Abstract
Background
Renal ischemia–reperfusion (RIR) is an important etiopathological mechanism of acute renal failure (ARF). Erythropoietin (EPO) has been candidate as a nephroprotectant agent. However, its nephroprotective effect when it is accompanied with estrogen has not been studied in female.
Methods
Fifty-six female rats were divided into seven groups. Each formed of 8 rats. Group I: control group. Group II: Female rats exposed to RIR (named RIR group).Group III: Female rats exposed to RIR and pretreated with EPO (named RIR + EPO group). Group IV: ovariectomized rats exposed to RIR (named OVR + RIR group). Group V: ovariectomized rats received estrogen (E) then exposed to RIR (named OVR + RIR + E group). Group VI: ovariectomized rats received EPO before RIR (named OVR + RIR + EPO group). Group VII: ovariectomized rats received E then received EPO before RIR (named OVR + RIR + E + EPO group).Serum creatinine, blood urea nitrogen (BUN) and renal blood flow (RBF) were measured. Tumor necrosis factor-α (TNF-α), Myeloperoxidase activity (MPO), nitric oxide (NO), endothelin-1(ET-1) and EPO levels were assessed in the renal tissue. Histopathology was assessed to detect renal damage score.
Results
RIR significantly increased the serum levels of creatinine and BUN with decrease in RBF. In addition it significantly increased TNF-α, MPO and endothelin-1 levels with decrease in NO and EPO levels in renal tissue. However, these parameters significantly reversed by EPO except RBF. Combination of E and EPO leads to significant decrease in the protective effect of EPO.
Conclusion
It seems that EPO could protect the kidney against RIR, while this protective effect was decreased when E was supplemented.
1 Introduction
ARF caused by RIR is an important clinical problem. Even though great progress has been made in patient care, there is still high morbidity and mortality associated with ARF.Citation1RIR injury occurs in various clinical settings including severe hypotension and subsequent resuscitation, shock, sepsis, renal transplantation and aorto-vascular Surgery.Citation2
Figure 3 Effect of EPO and E on scoring of renal tissue damage of all groups. Comparison between mean ± SD of scoring of renal tissue damage of group (I): control group, group II: (RIR group), group III: (RIR + EPO group), group IV: (OVR + RIR group), group V: (OVR + RIR + E group), group VI: (OVR + RIR + EPO group) and group (VII): (OVR + RIR + E + EPO). a = statistically significant compared to the corresponding value in group (I) (p < 0.001). b = statistically significant compared to the corresponding value in group (II) (p < 0.001). c = statistically significant compared to the corresponding value in group (III) (p < 0.001). d = statistically significant compared to the corresponding value in group (IV) (p < 0.001). # = statistically significant compared to the corresponding value in group (V) (p < 0.005). & = statistically significant compared to the corresponding value in group (V) (p < 0.01). @ = statistically significant compared to the corresponding value in group (VI) (p < 0.01).
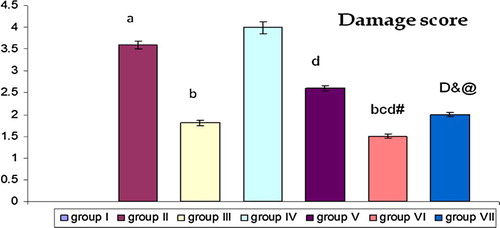
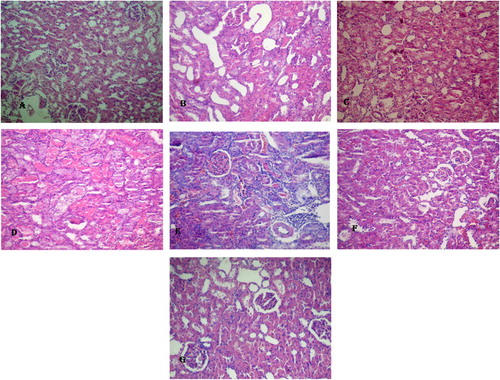
Figure 4 Histological results of all group. (A) A photomicrograph renal sections stained with H & E showed no histopathological changes in kidney of sham operated group. (B) Kidney sections of RIR group showed severe vacuolization and congestion with development of severe necrosis in tubular cells. (C) Kidney sections of RIR + EPO group showed moderate tubular cell vacuolization and necrosis. (D) Kidney sections of RIR + OVR group showed severe vacuolization and congestion with development of severe necrosis in tubular cells. (E) Kidney sections of RIR + OVR + E group showed mild vacuolization and congestion with mild necrosis in tubular cells. (F) Kidney sections of RIR + OVR + EPO group showed mild vacuolization and congestion with mild necrosis in tubular cells. (G) Kidney sections of RIR + OVR + E + EPO group showed moderate vacuolization and congestion with moderate necrosis in tubular cells (H & E 20×).
There is growing evidence which indicates that sex differences exist in kidney response to renal ischemic injury with increased male susceptibility to acute and chronic renal injury than that of female.Citation3Detailed cellular and molecular mechanisms of these differences are still unknown, and they involve genomic and non-genomic effects of sex hormones, particularly estrogen.Citation4
Endothelial dysfunction is an important component of initiating and continuing renal tubular epithelial injury and contributes to the pathogenesis of ischemic ARF.Citation5Endothelial injury may aggravate the inflammatory response through loss of normal nitric oxide (NO) production due to inhibition of endothelial nitric oxide synthase (eNOS). NO reduces leukocyte-induced injury by blocking leukocyte sequestration and activation. However, RIR also increases inducible nitric oxide synthase (iNOS), which potentiates injury. Also, the high output production by iNOS might suppress eNOS. This imbalance between the two NOS may be an important component of RIR injury.Citation6
Inflammation contributes to the pathogenesis of RIR injury in a variety of contexts. Inflammation can result in reduction in local blood flow to the outer medulla, with adverse consequences on tubule function and viability. There has been some controversy about the relative importance of various subgroups of leukocytes as neutrophils to RIR injury.Citation7
There is a controversy on the effect of E on renal function of ovariectomized rats. As some studies reported more deterioration by administration of E, others reported an improvement of renal function.
EPO is an essential growth factor of hemopoietic progenitor cells, but its extrahemopoietic effects imply additional therapeutic possibilities. Indeed, a wealth of experimental data is being generated with respect to the protective effect of EPO against the ischemic myocardium, liver, and renal injury.Citation8In addition; there exist sex differences in endogenous erythropoietin. The concentration of erythropoietin is higher in males than in females.Citation9
Experimental evidence suggests that E inhibits production of EPO in female rats, when rats have exposed to various intensities of ischemia, which is confirmed by production of lower amounts of EPO in normal females than in normal males.Citation10 Accordingly, the protective roles of EPO against RIR may change when E is accompanied by EPO. Therefore, this study was designed to find the protective role of EPO in RIR when it is accompanied by E.
2 Methods
2.1 Animals
Experiments were performed on 56 sexuallymature female rats (weighing 200–250 g). Experimental Animals were approved by the Ethical Committee of the Faculty of Medicine, Benha University, Egypt. Rats were fed with a standard laboratory diet and water ad libitum. Animals were left to acclimatize to the environment for two weeks prior to inclusion in the experiment.
The rats were divided into 7 different groups (n = 8).
Group I: Control sham-operated female rats (rats were subjected to midline laparotomy and dissection of renal pedicles without any renal ischemia) only receive Sesame oil by sc route for three weeks (control group).
Group II: female rats received Sesame oil by sc route for three weeks then exposed to bilateral RIR; ischemia was produced for 50 min, followed by 2 hs reperfusion (RIR group).Citation11
Group III: female rats received Sesame oil by sc rout for three weeks then treated with EPO 5000 U/kg single dose IP, 20 min before ischemia (named RIR + EPO group).Citation12
Group IV: female rats exposed to bilateral ovariectomy then after one week they received Sesame oil by sc rout for three weeks then exposed to RIR (named OVR + RIR).Citation13
Group V: female rats exposed to bilateral ovariectomy then after one week they received estrogen supplementation (25 μg/kg/day; SC) for three weeks then exposed to RIR (OVR + E + RIR).Citation14
Group VI: female rats exposed to bilateral ovariectomy then after one week they received Sesame oil by sc rout for three weeks then treated with EPO 5000 U/kg single dose IP, 20 min before ischemia (named OVR + RIR + EPO group).
Group VII: Ovariectomized rats received estrogen for 3 weeks, and then received EPO 20 min before ischemia (named OVR + RIR + E + EPO group).
2.2 Chemicals
Estrogen in the form of Folone ampoules, each 1 ml ampoule contains 5 mg estradiol benzoate in oily solution, was purchased from Misr Co., for Pharm. ind. S.A.E. (Cairo, Egypt). Sesame oil was purchased from Indian Co. (Cairo, Egypt). Thiopental sodium was purchased from Eipico Co. (Cairo, Egypt) and Erypro Safe (5000 i.u) (Erythropoietin) from Biocon (India) Ltd., from Pharma Co.
2.3 Preparation of E solution
E used in this study was a liquid in Folone ampoules (5 mg estradiol benzoate/ml) in oily solution. The injection solution was prepared by dissolving 1 ml of estradiol benzoate in 36 ml ethanol-sesame oil to give a concentration of 25 μgE in 0.2 ml final solution. All rats in all groups except that treated with E only received an oily solution as a vehicle (0.2 ml/kg/day SC).
2.4 RIR injury animal model
Rats were anesthetized by intraperitoneal injection with thiopental sodium (30 mg/kg). A Midline abdominal incision was made and both kidneys were exposed. Renal ischemia was induced by non-traumatic vascular clamps over the pedicles (arteries and veins) of the two kidneys for 50 min. Following the occlusion, the presence of ischemia was visually confirmed by observing blanching of the kidneys. During the period of ischemia, the edges of the abdominal incision were approximated to each other and covered by a cotton pad soaked with warm isotonic saline (37 °C) to prevent undue loss of body fluids. After 50 min, the clamp was removed for recirculation of blood flow then the kidneys were observed for an additional 1 min to see the color change indicative of blood reflow.Citation152 hs after reperfusion RBF was measured then blood samples were obtained via heart puncture. Serum samples were removed and stored at −20 °C until measurement. Right kidney was removed for preparation of renal tissue homogenate and the left kidney was removed and fixed in 10% formalin solution for pathological assessments. In sham-operated group, the same surgical procedures were done but without applying the clamps.
2.5 Ovariectomized rat model
The rat was anesthetized by thiopental sodium (30 mg/kg). The anesthetized rat was placed on the operating board in dorsal recumbency with its tail directed toward the surgeon. The ventral aspect of the lumbar region was shaved, and then cleaned with 75% ethanol, followed by thorough scrubbing with 10% povidone iodine (Betadine). 1 cm long longitudinal ventral midline incision was made above the symphysis pubis by a scalpel blade; the skin edges were laterally retracted, and the abdominal muscle layer and the peritoneum were incised. Both fallopian tubes were exposed and ligated; the ovary can usually be seen embedded in a pad of fat in the abdomen; then the ovaries were removed by cutting them with scissors, taking care not to rupture the ovarian capsules. The remaining tissues were replaced into the peritoneal cavity. The incision was then closed using a sterile 2/0 suture. The removed tissue was ensured to be the ovaries by histological sections. The rats were returned to their cages and left for about four weeks till RIR.Citation16
2.6 Mortality rate
Four rats died during the surgical procedures.
2.7 Assessment of renal function
Serum creatinine was assessed using the Jaffé picric acid procedureCitation17 with Sigma kit 555-A# (Sigma–Aldrich Chemical Co.). BUN was assessed by enzymatic methodCitation18 (modified Berthelot reaction) (dp international; Tuscaloosa: USA).
2.8 Measurement of renal blood flow by Doppler flow meter
The left kidney was exposed through a flank incision, and its artery was cleared of connective tissue so that an electromagnetic flowmeter probe (Carolina Medical Electronic, King, NC, USA) could be fitted.Citation19
2.9 Measurement of nitric oxide (NO)
Kidney tissue was homogenized in 5–10 ml cold buffer (50 mM potassium phosphate, pH 7.5. 1 mM EDTA) per gram tissue. Homogenate was centrifuged and supernatant was removed for assay. NO level was determined indirectly as its metabolic products (nitrate + nitrite ions) spectrophotometrically using Bio Assay Systems’ Quanti Chrom TM Nitric Oxide Assay Kit to measure NO production following reduction of nitrate to nitrite using improved Griess method.Citation20
2.10 Renal ET-1 assay
ET-1 was extracted from the kidney. The radioimmunoassay for ET-1 was performed as described previouslyCitation21
2.11 Estimation of renal tissue TNF-α
Estimation of renal tissue TNF-α was done by commercial sandwich Elisa kits for rats according to manufacturer’s instructions (Sigma–Aldrich Co., St Louis, MO, USA). The concentrations of TNF-α in kidney tissues were expressed as pg/mg protein.Citation22
2.12 Estimation of neutrophil accumulation
MPO activity was measured as an index of neutrophil accumulation. Tissue MPO activity was assessed using a commercial assay kit (Hycult Biotech Inc., Burlington, CA). The MPO activity was expressed as U/g of tissue.Citation22
2.13 Determination of renal EPO concentrations
Renal erythropoietin levels were determined by EPO ELISA kit and were expressed as pg/ml as described previously.Citation23
2.14 Histopathological procedures
The left kidney was fixed in 10% formalin solution and embedded in paraffin. The tissue slices were stained by hematoxylin and eosin (H & E) to examine the tissue damage based on the presence of tubular atrophy, hyaline cast, ischemic necrosis, vacuolization, and debris. According to the damage intensity, the samples were scored as 1–4 and 0 means normal (no tissue damage): 1. means low damage (up to 25% of tissue damage), 2. means mild damage (between 26% and 50% of tissue damage), 3. means moderate damage (between 51% and 75% of tissue damage) and 4. means severe damage (more than 75% of tissue damage).Citation24
2.15 Statistical analysis
All the data are presented as mean ± standard deviation (SD). Evaluation of differences between groups was performed using one-way analysis of variance (ANOVA) with SPSS 19.0 software. A P-value of less than 0.05 was considered statistically significant.
3 Results
3.1 Effect of erythropoietin and estrogen on serum creatinine, BUN and renal blood flow (RBF) in ovariectomized rats exposed to RIR
shows that serum creatinine and BUN were significantly elevated (p < 0.001) and RBF was significantly decreased (p < 0.001) in RIR group (group II) and OVR + RIR group (group IV) compared to control group. There was significant decrease (p < 0.001) in serum creatinine and BUN with non-significant increase in RBF in RIR + EPO group (group III) when compared with RIR group (group II). There was significant increase (p < 0.001) in serum creatinine and BUN and significant decrease (p < 0.001) in RBF in OVR + RIR group (group IV) when compared with RIR group (group II). Administration of E to ovariectomized rats exposed to RIR as in group V leads to significant decrease (p < 0.001) in serum creatinine and BUN and significant increase (p < 0.001) in RBF when compared to OVR + RIR group (group IV). Administration of EPO to ovariectomized rats exposed to RIR as in group VI leads to significant decrease (p < 0.001) in serum creatinine and BUN and significant increase (p < 0.001) in RBF when compared to OVR + RIR group (group IV), and leads to significant decrease (p < 0.001) in serum creatinine and BUN and significant decrease (p < 0.001) in RBF when compared to OVR + RIR + E group (group V). Administration of EPO and E to ovariectomized rats exposed to RIR as in group VII leads to significant decrease (p < 0.001) in serum creatinine and BUN and significant increase (p < 0.001) in RBF when compared to OVR + RIR (group IV) and leads to significant decrease (p < 0.001) in serum creatinine and BUN and significant decrease (p < 0.001) in RBF when compared to OVR + RIR + E group (group V). In addition it leads to significant increase (p < 0.001) in serum creatinine and BUN and significant increase (p < 0.001) in RBF when compared to OVR + RIR + EPO group (group VI); this indicates that administration of both E and EPO leads to significant decrease in the protective effect of erythropoietin on urea and creatinine. Also there was significant decrease (p < 0.001) in serum creatinine and BUN with significant increase (p < 0.005) in RBF in OVR + RIR + EPO group (group VI) when compared with RIR + EPO group (group III). Also effect of EPO and E on RBF in ovariectomized rats exposed to RIR is demonstrated in (A–G).
Figure 1 Effect of EPO and E on RBF in all groups. (A): A trace showing RBF of control group. (B) A trace showing RBF of RIR group. (C) A trace showing RBF of RIR + EPO group. (D) A trace showing RBF of OVR + RIR group. (E) A trace showing RBF of OVR + RIR + E group. (F) A trace showing RBF of OVR + RIR + EPO group. (G) A trace showing RBF of OVR + RIR + E + EPO group.
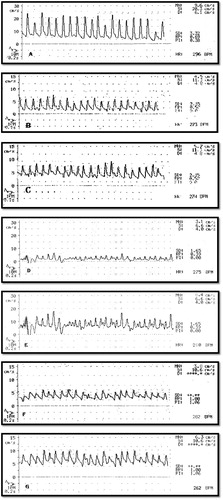
Table 1 Comparison between mean ± SD of serum creatinine (mg/dl), blood urea nitrogen (BUN) (mg/dl) and RBF cm/s in group (I): control group, group II: (RIR group), group III: (EPO + RIR group), group IV: (OVR + RIR group), group V: (OVR + RIR + E group), group VI: (OVR + RIR + EPO group) and group (VII): (OVR + RIR + E + EPO group).
3.2 Effect of erythropoietin and estrogen on NO (nmol/l), ET-1(ng/g) and erythropoietin (pg/ml) levels in renal tissue of ovariectomized rats exposed to RIR
shows that RIR as in group II caused significant decrease (p < 0.001) in NO and erythropoietin levels with significant increase (p < 0.001) in ET-1 levels in renal tissue when compared with the control group. There was significant increase (p < 0.001) in NO, ET-1 and Erythropoietin levels in renal tissue in RIR + EPO group (group III) when compared with RIR group (group II). RIR in ovariectomized rats leads to significant decrease (p < 0.001) in NO and erythropoietin levels with significant increase (p < 0.001) in ET-1 when compared with RIR group (group II). Administration of estrogen to RIR in ovariectomized rats as in E + OVR + RIR group (group V) leads to significant increase (p < 0.001) in NO and significant (p < 0.001) decrease in ET-1 and erythropoietin levels when compared with OVR + RIR group (group IV). Administration of EPO to RIR in ovariectomized rats as in EPO + OVR + RIR group leads to significant increase in NO (p < 0.001) and erythropoietin (p < 0.05) levels with non-significant increase in ET-1 when compared with OVR + RIR group (group III). And when compared with OVR + RIR + E group (group IV) there is significant decrease (p < 0.001) in NO and erythropoietin levels with significant increase in ET-1. Combination of EPO and E to RIR in ovariectomized rats as in OVR + RIR + E + EPO group leads to significant increase (p < 0.001) in NO and significant decrease (p < 0.001) in ET-1 and erythropoietin (p < 0.05) when compared with OVR + RIR group (group IV). While there was significant decrease (p < 0.001) in NO and significant increase (p < 0.001) in ET-1 when compared with group V, there was significant increase (p < 0.001) in NO and significant decrease (p < 0.001) in ET-1 and erythropoietin levels when compared with group VI.
Table 2 Comparison between mean ± SD NO (nmol/l), ET-1(ng/g) and EPO (Iu/l) levels in renal tissue of group (I): control group, group II: (RIR group), group III: (RIR + EPO group), group IV: (OVR + RIR group), group V: (OVR + RIR + E group), group VI: (OVR + RIR + EPO group) and group (VII): (OVR + RIR + E + EPO group).
3.3 Effect of EPO and E on TNF-α (pg/mg) and MPO (u/gm) levels in renal tissue of ovariectomized rats exposed to RIR
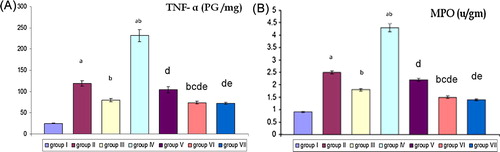
(A and B) shows that RIR as in group II caused significant increase (p < 0.001) in TNF-α and MPO levels in renal tissue when compared with the control group. Pretreatment of RIR with EPO as in RIR + EPO group leads to significant decrease (p < 0.001) in TNF-α and MPO when compared with RIR group (group II). RIR in ovariectomized rats as in OVR + RIR (group IV) leads to significant increase in (p < 0.001) TNF-α and MPO levels when compared with control group and RIR group (group II). Pretreatment of ovariectomized rats with E prior to RIR as in OVR + RIR + E (group V) leads to significant decrease (p < 0.001) of TNF-α and MPO levels when compared with OVR + RIR (group IV). Pretreatment of ovariectomized rats with EPO prior to RIR as in OVR + RIR + EPO (group VI) leads to significant decrease (p < 0.001) of TNF-α and MPO levels when compared with OVR + RIR (group IV) and OVR + RIR + E (group V). Pretreatment of ovariectomized rats with estrogen and EPO prior to RIR as in OVR + RIR + E + EPO (group VII) leads to significant decrease (p < 0.001) of TNF-α and MPO levels when compared with OVR + RIR (group IV), OVR + RIR + E (group V) and non-significant decrease when compared with OVR + RIR + EPO (group VI).
Figure 2 Effect of EPO and E on TNF-α (pg /mg) and MPO (u/gm) levels in renal tissue of all groups. (A and B) Comparison between mean ± SD of TNF-α (PG/mg) and MPO (u/gm) levels in renal tissue of group (I): control group, group II: (RIR group), group III: (RIR + EPO group), group IV: (OVR + RIR group), group V: (OVR + RIR + E group), group VI: (OVR + RIR + EPO group) and group (VII): (OVR + RIR + E + EPO). a = statistically significant compared to the corresponding value in group (I) (p < 0.001). b = statistically significant compared to the corresponding value in group (II) (p < 0.001). c = statistically significant compared to the corresponding value in group (III) (p < 0.001). d = statistically significant compared to the corresponding value in group (IV) (p < 0.001). e = statistically significant compared to the corresponding value in group (V) (p < 0.001).
3.4 Effect of EPO and E on damage score in renal tissue of ovariectomized rats exposed to RIR as shown in and (A–G)
Histologically there was significant increase (p < 0.001) in renal tissue damage in RIR (group II) when compared with the control group. Pretreatment RIR with EPO leads to significant decrease (p < 0.001) in renal tissue damage when compared with RIR group (group II). There was significant decrease (p < 0.001) in group (V, VI and VII) when compared with group IV. There was significant decrease in group VI when compared with group II (p < 0.001), group III (p < 0.001) and group V (p < 0.005). There was significant decrease (p < 0.01) in renal tissue damage of group VII when compared with group V and significant increase (p < 0.01) when compared with group VI, indicating that combination of estrogen and erythropoietin leads to decrease in the protective effect of erythropoietin on renal tissue damage.
4 Discussion
RIR has more destructive effects rather than ischemia alone. The main mechanisms that underlying RIR induced damages are microvascular dysfunctions, imbalance of vasoactive substances, oxidative stress, increased endothelial injury, and local activation of inflammation. Different processes are started to disturb structural and functional integrity of the kidney after RIR.Citation25
The main objective of this study was to determine the protective role of EPO against RIR induced renal injury and studying its effect on vasoactive substances and local activation of inflammation when accompanied with estrogen.
In the present study, rats exposed to RIR showed a significant increase in the serum creatinine and BUN levels with significant increase in renal damage score as shown by histopathological examination when compared to the control group. This is in accordance with the findings of many previous studies.Citation26,Citation27 RIR results in rapid loss of cytoskeletal integrity, cell polarity and shedding of the proximal tubule brush border. With severe injury, viable and nonviable cells are desquamated leaving regions where the basement membrane remains as the only barrier between the filtrate and the peritubular interstitium.Citation28
In the current study rats in the RIR group showed a significant decrease in RBF with decrease in NO and increase in ET1 when compared with the control group. These results can be explained by many studiesCitation29,Citation30, andCitation31 which reported that RIR causes direct endothelial damage with abnormal vascular tone due to increased sensitivity to vasoconstrictors and decreased vasodilatation in arterioles. Endothelial injury also causes cell swelling and narrowing of the vascular lumen, further reducing blood flow. Reperfusion paradoxically causes further impairment of flow. Increased solute delivery to the distal nephron, in part due to tubular epithelial injury, enhances vasoconstriction by activating tubuloglomerular feedback mechanisms. The resulting basal tone and persistent vasoconstriction contribute to decrease in RBF. Also Goligorsky et al.Citation32 proposed the key role of endothelial dysfunction in acute renal ischemia, suggesting that the defective production of endothelial NO may eventually lead to the destruction of tubular epithelial cells through vascular congestion. In addition Müller et al.Citation33 reported that RIR enhanced ET-1 production in renal tissues and explained that there is accumulating evidence indicating that ET-1 plays an important role in the pathogenesis of ischemic ARF.
This study showed that RIR in non-ovariectomized rats led to significant decrease in renal level of EPO when compared with the control group. This finding is in accordance with Plotnikov et al.Citation34 who revealed that Ischemia followed by reperfusion is a common pathological trigger for the kidney damage considering the high vulnerability of this organ to the transitions, which occur during cessation and restoration of blood flow leads to mitochondrial fragmentation, so decreasing the synthesis of EPO.
Concerning the inflammatory mechanism as a pathophysiology of RIR injury the current study showed that RIR caused significant increase in TNF-α and significant increase in MPO as a marker of neutrophil infiltration when compared with control group. These results coincide with those of Nasser et al.Citation35 and Burne-Taney et al.Citation36 who found that RIR caused increase synthesis of pro-inflammatory cytokine such as TNF-α. As injury of the endothelium that characterizes RIR enhanced leukocyte–endothelial cell adhesion with activation of platelets and the local coagulation pathway. The adherence of neutrophils to the vascular endothelium is the first step in the extravasation of these cells into injured tissue. After adherence and chemotaxis, infiltrating neutrophils can release reactive oxygen species that damage the tubular cells.Citation37
In the current study RIR in ovariectomized rats as in OVR + RIR group leads to significant deterioration of renal function and RBF with significant increase in renal damage score when compared with RIR in non-ovariectomized rats indicating the protective effect of the endogenous estrogen on renal function. In spite of significant increase in EPO level in ovariectomized rats there was significant deterioration of renal damage. We can explain that estrogen deficiency induced by ovariectomy abolished the protective effect of E on the kidney. These results were in agreement with the results of Hutchens et al.Citation38 as they reported that loss of ovarian steroids resulted in enhanced renal injury after renal ischemia. These results are in line with Pinheiro and SilvaCitation39 who reported that the RIR injury was exacerbated by ovariectomy. Conversely, Park et al.Citation40 showed that deprivation of estrogen in female animals by ovariectomy did not affect ischemic renal injury, as ovariectomy in this study was carried out 15 days before bilateral renal ischemia not four weeks before RIR as in this study.
The estrogen effect on renal EPO level was proved in our study by significant decrease in EPO level in E + RIR + OVR group when compared with RIR + OVR group. These results were in agreement with those of Peschle et al.Citation41 who demonstrated increased EPO production in female ovariectomized and male rats subjected to hypoxic stress and showed that normal female rats had lower EPO levels after hypoxia than normal males, or ovariectomized rats.
In the current study we demonstrated that OVR + E + RIR group showed better histological results and renal blood flow, compared to those of RIR. That might reflect that dose of treated E is higher than that of replacement. As we used in this study E at a dose of 25 μg/kg/day (SC for three weeks), which is more than the physiological dose used by Peschle et al.Citation41 who used E benzoate as replacement therapy in OVX rats at doses 2.5, 5 or10 mcg per day for 5 days.
By studying the effect of estrogen supplementation to ovariectomized rats exposed to RIR, it resulted in significant decrease in BUN, serum creatinine and renal damage score with significant increase in RBF when compared with OVR + RIR group. We attributed these results to the significant decrease of ET-1 and significant increase in NO in addition to its anti-inflammatory effect on renal tissue. And so estrogen caused significant increase in RBF and improved renal damage and renal function. These results were in line with the results of Müller et al.Citation42 and Masanori et al.Citation43 who showed that 17b-oestradiol was capable of preventing the renal dysfunction and tissue injury induced by RIR in male rats. They also found that the effects of 17b-oestradiol were accompanied by a decrease in renal content of ET-1, a deleterious mediator in the pathogenesis of ischemic ARF. Thus, 17b-oestradiol appears to suppress the enhanced ET-1 production in renal tissues and the consequent renal damage in this model of ARF.
In this study RIR in female either ovariectomized or leads to significant increase in ET-1 level in renal tissue indicating its role in the pathogenesis of RIR injury. These results were in line with the results of Kuro et al.Citation44 and Masanori et al.Citation43 who showed that endothelin-1 is implicated in renal ischemia. In addition RIR leads to significant decrease in nitrate level in renal tissue reflecting decrease in NO. These results were in agreement with results obtained by Thomas and TanyaCitation45 who found that renal NO generation was significantly lower in acute kidney injury.
To the best of our knowledge, this is the first report that analyzes the effect of E on the inflammatory response of RIR. This study revealed significant decrease in TNF-α and MPO activity in renal tissue after E administration indicating its anti-inflammatory effect. This may be due to the effect of E on increasing NO. NO decreases inflammation and inhibits adhesion of neutrophils to TNF-α activated endothelial cells.Citation46 Estrogen treatment (pregnancy levels) inhibited burn-induced elevation in serum TNF α levels and the increase of MPO activity in liver and lung.Citation47
By studying the effect of EPO supplementation at a dose of 5000 U/kg single dose IP, 20 min before ischemia reperfusion either in ovariectomized or in non-ovariectomized rats, we found a significant decrease in BUN, serum creatinine and renal damage score with significant increase in RBF and renal EPO level when compared with OVR + RIR group or RIR group. Our findings are in agreement with, numerous studies that revealed that administration of EPO protected tissue and whole-organ function in various experimental settings of RIR.Citation48–Citation50
In the current study EPO administration either in ovariectomized or in non-ovariectomized rats leads to significant increase in RBF when compared with OVR + RIR group and RIR group and this can be explained in the current study by enhancing the release of NO with decreasing TNF-α and MPO levels .These results were in agreement with the results of Moore and BellomoCitation51 who revealed that pretreatment with EPO in cisplatin induced acute renal failure leads to significant increase in RBF. NO is one of signaling pathways associated with EPO. It has been reported that EPO stimulates vascular NO production directly or indirectly through stimulation of the endothelial NO synthase and increasing shear stress in endothelial cells.Citation52
EPO administration in ovariectomized rats exposed to RIR showed significant decrease in RBF when compared with E administration in ovariectomized rats exposed to RIR. This may be due to the enhanced ET-1 release by EPO as shown in this study. These results were in agreement with those of Carlini et al.Citation53 who found that EPO has a direct stimulatory effect on ET-1 release through an increase in its synthesis.
EPO supplementation before ischemia reperfusion either in ovariectomized or in non-ovariectomized rats resulted in significant decrease in TNF-α and MPO when compared with OVR + RIR group or RIR group. These findings are in agreement with, several studiesCitation53,Citation54 which have demonstrated that complement system and inflammatory pathway are activated by RIR injury. Pro-inflammatory cytokine and chemokine production start to increase in damaged tissue. These chemokines attract neutrophils and macrophages to the injured kidney and pretreatment with EPO significantly decreases polymorphonuclear leukocyte infiltration and tissue MPO activity. Other study showed that EPO treatment inhibits renal inflammation during RIR damage by decreasing proinflammatory cytokines, TNF-α, IL-6 and NF- B activation.Citation55
To the best of our knowledge, this is the first report that analyzes the effect of combination of estrogen with EPO on a RIR model. This study revealed significant decrease in the protective effect of EPO as regards serum creatinine, BUN, renal damage score when combined with estrogen as in OVR + RIR + E + EPO group when compared with the group that receives EPO only (OVR + RIR + EPO group). This can be explained by significant decrease in serum EPO level in OVR + RIR + E + EPO group when compared with the group that receives EPO only (OVR + RIR + EPO group). This indicates that E significantly decreases EPO renal level, so decreasing its renal protective effect. As well as RIR in ovariectomized rats showed significant increase in EPO when compared with RIR group and combination of EPO and estrogen in EPO + E + RIR + OVR leads to significant decrease in EPO when compared with EPO + RIR + OVR group. This means that estrogen deficiency induced by ovariectomy led to increase in renal EPO level, while Combination of estrogen and erythropoietin which is more than the physiological dose with EPO as in EPO + E + RIR + OVR leads to significant decrease in EPO renal level when compared with EPO + RIR + OVR group. These results were in agreement with those of Zahra et al.Citation56 who reported that E decreases hypoxic induction of plasma EPO, and reduces EPO gene expression in kidneys.Citation57This can be explained by Mukundan et al.Citation58 who revealed that 17β-estradiol attenuates EPO expression in part by interfering with hypoxic increases in both expression and activity of hypoxia inducible factor-1 α protein (HIF-1α) which is a heterodimeric transcription factor that is responsible for activation of many hypoxia-inducible genes including EPO through an estrogen receptor-dependent mechanism. Also Mukundan et al.Citation59 reported that 17 β -Estradiol decreased hypoxic induction of plasma EPO and renal EPO mRNA expression in OVX rats by increasing NO production. NO attenuates HIF-1 activity/stability during hypoxia thereby decreasing expression of hypoxia induced genes.Citation60 Another mechanism explained by Bishop et al.Citation61, who showed that 17β-estradiol might directly alter intracellular iron and zinc distribution, affecting the oxygen-sensing system, so suppresses Epo gene induction.Citation62
In the current study there was non-significant difference in the level of TNF-α and MPO activity in OVR + RIR + E + EPO group when compared with the group that receives EPO only (OVR + RIR + EPO group). This may be due to the anti-inflammatory effect of E, that may exceed its ability to decrease the level of EPO and its anti-inflammatory effect.
Treatment with EPO in EPO + RIR + OVR leads to significant increase in EPO when compared with RIR + EPO group. These results go parallel with those of Citation9 who showed that women on hemodialysis therapy require a greater dose of EPO to attain a hematocrit equivalent with men and because they show a lower level of EPO and higher exogenous EPO also would be needed to reach the same protective effect that they found in males. Also they observed that the protective effect of EPO against RIR injury is sex related and more pronounced in male than in female rats.
5 Conclusion
Endogenous estrogen has some protective effect on renal damage in female rats. In addition treatment with EPO and E demonstrated a protective role against RIR injury in female rats; however, combination of both leads to decrease in the protective effect of EPO alone. So treatment with E should be decreased or stopped in menopausal female supplemented with EPO in acute renal injury.
6 Conflict of interest
The authors declare that there is no conflict of interest.
Acknowledgments
Our deep gratitude and appreciation to physiology and pharmacology departments for their help and support, as well as to pathology department, Faculty of Medicine, Benha University for valuable constructive cooperation in the histopathological part.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 30 December 2015
References
- N.PericoD.CattaneoM.H.SayeghG.RemuzziDelayed graft function in kidney transplantationLancet364200418141827
- H.T.HassounD.N.GrigoryevM.L.LieIschemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomyAm J Physiol293120073040
- B.A.MolitorisT.A.SuttonEndothelial injury and dysfunction: role in the extension phase of acute renal failureKidney Int662004496499
- B.OstadalI.NetukaJ.MalyJ.BesikI.OstadalovaGender differences in cardiac ischemic injury and protection: experimental aspectsExp Biol Med234200910111019
- J.NeugartenA.AcharyaS.R.SilbigerEffect of gender on the progression of nondiabetic renal disease: a meta-analysisJ Am SocNephrol112000319329
- P.K.ChatterjeeN.S.PatelE.O.KvaleS.CuzzocreaP.A.BrownK.N.StewartInhibition of inducible nitric oxide synthase reduces renal ischemia/reperfusion injuryKidney Int612002862871
- Joseph V.BonventreMechanisms of acute kidney injury and repair2010SpringerBerlin Heidelberg 2.1, pp. 13–20
- P.K.ChatterjeeNovel pharmacological approaches to the treatment of renal ischemia-reperfusion injury: a comprehensive reviewNaunyn Schmiedebergs Arch Pharmacol3762007143
- A.ProkaiA.FeketeN.F.BankiRenoprotective effect of erythropoietin in rats subjected to ischemia/reperfusion injury: gender differencesSurgery15020113947
- H.MukundanT.C.RestaN.L.Kanagy17β-estradiol decreases hypoxic induction of erythropoietin gene expressionAm J Physiol28322002496504
- V.MullerG.LosonczyU.HeemannA.VannayA.FeketeG.ReuszSexual dimorphism in renal ischemia-reperfusion injury in rats: Possible role of endothelinKidney Int62200213641370
- Nasser.AhmadiaslShokofehBanaei, and AlirezaAlihemmati. Combination Antioxidant Effect of Erythropoietin and Melatonin on Renal Ischemia-Reperfusion Injury in Rats. Iran J BasicMed Sci1612201312091216
- O.KwonS.M.HongG.RameshDiminished NO generation by injured endothelium and loss of macula densa nNOS may contribute to sustained acute kidney injury after ischemia-reperfusionAm J Physiol Renal Physiol29620092533
- P.L.YuC.I.WuT.S.LeeW.H.PanP.S.WangS.W.WangAttenuation of estradiol on the reduction of striatal dopamine by amphetamine in ovariectomized ratsJ Cell Biochem108200913181324
- J.M.Mejía-ViletV.RamírezC.CruzN.UribeG.GambaN.A.BobadillaRenal ischemia-reperfusion injury is prevented by the mineralocorticoid receptor blocker spironolactoneAm J Physiol Renal Physiol29320077886
- A.FloresA.I.GallegosJ.VelascoF.D.MendozaC.MontielP.M.EverardoThe acute effects of bilateral ovariectomy or adrenalectomy on progesterone Testosterone and estradiol serum levels depend on the surgical approach and the day of the estrous cycle when they are performedReprod Biol Endocrinol6200817
- C.J.PattonS.R.CrouchBlood urea estimationAnal Chem491977464
- V.FlavioJ.C.WilliamLong term renal function in kidney donorsTransplantation3661983626
- J.R.HaywoodR.A.SahfferC.FAstenowRegional blood flow measurement with pulsed Doppler flowmeter in conscious ratsAm J Physiol19812421981273278
- K.HasegawaS.WakinoS.TatematsuK.Yoshio-kaK.HommaN.SuganoRole of asymmetric dimethylarginine in vascular injury in transgenic mice overexpressing dimethylarginie dime-thylaminohydrolaseCirc Res10122007210
- Y.MatsumuraR.IkegawaM.TakaokaS.MorimotoConversion of porcine big endothelin to endothelin by an extract from the porcine aortic endothelial cellsBiochem Biophys Res Commun1671990203210
- Bassem.RefaatTariqHelal AshourAdel GalalEl-ShemiRibavirin induced anemia: the effect of vitamin D supplementation on erythropoietin and erythrocyte indices in normal Wistar ratInt J ClinExp Med79201426672676
- Kai-HsinChangMary MStevensonM. STEVENSON Effect of anemia and renal cytokine production on erythropoietin production during blood-stage malariaKidney Int65200416401646
- BidyaDharSahuAnil KumarKalvalaMeghanaKoneruJerald MaheshKumarMadhusudanaKunchaShyam SunderRachamallaAmeliorative Effect of Fisetin on Cisplatin-Induced Nephrotoxicity in Rats via Modulation of NF-κB Activation and Antioxidant DefencePLoS One920149
- M.LegrandE.G.MikT.JohannesD.PayenC.InceRenal hypoxia and dysoxia after reperfusion of the ischemic kidneyMol Med147–82008502516
- J.KimH.JangK.M.ParkReactive oxygen species generated by renal ischemia and reperfusion trigger protection against subsequent renal ischemia and reperfusion injury in miceAm J Physiol Renal Physiol2982010158166
- D.P.BasileE.C.LeonardD.TonadeJ.L.FriedrichS.GoenkaDistinct effects on long-term function of injured and contralateral kidneys following unilateral renal ischemia-reperfusionAm J Physiol Renal Physiol3022012625635
- A.ZukJ.V.BonventreK.S.MatlinExpression of fibronectin splice variants in the postischemic rat kidneyAm J Physiol Renal Physiol28062001F1037F1053
- T.A.SuttonH.E.MangS.B.CamposR.M.Sandov-ALM.C.YoderB.A.MolitorisInjury of the renal microvascular endothelium alters barrier function after ischemiaAm J Physiol Renal Physiol2852003191198
- J.V.BonventreA.ZukIschemic acute renal failure: an inflammatory disease?Kidney Int6622004480485
- J.V.BonventreJ.WeinbergRecent advances in the pathophysiology of ischemic acute renal failureJ Am Soc Nephrol14200321992210
- M.S.GoligorskyS.V.BrodskyE.NoiriNitric oxide in acute renal failure: NOS versus NOSKidney Int612002855861
- V.l.MüllerG.LosonczyU.HeemannA.VannayA.FeketeG.ReuszSexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelinKidney Int624200213641371
- E.Y.PlotnikovA.A.ChupyrkinaS.S.JankauskasI.B.PevznerD.N.SilachevV.P.SkulachevMechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusionBiochimBiophys Acta181220117786
- A.NasserB.ShokofehA.AlirezaB.BehzadA.EhsanThe anti-inflammatory effect of erythropoietin and melatonin on renal ischemia reperfusion injury in male ratsAdv Pharm Bull4120144954
- M.J.Burne-TaneyJ.KoflerN.YokotaM.WeisfeldtR.J.TraystmanH.RabbAcute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injuryAm J Physiol Renal Physiol28512003F87F94
- Z.HeB.DursunD.-J.OhL.LuS.FaubelC.L.EdelsteinMacrophages are not the source of injurious interleukin-18 in ischemic acute kidney injury in miceAm J Physiol29632009535542
- M.P.HutchensT.NakanoY.KosakaJ.DunlapW.ZhangP.S.HersonEstrogen is renoprotective via a nonreceptor-dependent mechanism after cardiac arrest in vivoAnesthesiology1122010395405
- S.V.PinheiroA.C.SilvaAngiotensin converting enzyme angiotensin-(1–7), and receptor mas axis in the kidneyInt. J. Hypertens201218
- K.M.ParkJ.I.KimY.AhnA.J.BonventreJ.V.BonventreTestosterone is responsible for enhanced susceptibility of males to ischemic renal injuryJ Biol Chem27920045228252292
- C.PeschleI.A.RappaportG.F.SassoM.CondorelliGordon AS The role of estrogen in the regulation of erythropoietin productionEndocrinology9221973358362
- V.l.MüllerG.LosonczyU.HeemannA.VannayA.FeketeG.ReuszSexual dimorphism in renal ischemia-reperfusion injury in rats: possible role of endothelinKidney Int624200213641371
- T.MasanoriY.MikihiroF.ToshihideO.MamoruO.Yasuoestrogen protects against ischaemic acute renal failure in rats by suppressing renal endothelin-1 overproductionClin Sci1032002434437
- T.KuroK.KohnouY.KobayashiSelective antagonism of ETA but not ETB receptor is protective against ischemic acute renal failure in ratsJpn J Pharmacol822000307316
- E.ThomasM.TanyaImmunoregulatory role of TNF in inflammatory kidney diseasesKidney Int762009262276
- Rainer H.StraubThe complex role of estrogens in inflammationEndocrine Rev Volume2852013
- E.SpandouI.TsouchnikasG.KarkavelasErythropoietin attenuates renal injury in experimental acute renal failure ischaemic/reperfusion modelNephrol Dial Transplant212006330336
- L.V.d’UscioL.A.SmithA.V.SanthanamEssential role of endothelial nitric oxide synthase in vascular effects of erythropoietinHypertension49200711421148
- A.V.SanthanamL.A.SmithK.A.NathZ.S.KatusicIn vivo stimulatory effect of erythropoietin on endothelial nitric oxide synthase in cerebral arteriesAm J Physiol Heart CircPhysiol2912006781786
- Maryam Moeini 1,2, Mehdi Nematbakhsh1,3,4, Mohammad Fazilati2, Ardeshir Talebi5, Ali Asghar Pilehvarian2, Fariba Azarkish1,2, FatemehEshraghi Jazi1, Zahra Pezeshki. Protective Role of Recombinant Human Erythropoietin in Kidney and Lung Injury Following Renal Bilateral Ischemia Reperfusion in Rat Model Int J Prev Med 2013; 4: 648–55.
- E.MooreR.BellomoErythropoietin (EPO) in acute kidney injuryAnnals Intensive Care120113
- LarysaSautinaYuriSautinElaineBeemZhuoZhouAnnaSchulerJennaferBrennanInduction of nitric oxide by erythropoietin is mediated by the β common receptor and requires interaction with VEGF receptor. 2Blood1152010896905
- Raul G.CarliniAdriana S.DussoChamberlain I.ObialoUlises M.AlvarezMarcosRothsteinRecombinant human erythropoietin (rHuEPO) increases endothelin-1 release by endothelial cellsKidney Int43199310101014
- N.S.PatelE.J.SharplesS.CuzzocreaP.K.ChatterjeeD.BrittiM.M.YaqoobPretreatment with EPO reduces the injury and dysfunction caused by ischemia/reperfusion in the mouse kidney in vivoKidney Int6632004983989
- E.AtesA.U.YalcinS.YilmazT.KokenC.TokyolProtective effect of erythropoietin on renal ischemia and reperfusion injuryANZ J. Surg7512200511001105
- Zahra P., Mehdi N., Safoora M., Fatemeh E., Ardeshir T., Hamid N., Tahereh S., Azam M. and Farzaneh A. Estrogen Abolishes Protective Effect of Erythropoietin against Cisplatin-Induced Nephrotoxicity in Ovariectomized Rats ISRN Oncology Volume 2012, Article ID 890310, 7 pages.
- V.TodorovB.GessA.GödeckeC.WagnerJ.SchräderA.KurtzEndogenous nitric oxide attenuates erythropoietin gene expression in vivoPflugersArchiv Euro J Physiol43942000445448
- HarshiniMukundanNancy L.KanagyThomas C.Resta17-β Estradiol Attenuates Hypoxic Induction of HIF-1α and Erythropoietin in Hep3B CellsJ Cardiovasc Pharmacol441200493100
- Harshini.MukundanThomas C.RestaNancy I.Kanagy17-Estradiol decreases hypoxic induction of erythropoietin gene expressionAm J Physiol Regul Integrative Comp Physiol2832002R496R504
- K.SogawaK.Numayama-TsurutaM.EmaM.AbeH.AbeY.Fujii-KuriyamaInhibition of hypoxia-inducible factor 1 activity by nitric oxide donors in hypoxiaProc Natl Acad Sci USA95199873687373
- G.M.BishopL.E.SwanS.R.RobinsonAltered cellular distribution of iron in rat cerebral cortex during the oestrous cycleJ Neural Transm1112004159165
- H.HoriguchiF.KayamaE.OgumaW.G.WillmoreP.HradeckyH.F.BunnCadmium and platinum suppression of erythropoietin production in cell culture: clinical implicationsBlood96200037433747
