techniques. Moreover, we have also investigated the antibacterial activities of these complexes. The knowledge of coordination behavior of thioamides toward Palladium(II) would be useful to understand the interactions of heavy metals to nucleotides and related compounds, which may have antitumor activity. The structures of the ligands used in this study are shown in .
2 Experimental
2.1 Chemicals
Palladium(II) chloride was purchased from Degussa AG 40474, Dusseldorf, Germany. The ligands, Thiourea (Tu), Methylthiourea (Metu), N,N′-Dimethylthiourea (Dmtu), Tetramethylthiourea (Tmtu), 2-Mercaptopyridine (Mpy), 2-Mercaptopyrimidine (Mpm), and Thionicotinamide (Tna) were purchased from ACROS Organics, USA. Imidazolidine-2-thione (Imt) was prepared according to the published procedure.Citation12
2.2 Synthesis of the complexes
Potassium tetrachloropalladate (II) was prepared as described in the literature.Citation20 Slight excess of two equivalents of Potassium chloride 1:2.25 ratio was added into the solution of Palladium chloride in 25 cm3 of distilled water with stirring for half an hour. The mixture was cooled in ice after few minutes yellowish-brown crystals of Potassium tetrachloropalladate (II) were formed which was separated by filtration and recrystallized from water containing few drops of HCl.
All the complexes were prepared by adding 2 equivalents of thioamides already dissolved in 15 mL methanol solution of K2[PdCl4] (0.326 g) in 15 mL of water (). After half an hour stirring 2 equivalents of aqueous solution of Potassium iodide were added and stirred the solutions for one hour. On mixing dark brown or reddish color solutions were obtained except for [Pd(Metu)4]I2 complex, where a shows a pale yellow color solution was obtained. All these solutions were filtered and washed with methanol and were kept at room temperature for three to five days. Solid products were obtained from water–methanol mixture on slow evaporation. The experimental yield of the products was around 60–75%. The proposed structures of the complexes are shown in . The elemental analysis and melting points (MP) of the complexes are given in .
Table 1 Proposed structure of the synthesized compounds.
Table 2 CHNS analysis, and MP of [Pd(L)4]I2 complexes.
2.3 Measurements
Elemental analysis was carried out on a Leco CHNS-932 Leco Corporation USA. Melting point was recorded on an Electrothermal IA 9000 Series, Essex SS2 5PH UK. FT-IR spectra were recorded on a Thermo Nicolet Nexus 6700 USA. The 1H and 13C NMR spectra of the ligands and their complexes in DMSO-d6 were obtained on Bruker Avance 300 MHz NMR Spectrometer operating at frequencies of 300.00 MHz and 75.47 MHz respectively at 300 K. The spectral conditions were as follows: 32 K data points, 1.822 s acquisition time, 2.00 s pulse delay and 6.00 μs pulse width. The 13C chemical shifts were measured relative to TMS.
2.4 Antibacterial activities of the complexes
The antibacterial activity of all synthesized metal complexes has been investigated against four bacteria, two G (+) bacterial strains i.e., Staphylococcus aureus ATCC 25923, and Bacillus subtilis DSM 3256, and two G (−) bacterial strains i.e., Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 10197 by the agar well diffusion method. Imipenem was used as standard antibiotic which is β-Lactam antibiotic effective against G (+ve) as well as G (−ve) bacteria. 3 mg of the test samples (ligands & complexes) was dissolved in 1 mL of DMSO. 2–3 mL nutrient broth (0.8 g/100 mL) was prepared in distilled water with pH 7 and was autoclaved at 121 °C, 15 psi pressure and for 20 min. 24 h fresh culture grown on nutrient broth at pH 7 was used for sensitivity testing. To compare the turbidity of bacterial cultures McFarland BaSO4 solution was used as turbidity standard. To perform antibacterial assay, nutrient agar medium was prepared by dissolving 2 g/100 mL in distilled water with pH 7 and the medium was autoclaved. The nutrient agar medium was poured in Petri dishes and allowed to solidify. Using sterile cotton swabs lawns of test cultures were prepared on labeled plates. Four wells per plate were prepared with the help of sterile cork borer (2 mm). Using micropipette, 30 μL of test solution was poured in respective labeled wells. Now, these experimental plates were incubated for 24 h and zones of inhibition (%) were measured and compared with standard antibiotic imipenem having zone inhibition 21 mm, 18 mm, 16 mm and 18 mm, respectively.Citation37,Citation38
2.5 Petra/Osiris/Molinspiration (POM) analyses
Petra/Osiris/Molinspiration (POM) analysis is one of the well-known approaches that has been used regularly to produce the two dimensional models to identify and to indicate the type of pharmacophore site that affects biological activity with a change in the chemical substitution. The advantages of POM are the ability to predict the biological activities of the molecules and to represent the relationships between steric/electrostatic property and biological activity in the form of pharmacophore site, which gives key features on not only the ligand–receptor interaction, but also on the topology of the receptor.Citation39
3 Results and discussion
3.1 Elemental analysis (CHN)
Elemental analysis data are given in . It can be seen that the observed values are in very good agreement with the calculated values that show the purity of the synthesized compounds.
3.2 IR studies
Selected IR spectroscopic vibrational bands for the free ligands and their Palladium(II) complexes are given in . Low frequency shifts in the ν(C=S) band at 506–739 cm−1, ν(N–H) band at 1487–1675 cm−1, ν(C–N) band at 1307–1602 cm−1 and high frequency shifts in ν(N–H) band at 3023–3379 cm−1 shifted to 562–762 cm−1, 1498–1619 cm−1, 1317–1573 cm−1 and 2917–3468 cm−1 respectively in the complexes were observed indicating coordination through sulfur compared with free ligands. The peaks at 301–335 cm−1 and 260–279 cm−1 were attributed to the ν(Pd–S) and ν(Pd–I) vibrations, respectively.Citation19,Citation40,Citation41
Table 3 Selected IR absorption (cm−1) for free ligands and their Palladium(II) iodide complexes.
3.3 NMR studies
The 1H NMR data of the ligands and their complexes are given in . In 1H NMR spectra of the complexes, the N–H signal of thiones becomes less intense upon coordination and shifted downfield by 0.03–1.74 ppm from their positions in free ligands. The downfield shifting of the N–H proton is related to an increase of the π electron density in the C–N bond upon complexation.Citation22 The appearance of N–H signal shows that the ligands are coordinated to Palladium(II) via the thioamide group. The N–H protons of Dmtu are equivalent but after coordination they become nonequivalent as NH2 appears as triplets in Dmtu.
Table 4 1H NMR data of the ligands and their Palladium(II) iodide complexes.Table Footnotea
The 13C NMR chemical shifts for the ligands and [Pd(L)4]I2 complexes are given in . Upfield shifts are observed in the C=S resonance of ligands upon its complexation with Palladium(II). The upfield is attributed to the lowering of C=S bond order and shifting of N → C electron density producing a partial double bond character in the C–N bond, as observed in the other metal complexes of thiourea.Citation14,Citation19,Citation20,Citation36 In all complexes the C-2 resonance appears upfield by 2.97–10.12 ppm compared with the free ligands. Among these complexes the Mpy complexes are the most stable complex due to its most and significant shift in the C-2 resonance.
Table 5 13C NMR data of the ligands and their Palladium(II) iodide complexes. Table Footnotea
3.4 Antibacterial studies
Metal complexes with thioamides are capable of inhibiting bacterial growth and activity by interfering with the metabolic process in the bacteria.Citation1–Citation3,Citation14,Citation20,Citation33,Citation36,Citation38,Citation41,Citation42 In the present work, we have synthesized thioamides Palladium(II) iodide complexes and their. Antibacterial activities have been determined against four strains of bacteria S. aureus (ATCC 25923), B. subtilis (DSM 3256), E. coli (ATCC 25922), and P. aeruginosa (ATCC 10197). The results are shown in . Better antibacterial activities were observed as compared to a standard drug. The Palladium(II) complexes containing thiourea, mercaptopyridine and mercaptopyrimidine exhibited good activity against all bacteria S. aureus, B. subtilis, E. coli and P. aeruginosa. The complexes of methylthioure, a tetramethylthiourea and imidazolidine-2-thione exhibited relatively low activity against all of these bacteria. The complexes of dimethylthiourea and thionicotinamide are ineffective against all bacteria i.e. all bacteria show resistance against these two complexes. The antibacterial activity of complexes may be attributed to their inhibition in the metabolic pathway which alter the replication of DNA. Moreover the Palladium(II) complexes have the ability to form hydrogen bonding with the constituents of cell and cell wallCitation43 or direct interaction with membrane, enzyme and proteins. The ligands have the capability to carry metal to the target (DNA) and allow it to interact with it. Weak coordinated ligands may be the best carrier for metal ions to the biological systems. The inactivity or decreased biological activity of some of the complexes may be related to the strongly coordinated ligands with the metal ions.Citation44,Citation45 It has been observed that antibacterial activity decreases on complexation, while some ligands are biologically inactive but upon coordination showed biological activity. The complexes which exhibit significant biological activity show their potential to be used as antibacterial agents.
Table 6 Antibacterial activity of the ligands and their Palladium(II) iodide complexes.
3.5 Petra/Osiris/Molinspiration (POM) analyses
Petra/Osiris/Molinspiration (POM) analyses were performed to investigate the potential pharmacophore sites of the synthesized compounds and reference drug (imipenem).
Modern drug discovery is based in large part on high throughput screening of small molecules against macromolecular disease targets requiring that molecular screening libraries contain drug-like or lead-like compounds. We have analyzed the known standard references (SR) for drug-like properties. With this information in hand, we have established a strategy to design specific drug-like compounds.
3.5.1 Petra analysis
On the basis of the new finding, we can conclude that the synthesized compounds have the C=S, –NH2, NH and –N(CH3)2 moieties which possess a potential for antibacterial/antifungal activities. For antiviral/antifungal activity, the compound possesses (Xδ—Yδ+) pharmacophore site and also it was hypothesized that the difference in charge between X and Y of the same dipolar pharmacophoric site should facilitate the inhibition of bacteria more than viruses.Citation46
3.5.2 Osiris calculations
Remarkably well behaved mutagenicity of divers synthetic molecules classified in database of Celeron Company of Swiss can be used to quantify the role played by various organic groups in promoting or interfering with the way a drug can associate with DNA. The Osiris calculations are tabulated in . Toxicity risks (mutagenicity, tumorigenicity, irritation, and reproduction) and physicochemical properties (cLog P, solubility, drug-likeness and drug-score) of compounds were calculated by the methodology developed by Osiris. The toxicity risk predictor locates fragments within a molecule, which indicate a potential toxicity risk. Toxicity risk alerts are an indication that the drawn structure may be harmful concerning the risk category specified. From the data evaluated in it is observed that all the complexes are supposed to be slightly mutagenic, tumorigenic, irritant with slight reproductive effects when run through the mutagenicity assessment system in comparison with the standard/reference drugs. The reference drug (imipenem) is non-mutagenic, non-tumorigenic, non-irritant with no reproductive effect as shown in . Low hydrophilicities and low cLog P values may cause good absorption or permeation. It has been shown that for compounds to have a reasonable probability of good absorption, their cLog P value must not be greater than 5.0. On this basis, all the compounds possessed cLog P values are quite less than 5. The aqueous solubility of a compound significantly affects its absorption and distribution characteristics. Typically, a low solubility goes along with a bad absorption and therefore the general aim is to avoid poorly soluble compounds. Our estimated Solubility (S) value is a unit stripped logarithm (base 10) of a compound’s solubility in mol/L. There are more than 80% of the drugs on the market having an (estimated) S value greater than −4.Citation47–Citation49 In our case the S values for studied compounds are in the range of −1.08 and −3.13. Further, shows drug likeness of the compounds which are almost in the comparable zone with that of standard drug used for comparison. The compounds also possess the drug-score though smaller than that of the reference drug.
Table 7 Osiris calculations of toxicity risks and drug-score of the selected compounds.
3.5.3 Molinspiration calculations
cLog P (octanol/water partition coefficient) is calculated by the methodology developed by Molinspiration as a sum of fragment-based contributions and correction factors (). The method is very stout and is able to process practically all organic and most organometallic molecules. Molecular Polar Surface Area (TPSA) is calculated as a sum of fragment contributions. S-, N-, I and Pd centered polar fragments are considered. PSA has been shown to be a very good descriptor characterizing drug absorption, including intestinal absorption, bioavailability, Caco-2 permeability and blood–brain barrier penetration. Prediction results of the studied compounds (molecular properties (TPSA and GPCR ligand)) are valued (). Lipophilicity (cLog P value) and polar surface area (TPSA) values are two important properties for the prediction of per oral bioavailability of drug molecules.Citation47–Citation49 Therefore cLog P and TPSA values for compounds were calculated using molinspiration software programs and compared with the values obtained for standard drugs imipenem (for antibacterial activity). The calculated cLog P values for the investigated compounds are in the range of −0.13 to 0.31 which are in the acceptable range. So these compounds are expected to present good bioavailability.Citation47–Citation49
Table 8 Molinspiration property and Molinspiration bioactivity score data of the selected compounds and reference drugs.
The polar surface area (TPSA) is calculated from the surface areas that are occupied by oxygen and nitrogen atoms and by hydrogen atoms attached to them and/or metal atom. Thus, the TPSA is closely related to the hydrogen bonding potential of a compound. Molecules with TPSA values around of 160 or more are expected to exhibit poor intestinal absorption. It is to be noted that cLog P and TPSA values are the two important parameters, although not sufficient criteria for predicting oral absorption of a drug. All the compounds (except Pd(Metu)4]I2 and Pd(Tu)4]I2) have one violation from the Rule of 5. Two or more violations of the Rule of 5 suggest the probability of problems in bioavailability of the standard drug.Citation48,Citation49 Properties such as hydrophobicity, electronic distribution, hydrogen bonding characteristics, molecule size, flexibility and presence of various pharmacophores features influence the behavior of molecule in a living organism, including bioavailability, transport properties, affinity to proteins, reactivity, toxicity, metabolic stability and many others. Activity of the synthesized compounds and standard drug was rigorously analyzed under four criteria of known successful drug activity in the areas of GPCR ligand activity, ion channel modulation, Kinase inhibition activity, and nuclear receptor ligand activity. Results are shown in by means of numerical assignment. Thus, these compounds are expected to have near similar activity to the standard drug used based upon these four rigorous criteria (GPCR ligand, ion channel modulator, Kinase inhibitor and nuclear receptor ligand).Citation47–Citation49
4 Conclusion
The present study shows that the thioamide ligands coordinate with Palladium(II) iodide in the thione form in solution as well as in the solid state. All ligands behave as S-donors and are binding in a terminal mode and no bridging of groups between metal centers is found. The complexation was confirmed by the downfield shift of the N–H proton as a result of increase of π electron density in the C–N bond. In the 13C NMR spectra of the complexes upfield shifts in the C=S resonance also support the binding of ligands to Palladium(II). The shift difference of the C=S resonance may be related to the strength of metal-sulfur bond. Some of the screened compounds have shown good antibacterial activity which has potential for their use in future as antibacterial agents.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgment
Financial support from Higher Education Commission Islamabad Pakistan is gratefully acknowledged.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
References
- G.De MunnoB.GabrieleG.SalernoX-ray structure of palladium(II) tetrakis-thiourea iodide, a catalyst for carbonylation reactionsInorg Chim Acta2341995181183
- T.S.LobanaS.KhannaR.J.ButcherA.D.HunterM.ZellerSynthesis, crystal structures and multinuclear NMR spectroscopy of copper(I) complexes with benzophenone thiosemicarbazonePolyhedron25200627552763
- S.A.TirmiziS.NadeemA.HameedM.H.S.WattooA.AnwarZ.A.AnsariSynthesis, spectral characterization and antibacterial studies of Palladium(II) complexes of heterocyclic thionesSpectroscopy232009299306
- M.MufakkarS.AhmadI.U.KhanH.K.FunS.ChantraprommaTris (N,N′-dibutylthiourea-S) iodidocopper (I) 0.6-hydrateActa CrystallogrE632007m2384
- A.A.IsabS.AhmadM.ArabSynthesis of Silver(I) complexes of thiones and their characterization by 13C, 15N and 107Ag NMR spectroscopyPolyhedron21200212671271
- W.AshrafS.AhmadA.A.IsabSilver cyanide complexes of heterocyclic thionesTransition Met Chem292004400404
- M.HanifS.AhmadM.AltafH.Stoeckli-EvansPoly[bis(μ2-cyanido)-κ2C:N; κ2N:C-(μ2-N,N,N′,N′-tetramethylthiourea-κ2S:S)diSilver(I)]Acta CrystallogrE632007m2594
- F.B.StockerD.BrittonV.G.YoungCrystal structures of a family of Silver cyanide complexes of thiourea and substituted thioureasInorg Chem39200034793484
- C.PakawatchaiK.SivakumarH.K.FunBis (N,N′-dimethylthiourea-S) Silver (I) perchlorate and tris (N,N′-dimethylthiourea-S) Silver (I) perchlorateActa CrystallogrC52199619541957
- H.K.FunI.A.RazakC.PakawatchaiS.ChantraprommaS.SaithongTris (N,N′-diethylthiourea-S) iodocopper (I) and Tris (N,N′-diethylthiourea-S) iodoSilver (I)Acta CrystallogrC541998453456
- J.S.CasasE.G.MartinezA.SanchezA.S.GonzalezJ.SordoU.CasellatoComplexes of Ag(I) with 1-methyl-2(3H)-imidazolinethione. The crystal structure of tris[1-methyl-2(3H)-imidazolinethione]-Silver(I) nitrateInorg Chim Acta2411996117123
- F.B.StockerD.Britton1,2-Di-cyano-1,2-bis-(imidazolidine-2-thione)-digold(I) and 2,2-di-cyano-1,1-bis-(di-methyl-thio-urea)-digold(I)Acta CrystallogrC562000798800
- S.AhmadA.A.IsabH.P.PeranowskiLigand scrambling reactions of cyano (thione) gold (I) complexes and determination of their equilibrium constantsCan J Chem80200212791284
- A.A.IsabM.B.FettouhiS.AhmadL.OuahabMixed ligand gold(I) complexes of phosphines and thiourea and X-ray structure of (thiourea-κS)(tricyclohexylphosphine)gold(I)chloridePolyhedron22200313491354
- I.LakomskaP.LeszekS.JerzyK.LechP.MarzenaN.AnnaMultinuclear NMR spectroscopy and antiproliferative activity in vitro of platinum(II) and Palladium(II) complexes with 6-mercaptopurineJ Mol Struct7072004241247
- N.HadjiliadisT.TheophanidesSynthesis of platinum 6-thiopurine riboside complexesInorg Chim Acta151975167178
- S.KrischnerY.K.WeiD.FrancisJ.G.BergmanAnticancer and potential antiviral activity of complex inorganic compoundsJ Med Chem91966369372
- P.RebolledoM.VieitesD.GambinoO.E.PiroE.E.CastellanoC.L.ZaniPalladium (II) complexes of 2-benzoylpyridine-derived thiosemicarbazones: spectral characterization, structural studies and cytotoxic activityJ Inorg Biochem992005698706
- I.MatesanzP.SouzaNovel cyclopalladated and coordination Palladium and platinum complexes derived from α-diphenyl ethanedione bis(thiosemicarbazones): structural studies and cytotoxic activity against human A2780 and A2780cisR carcinoma cell linesJ Inorg Biochem101200713541361
- S.NadeemM.K.RaufM.EbiharaS.A.TirmiziS.AhmadTetrakis (thiourea-S) Palladium (II) dithiocyanateActa CrystallogrE642008m698m699
- S.NadeemM.K.RaufS.AhmadM.EbiharaS.A.TirmiziS.A.BashirSynthesis and characterization of Palladium(II) complexes of thioureas. X-ray structures of [Pd(N,N′-dimethylthiourea)4]Cl2·2H2O and [Pd(tetramethylthiourea)4]Cl2Tran Met Chem342009197202
- Z.AthertonD.M.L.GoodgameS.MenzerD.J.WilliamsFormation of chain and large-ring polymeric metal complexes by the “extended reach” sulfur donor ligands N,N′-ethylenebis(pyrrolidine-2-thione) and N,N′-p-phenylenedimethylenebis(pyrrolidine-2-thione)Inorg Chem371998849858
- M.FettouhiM.I.M.WazeerA.A.IsabCrystal structure of bis(3,4,5,6-tetrahydropyrimidine-2(1H)-thione-S)gold(I)chloride,[Au(C4H8N2S)2ClZ Kristallogr NCS219200412
- M.FettouhiM.I.M.WazeerA.A.IsabZinc halide complexes of imidazolidine-2-thione and its derivatives: X-ray structures, solid state, solution NMR and antimicrobial activity studiesJ Coord Chem602007369377
- A.BeheshtiW.CleggS.H.DaleR.HyvadiSynthesis, crystal structures, and spectroscopic characterization of the neutral monomeric tetrahedral [M(Diap)2(OAc)2]·H2O complexes (M = Zn, Cd; Diap = 1,3-diazepane-2-thione; OAc = acetate) with N–H⋯O and O–H⋯O intra- and intermolecular hydrogen bonding interactionsInorg Chim Acta360200729672972
- M.I.M.WazeerA.A.IsabM.FettouhiNew cadmium chloride complexes with imidazolidine-2-thione and its derivatives: X-ray structures, solid state and solution NMR and antimicrobial activity studiesPolyhedron26200717251730
- U.RajalingamP.W.A.DeanH.A.JenkinsSolution multinuclear (31P, 111Cd, 77Se) magnetic resonance studies of cadmium complexes of heterocyclic aromatic thiones and the structure of [tetrakis(2(1H)-pyridinethione)cadmium] nitrate, [Cd(C5H5NS)4](NO3)2Can J Chem782000590597
- U.RajalingamP.W.A.DeanH.A.JenkinsM.JenningsJ.M.HookCadmium complexes of thiones. Part II.1 A synthetic, solution, and solid-state MAS 111/113Cd NMR study of cadmium complexes of 1,3-thiazolidine-2-thione, and the structures of [tetrakis(1,3-thiazolidine-2-thione)cadmium] trifluoromethanesulfonate ([Cd(C3H5NS2)4](CF3SO3)2) and [tetrakis(1,3-thiazolidine-2-thione)cadmium][tetrakis(nitrato-O,O′)cadmate] ([Cd(C3H5NS2)4][Cd(O2NO)4])Can J Chem79200113301337
- N.A.BellW.CleggS.J.ColesC.P.ConstableR.W.HarringtonM.B.HursthouseComplexes of heterocyclic thiones and group 12 metals: Part VI. Preparation and characterisation of complexes of cadmium(II) halides with 1-methylimidazoline-2(3H)-thione, 1,3-thiazolidine-2-thione and 1,3-benzothiazoline-2-thione. Crystal structures of polymeric (1,3-thiazolidine-2-thione)cadmium(II) chloride, bis(1,3-thiazolidine-2-thione)cadmium(II) iodide and monomeric bis(1-methylimidazoline-2(3H)-thione)cadmium(II) bromideInorg Chim Acta357200420912099
- M.J.MolotoM.A.MalikP.O.BrienM.MotevalliG.A.KolawoleSynthesis and characterisation of some N-alkyl/aryl and N,N′-dialkyl/aryl thiourea cadmium(II) complexes: the single crystal X-ray structures of [CdCl2(CS(NH2)NHCH3)2]n and [CdCl2(CS(NH2)NHCH2CH3)2]Polyhedron222003595603
- A.TadjarodiF.AdhamiZ.GharehdaghiSynthesis and crystal structure of a new Cadmium(II) complex with 1,3-thiazolidine-2-thione and bipyridine ligandsAnal Sci X-ray Struct Anal Online232007x35x36
- E.S.RaperJ.R.CreightonN.A.BellW.CleggL.Cucurull-SanchezComplexes of heterocyclic thiones and group twelve metals Part 1. Preparation and characterisation of 1:1 complexes of mercury(II) halides with 1-methylimidazoline-2(3H)-thione: the crystal structure of [(μ2-dibromo) bis(trans{(bromo) (1-methyl-imidazoline-2(3H)-thione)}mercury(II))] at 160 KInorg Chim Acta27719981420
- Z.PopovicG.PavlovicD.Matkovic-CalogovicZ.SoldinM.RajicD.Vikic-TopicMercury(II) complexes of heterocyclic thiones. Part 1. Preparation of 1:2 complexes of mercury(II) halides and pseudohalides with 3,4,5,6-tetrahydropyrimidine-2-thione. X-ray, thermal analysis and NMR studiesInorg Chim Acta3062000142152
- N.A.BellT.N.BranstonW.CleggJ.R.CreightonL.Cucurull-SánchezM.R.J.ElsegoodComplexes of heterocyclic thiones and Group 12 metals: Part 3. Preparation and characterisation of 1:2 complexes of mercury(II) halides with 1-methylimidazoline-2(3H)-thione: the crystal structures of [(HgX2)(1-methylimidazoline-2(3H)-thione)2] (X = Cl, Br, I) at 160 KInorg Chim Acta3032000220227
- Z.PopovicZ.SoldinD.Matkovic-CalogovicG.PavlovicM.RajicG.GiesterMercury (II) complexes with heterocyclic thiones — preparation and characterization of the 1:1 and 1: 2 mercury (II) complexes with benzo-1,3-imidazole-2-thioneEur J Inorg Chem12002171180
- M.I.M.WazeerA.A.IsabComplexations of Hg(CN)2 with imidazolidine-2-thione and its derivatives: solid state, solution NMR and antimicrobial activity studiesSpectrochim Acta A68200712071212
- S.AhmadA.A.Isab13C NMR studies of the interaction of Gold(I) thiomalate with 6-mercaptopurine and its derivativesJ Coord Chem552002189203
- S.U.KazmiS.N.AliS.A.JamalAtta-ur-RehmanNew bioactive compounds of plant originJ Pharm Sci41991113123
- T.K.MohantaJ.K.PatraS.K.RathD.K.PalH.N.ThatoiEvaluation of antimicrobial activity and phytochemical screening of oils and nuts of Semicarpus anacardiumSci Res Essays22007486
- M.SirajuddinS.AliV.McKeeH.UllahSynthesis, spectroscopic characterization and in vitro antimicrobial, anticancer and antileishmanial activities as well interaction with Salmon sperm DNA of newly synthesized carboxylic acid derivative, 4-(4-methoxy-2-nitrophenylamino)-4-oxobutanoic acidSpectrochim Acta Part A1382015569578
- A.ReissM.MureseanuDevelopment and validation of liquid chromatographic method for naproxen and esomeprazole in binary combinationJ Chil Chem Soc572012456459
- J.JolleyW.I.CrossR.G.PritchardC.A.McAuliffeK.B.NolanSynthesis and characterisation of mercaptoimidazole, mercaptopyrimidine and mercaptopyridine complexes of platinum(II) and platinum(III). The crystal and molecular structures of tetra(2-mercaptobenzimidazole)- and tetra(2-mercaptoimidazole)platinum(II) chlorideInorg Chim Acta31520013643
- S.A.TirmiziS.NadeemA.HameedM.H.S.WatooA.AnwarZ.A.AnsariSynthesis, spectral characterization and antibacterial studies of Palladium (II) complexes of heterocyclic thionesSpectroscopy232009299306
- H.U.ArslanN.K.FlorkeThe crystal and molecular structure of 1-(biphenyl-4-carbonyl)-3-p-tolyl-thioureaActa Chim Slov512004787792
- A.PapageorgiouA.IakovidouD.MourelatosE.MioglouL.BoutisA.KotsisAntineoplastic and cytogenetic effects of complexes of Pd(II) with 4N-substituted derivatives of 2-acetyl-pyridine-thiosemicarbazoneAnticancer Res171997247251
- J.P.ScovillD.L.KlaymanD.G.Franchino2-Acetylpyridine thiosemicarbazones. 4. Complexes with transition metals as antimalarial and antileukemic agentsJ Med Chem25198212611264
- J.SheikhA.ParvezV.IngleH.JunejaR.DongreZ.H.ChohanSynthesis, biopharmaceutical characterization, antimicrobial and antioxidant activities of 1-(4′-O-β-d-glucopyranosyloxy-2′-hydroxyphenyl)-3-aryl-propane-1,3-dionesEur J Med Chem46201113901399
- A.ParvezJ.MeshramV.TiwariJ.SheikhR.DongreM.H.YoussoufiPharmacophores modeling in terms of prediction of theoretical physico-chemical properties and verification by experimental correlations of novel coumarin derivatives produced via Betti’s protocolEur J Med Chem45201043704378
- N.UddinM.SirajuddinN.UddinH.UllahS.AliM.TariqSynthesis, spectroscopic characterization, biological screenings, DNA binding study and POM analyses of transition metal carboxylatesSpectrochim Acta A1402015563574