Abstract
Background
It is usually hard to detect pancreatic lesions early as the pancreas lays retro peritoneum so it cannot be assessed during a routine physical exam. By the time a person has symptoms, the disease has already established morphological imaging CT changes.
Objective
The objective of our study was to clarify the role of multidetector computerized tomography (MDCT) in different adult acquired pancreatic diseases and assess the efficacy of surgical pancreatic tumors resectability preoperative.
Materials & methods
The study included thirty adult patients suspected to have pancreatic diseases (18 males and 12 females); their age range was 45–90 years with a mean age of 68 years. All patients underwent triple-phase multi-detector row CT using a 16-slice machine. The presence of inflammation, masses, and vascular invasion was evaluated and interpreted images were obtained during each phase. Results were compared with surgery, histopathology or follow-up.
Results
Of 30 patients, 15 had pancreatic malignancies (14 adenocarcinoma of which 6 were resectable and 8 were irresectable, 1 distant metastasis) proven at biopsy and/or surgery, 11 patients had pancreatitis (acute and chronic), three patients had cystic benign tumors (2 mucinous cystadenoma, 1 serous cystadenoma), and one patient had neuroendocrine tumor (insulinoma).
Conclusion
Contrast enhanced multiphase pancreatic imaging by MDCT with its post processing techniques represents the imaging modality of choice for the diagnosis of different adult acquired pancreatic diseases.
1 Introduction
Imaging of the pancreas is a challenging investigation because of its anatomic location in the retro peritoneum and its intricate relationship with major blood vessels and bowel. CT had been the initial imaging modality of choice for evaluation of pancreatic pathology. Improvements in CT technology during the past decade, with fast image acquisition and improved spatial resolution, had increased the accuracy of CT lesion detection and characterization. Excellent visualization of the pancreatic parenchyma in various phases of contrast enhancement facilitates early detection of small pancreatic lesions. Post processing techniques provide a vascular road map for the operating surgeon.Citation1
Acute pancreatitis is a disease with a broad spectrum of findings that varies in severity from mild interstitial or edematous pancreas to severe form with significant local and systemic complications that are associated with a substantial degree of morbidity and mortality.Citation2
Contrast enhanced computed tomography (CECT) is useful in determining the underlying cause of pancreatitis, grading the severity and detection of local complications such as necrosis, abscess, or pseudo cysts.Citation3
CT outclasses all imaging modalities in detecting calcifications, any specific sign of advanced chronic pancreatitis. CT can also detect most complications such as pseudocysts, calculi in the pancreatic duct or inflammatory masses.Citation4
Pancreatic cancer is one of the most lethal diseases, with a poor prognosis. Surgical resection remains the only potentially curative treatment. Therefore, accurate staging to select patients who may benefit from resection is essential. In patients with resectable tumors, accurate diagnoses of tumor extension, vessel involvement, and the presence or absence of liver metastasis are necessary before operation.Citation5
The latest multi-detector computed tomography (MDCT) technology provides three-dimensional multi-planar reconstruction techniques enabling determination of tumor involvement of the common bile duct (CBD), pancreatic duct, and peripancreatic vasculature, which is expected to improve the preoperative determination of surgical resectability, particularly in relation to vascular invasion.Citation6
2 Materials & methods
2.1 Subjects
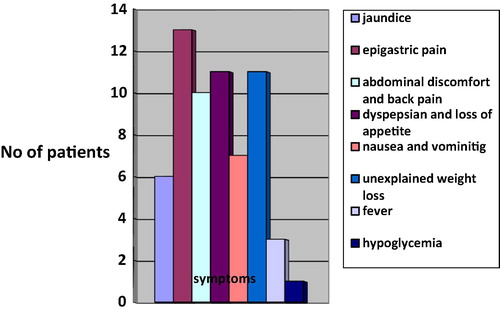
This study was conducted on 30 selected patients (18 males and 12 females) suspected on clinical symptoms () and laboratory findings of having different acquired pancreatic diseases listed in . The age range distribution among the examined patients is demonstrated in . The mean age of patients was 68 years.
Table 1 MDCT diagnosis in relation to the number of patients.
Table 2 The age range distribution in the 30 included patients.
Study was conducted in some private radiology centers and in Ain Shams Specialized Hospital starting from January 2012 to May 2015. Patients’ Exclusion criteria included patients with congenital pancreatic disease, contraindication to iodinated contrast media (chronic renal impairment or previous allergy to the contrast media), and pregnant women. An informed consent was obtained from all patients after full explanation of the benefits and risks of this procedure.
2.2 Methods
All patients were subjected to careful history taking, general and abdominal examination, laboratory and serological examinations, abdomino-pelvic ultrasound and multi-detector computed tomography.
This study was performed using a 16 slice multi-detector CT (Toshiba activeon). Patients fasted for about 4–6 h before examination. Reassurance and brief explanation of the procedure was done. All patients were examined in supine position, each patient was instructed to remain stable and suspend breathing during scanning time.
Opacification of the gastrointestinal tract with oral contrast material was done before CT scanning using one liter of diluted 2–4% non-ionic contrast material divided into three doses.
An initial unenhanced scan was obtained in all patients starting from the level of the diaphragm down to the iliac crest using a 5-mm slice thickness at 5-mm increments. An unenhanced scan demonstrates dense common bile duct calculi and pancreatic calcifications.
After the end of pre contrast CT examination, post contrast scan was done. IV contrast medium (Ultravist 300 mg) was injected at the rate of 4 ml per second using a power injector. The contrast enhanced scans were obtained in a late arterial phase (40s post-injection), and a portal venous phase (70s post-injection). Contrast-enhanced acquisitions were performed cranio-caudally with thin collimation (1 mm).
The pancreatic parenchymal phase provides excellent enhancement of the arterial system. The portal venous phase is ideal phase for detecting liver metastases, as well as for creating reconstructed images of the venous structures, which might be essential for surgical planning.
2.3 The scan parameters and image analysis
The scan parameters were tube current 120 kV and 400 mA, slice thickness 5 mm, collimation of 0.6 mm, pitch 0.6, 0.6-s gantry rotation time and table speed of 7.5–10 mm per rotation during a single breath-hold acquisition of 15–25 s.
Image interpretation was done on an independent workstation and included the axial source images and the reformatted (MPR, MIP, CPR and volume rendering) images which helped in the definition of the location and extent of the lesions. All TDM was interpreted by the same radiologists working in private radiology centers and in Ain Shams Specialized Hospital.
2.3.1 In cases of acute pancreatitis
The CT scan was assessed in case of non-enhancement and normal enhancement pattern of pancreas. Non-enhancement of pancreas represented pancreatic necrosis. The extent of pancreatic necrosis was estimated at ⩽30%, more than 30% but less than 50% and ⩾50%.
CT scans were also assessed for peripancreatic inflammation, mesenteric stranding, peripancreatic fluid collection, pseudocyst, pancreatic abscess, and inflammatory changes of the adjacent organs and vessels.
The severity of the pancreatitis for each case was assessed using the CT severity index (CTSI) developed by Balthazar et al. (). CTSI includes grading of pancreatitis (A–E) and the extent of pancreatic necrosis. The maximum score that can be obtained is 10.Citation7
Table 3 CT severity index (CTSI) for grading of acute pancreatitis by Balthazar et al.
2.3.2 In cases of chronic pancreatitis
CT scans were assessed for intraductal calcifications, main pancreatic duct dilatation and parenchymal atrophy. It was also assessed for any peri pancreatic collections and masses.
2.3.3 In cases of pancreatic neoplasm
Any pancreatic lesion was assessed according to tumor site, size, and invasion of adjacent organs, CBD and pancreatic ducts caliber, major vascular invasion, and lymph node metastasis. Staging of the pancreatic mass was done according to TNM system (). Classic criteria for defining non-resectability () included extrapancreatic invasion of adjacent tissues and organs other than the duodenum; occlusion, stenosis, or semicircular encasement of any major peripancreatic vessel (celiac, hepatic, or superior mesenteric artery or portal or superior mesenteric vein); hepatic metastases; peritoneal carcinomatosis; and lymph node or distant metastases ().
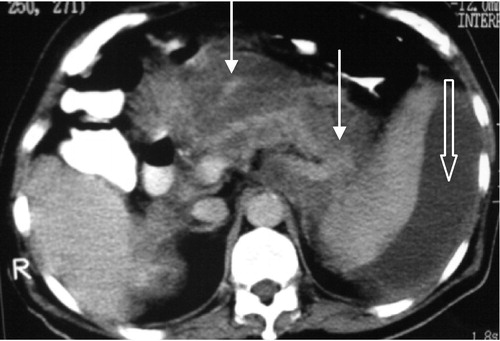
Figure 2 Irresectable pancreatic body adenocarcinoma T4N1 M1. Axial post contrast CT images (porto-venous phase) show pancreatic body hypodense mass lesion inducing focal contour bulge (white arrow) with loss of fat plane between it and the posterior wall of the stomach (thick arrow). There is also right hepatic lobe hypodense metastatic focal lesion (thin curved black arrow) and peri pancreatic nodal involvement (thin curved white arrow). The distal pancreatic duct is seen dilated (thick curved white arrow).
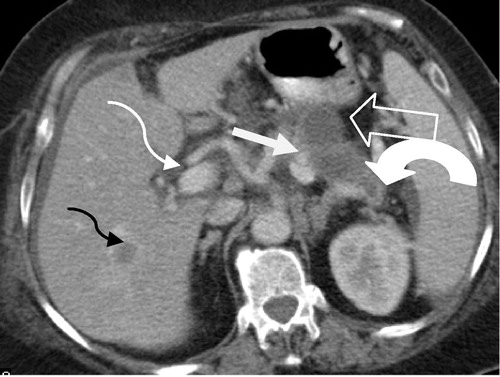
Figure 3 Irresectable pancreatic body carcinoma (T4 N0M1). Axial CT image shows hypodense heterogenous pancreatic body mass (white arrow) encasing the celiac trunk (black arrow) and atrophy of the pancreatic tail (arrowhead). Liver with multiple hypodense lesions due to metastasis (thick black arrow).
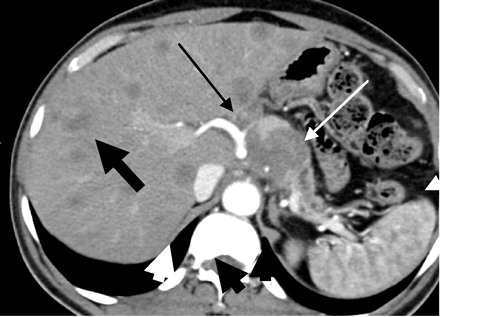
Table 4 TNM Classification for the staging of pancreatic cancer.Citation25
3 Results
3.1 In case of pancreatitis
Eleven cases of pancreatitis were detected; eight patients were diagnosed as acute pancreatitis (7 males and 1 female) and three patients were diagnosed as chronic pancreatitis (2 male and one female).
All cases of acute pancreatitis in our study were diagnosed on the basis of the clinical data and elevated serum amylase.
The causes of acute pancreatitis in the 8 patients were as follows: Gall Bladder stones (6 patients), alcohol (1 patient), and idiopathic (1 patient).
Acute pancreatitis was graded by MSCT according to the currently accepted CT severity index into 3 categories: Mild (CT severity index 0–3), Moderate (CT severity index 4–6), Severe (CT severity index 7–10) ().
Table 5 CT severity index in case of acute pancreatitis.
Six patients showed complications resulting from acute pancreatitis in the form of variable degrees of pancreatic necrosis (). One patient showed pancreatic abscess (), three patients showed pseudocysts (), three patients complicated with organ failures, and one patient showed sepsis. Percutaneous drainage of pancreatic pseudocysts was performed in two patients. Percutaneous drainage of an abscess was performed in one patient. Pseudocysts in two patients showed spontaneous resolution during the course of medical treatment for acute pancreatitis. The size of these cysts did not exceed 5 cm in diameter.
Figure 4 Acute necrotizing pancreatitis CT SI 9. Note a fluid attenuation collection in the pancreatic bed with minute islands of preserved fatty tissue (thin arrow) and well defined rim, suggestive of walled off necrosis with fat necrosis involving the pancreas and peri pancreatic tissue. Few small areas of glandular enhancement are only seen at the region of the body and tail (thick arrow).
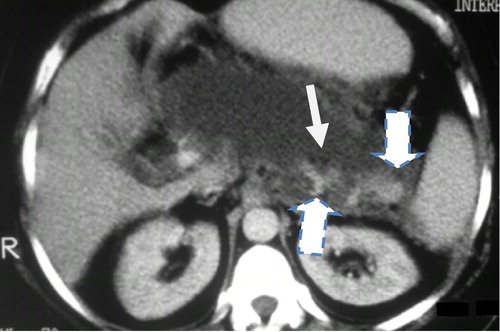
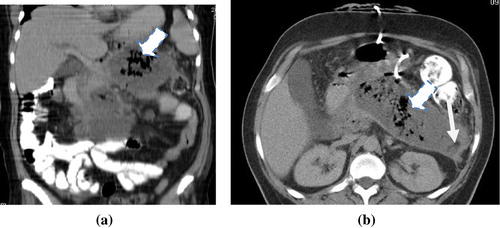
Figure 5 (a and b) Acute necrotizing pancreatitis with pancreatic necrosis and abscess formation. CTSI 10 Axial and coronal MDCT images showed Fluid attenuation collection at the pancreatic body, tail and part of the head with well-defined wall. It shows small fat density (thin arrow) and air (thick arrow) loculi, suggestive of infected pancreatic walled off necrosis and abscess formation. Percutaneous catheter is seen inside the collection for drainage.
3.2 Chronic pancreatitis
Three cases of chronic pancreatitis were diagnosed (2 males and 1 female). Their age ranges from 45 to 60 years (mean, 52.5 years). All patients had a history of repeated attacks of pancreatitis.
Variable degrees of irregular and beaded pattern of pancreatic duct dilatation were noted in all cases of chronic pancreatitis. Pancreatic intraductal stones were observed in two cases of the study. Pancreatic parenchymal atrophy was present in all cases of chronic pancreatitis in this study. It was graded as mild in two cases and moderate in one case.
Pancreatic calcification was detected in cases of chronic pancreatitis with variable size (tiny stippled calcifications to large coarse calcifications) and distribution from localized involving one portion of the gland to diffusely distributed ().
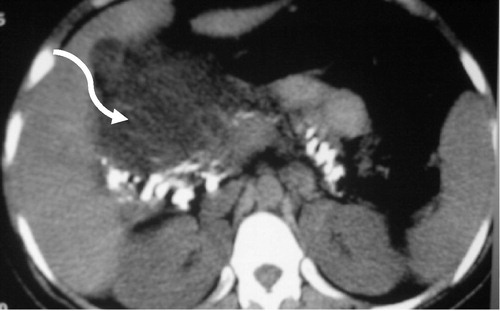
Figure 7 Chronic calcific pancreatitis with intra ductal stone and pseudopancreatic cyst. Axial CT images demonstrate atrophic pancreas with innumerable diffuse calcification. A well-defined homogenous fully encapsulated collection, representing pseudopancreatic cyst is seen related to the pancreatic head region (curved arrow).
3.3 Neoplastic pancreatic lesions
This included 19 cases that were classified into two main groups according to their pathological findings ():
| 1. | Group I: Pancreatic adenocarcinoma. | ||||
| 2. | Group II: Miscellaneous. | ||||
Table 6 Distribution of neoplastic pancreatic lesions according to pathological findings.
3.3.1 Group I (pancreatic adenocarcinoma)
This group included 14 patients, their age ranged from 50 to 72 years with a mean age of 62 years. Variable degrees of MDCT findings are listed in .
Table 7 The MDCT findings in pancreatic adenocarcinoma.
Three patients showed ill-defined heterogeneous focal lesion in the pancreas, and one had peri pancreatic lymphadenopathy. Three patients showed focal lesion of heterogeneous echogenicity at the head of the pancreas (), with dilatation of CBD, and/or intrahepatic biliary radicles. Three patients showed only dilatation of CBD and intrahepatic biliary radicles and the pancreatic area were obscured by gases. Two patients showed focal lesion of heterogeneous echogenicity at the pancreas, with dilatation of intrahepatic biliary radicles, and hypoechoic focal lesions of the liver. Two patients showed focal lesion of the pancreas with hypoechoic areas of the liver (mostly metastatic).
Figure 8 (a and b) Irresectable pancreatic head adenocarcinoma T4 N0M1. (a) Axial post contrast CT images (porto-venous phase) pancreatic head heterogeneously enhancing irregular mass lesion inducing contour bulge (white arrow) invading the CBD with consequent intra hepatic biliary dilatation (thin curved white arrow). There is also right hepatic lobe hypodense metastatic focal lesion (thin curved black arrow). (b) Coronal MIP reconstruction clearly shows the vascular invasion of the portal vein.
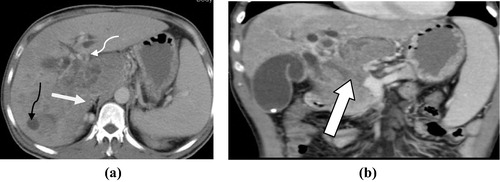
Percutaneous CT guided biopsy was done in 4 patients, intra-operative true-cut biopsy was done in 2 patients, percutaneous ultrasound guided biopsy was done in 2 patients and ERCP biopsy was done in 6 patients. All fourteen patients were proved to be adenocarcinoma by histopathological examination.
Six cases that were suggested to be resectable by MDCT criteria in our study proved to be surgically resectable intra operative. However, surgical resection was aborted in only one case out of 6 cases due to unsuspected peritoneal deposits.
3.3.2 Group II (miscellaneous)
This group included 5 patients with two patients were diagnosed as mucinous cystadenoma at the tail of the pancreas by MSCT criteria. The two cases were females. The diagnosis was confirmed in one case by ultrasound guided aspiration cytology and proved to be mucinous cystadenoma. Follow-up was done in the other case and showed stationary course.
One male patient showed pancreatic head irregular small cystic lesion and MSCT diagnosis was pancreatic serous cystadenoma. Follow-up was done after 6 months and showed stationary course.
One female patient 45 years old presented with episodes of hypoglycemia. MSCT showed hyper vascular well defined small lesion which enhanced more intensely than the normal pancreatic parenchyma in all phases, and the final diagnosis was established as benign insulinoma ().
Figure 9 Benign pancreatic head insulinoma. Axial CT cut pancreatic-phase shows a 1 cm small, typically hyperenhancing nodule in the pancreatic head (arrow).
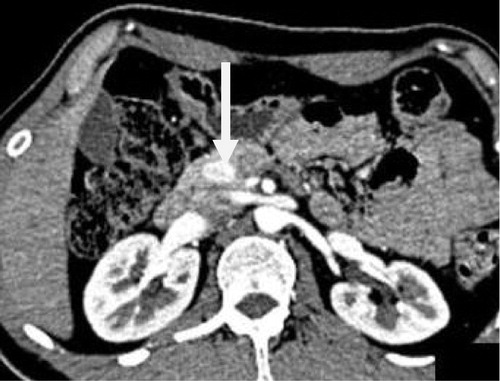
One female patient was diagnosed by MSCT as pancreatic metastasis at the neck of the pancreas as well as at the operative bed that showed the same pattern of enhancement. She had a known history of right nephrectomy for renal cell carcinoma. Biopsy from the operative bed revealed recurrent renal cell carcinoma (see ).
Table 8 Classification of patients according to resectability.
4 Discussion
Imaging with CT had become a frontline technique for initial diagnosis, evaluation of complications, and long-term follow-up of a variety of diseases of the pancreas.Citation8
Almost all life-threatening complications occur with necrotizing pancreatitis, including secondary bacterial contamination and multi-organ failure.Citation9 In our study 2 patients showed mild form of pancreatitis. In one patient (50%) fluid collections were detected and resolved within one week. This agrees with a study done by Lenhart and BalthazarCitation10, in which fluid collections were detected in 73 patients (43.2%) and almost totally resolved within 7–10 days in most patients.
No complications were detected in our study in the mild form of acute pancreatitis. This is in contrary to the study done by Lenhart and BalthazarCitation10 who found Complications developed in nine patients with acute non necrotizing pancreatitis with incidence of 5.3%.
The internationally accepted CT severity index, which is based on scoring the presence and degree of pancreatic inflammation and pancreatic necrosis, not only allows accurate differentiation of mild from severe pancreatitis but also numerically correlates with the patient’s prognosis.Citation11
Our results confirmed that the currently accepted CT severity index is indeed a powerful tool with which we can predict morbidity in patients with acute pancreatitis. When comparing patients with mild pancreatitis and those with severe pancreatitis, we documented a statistically significant correlation between the numeric score obtained with the currently accepted index and the presence of infection, the need for surgery and percutaneous interventions, and the length of the hospital stay.
The reported mortality rate for patients with acute pancreatitis varies greatly. A study by CasasCitation12 demonstrated that CTSI ⩾ 5 is the index for patients under the danger of death. In Bradley’s studyCitation13, the CTSI > 8 is the index for death. According to Simchuk et al.Citation14, the CTSI < 3 had a 3% mortality rate, whereas patients with a CTSI > 7 had a mortality rate of 17%. In our study, one patient out of the eight patients died (12.5%) and had a CTSI = 10 and no mortality was detected in mild and moderate form of acute pancreatitis.
In our study we found that main pancreatic duct dilatation, intra ductal calcification and variable degrees of pancreatic atrophy were detected in all cases of chronic pancreatitis. Pancreatic pseudocyst was found in 33% of cases. This agreed with Remer and BakerCitation15 who found dilatation of the main pancreatic duct in 68% of patients, parenchymal atrophy in 54% of patients, pancreatic calcifications in 50% of patients, and fluid collections in 30% of patients.
In our work we used the pancreatic parenchymal phase and the portal venous phase. Also Sahani et al.Citation16 recommended the same biphasic technique. 14 patients out of 19 had pancreatic adenocarcinoma i.e. (75%) this comes in agreement with this of Carbognin et al.Citation17 who decided that ductal adenocarcinoma accounts from 80% to 90% of all tumors of the pancreas.
8 male patients and 6 female patients had pancreatic carcinoma and this was as the same as David’s et al. studyCitation18 which stated that higher rates of pancreatic carcinoma are seen in men than in women and this is in contrast to John’s et al.Citation19 who found that recently more women are developing pancreatic malignancy.
Scaglione et al.Citation20 reported that 60% of pancreatic tumors occupied the head of pancreas, 10% the body, about 5% the tail and the remaining 25% were diffusely involved. In our study 57% of tumors occupied the head of the pancreas, 29% the body, and 14% the tail. That explains why 42% of patients had a clinical history of jaundice because 57% of the masses occupied pancreatic head.
The size of the pancreatic head tumors in our study was between 2 and 4 cm and that was similar to Scaglione et al.Citation20 who reported that at the level of the pancreatic head, the average size was 2–3 cm.
Scaglione et al.Citation20 stated that pancreatic carcinoma tended to be hypo dense masses distorting pancreatic contour. In our study 11 patients showed hypo dense lesions, while 3 cases showed heterogeneous focal lesions.
In this study, the most common reported associated extra pancreatic finding was dilated CBD and intra hepatic biliary radical, which was seen in 6 patients (42%); this is seen in 75% of patients with head masses. John et al.Citation19 stated that ductal dilatation occurs in 58% of patients with pancreatic neoplasm and ductal dilatation proximal to the obstructing tumors was detected in approximately 88% of pancreatic head tumors.
Liver metastases were detected by multi slice CT in 7 patients (50%). This comes in agreement with MurfittCitation21 who stated that metastasis to the liver occurred in approximately 17–55% of the patients.
Chest distant metastatic finding was a common association with pancreatic neoplasm in the current study. This was reported in 28.5% of the patients. Osteolytic bony lesion was found in 7%. This comes in agreement with the statement of KloppelCitation22 who stated that metastasis to lung, pleura and bone is only seen in advanced tumor stages.
In our study, 5 cases were resectable out of 14 cases (35%). Near to this is the study of Grenacher and KlauBCitation23 which stated that only 20% of all patients believed to have a surgically resectable disease.
Resection is aborted in one case out of 6 cases (about 16% of the suspected resectable cases) due to unsuspected peritoneal deposits, whereas Zamboni et al.Citation24 decided that resection was aborted in 11% of their suspected resectable cases.
Darren et al.Citation25 stated that False-negative results almost often occur because of the unsuspected liver surface metastases, peritoneal deposits, or unsuspected vascular invasion.
Frate et al.Citation26 stated that MSCT has an accuracy rate for staging of pancreatic adenocarcinoma of virtually 100%. In our study the overall accuracy of tumor staging by MSCT was 84%. Whereas Zamboni et al.Citation24 stated that the accuracies ranged from 85% to 95%, Scaglione et al.Citation20 decided that the accuracy of MSCT in staging of pancreatic cancer is as high as 93%.
In our study insulinoma appeared as well defined small lesion which enhanced more intensely than the normal pancreatic parenchyma in all phases. This coincides with McLeanCitation27 study which stated that insulinomas are typically hyper attenuating on at least one phase of contrast enhancement typically on the late arterial [25 s] or pancreatic phase [35–40 s] of imaging but occasionally in the portal venous phase.
In our study there were two cases of pathologically confirmed pancreatic mucinous cystadenoma. Both patients were female and in relatively younger age group (53 and 57 years). Aslam and YeeCitation28 stated that a mucinous cystic neoplasm occurs predominantly in women.
One case was diagnosed by MDCT as serous cystadenoma. This was a 54 year old male. This is in contrast to Dewhurst et al.Citation29 who stated that about 80% occur in women who are more than 60 years old. This is possibly attributed to small number of cases enrolled in our study.
One case of pancreatic metastasis was from renal cell carcinoma. PaspulatiCitation1 stated that the common primary tumors that metastasize to the pancreas are from lung, breast, kidney, and melanoma. Mechó et al.Citation30 stated that pancreatic metastases are uncommon, representing 4.5% of pancreatic tumors.
5 Conclusion
Contrast enhanced multiphase pancreatic imaging by MDCT with its post processing techniques represents the imaging modality of choice for diagnosis of different adult acquired pancreatic disease.
Conflict of interest
The authors declare that there are no conflict of interests.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 22 June 2016
References
- R.M.PaspulatiMultidetector CT of the pancreasRadiol Clin North Am4320059991020
- A.AlhajeriS.ErwinAcute pancreatitis, value and impact of CT severity indexAbdom Imag3320081820
- SaokarAnuradhaRabinowitz B.ChadSahani V.DushyantCross-sectional imaging in acute pancreatitisRadiol Clin North Am452007447460
- VikasChaudharyBanoShahinaImaging of the pancreas: recent advancesIndian J Endocrinol Metab152011S25S32
- S.SatoiH.YamamotoS.TakaiClinical impact of multidetector row computed tomography on patients with pancreatic cancerPancreas341752007179
- A.RafiqueA clinical algorithm for the assessment of pancreatic lesions, utilization of 16- and 64-section multidetector CT and endoscopic ultrasoundClin Radiol62114220071153
- E.J.BalthazarP.C.FreenyE.VansonnenbergImaging and intervention in acute pancreatitisRadiology1931994297306
- Mustafa R.BashirRajan T.GuptaMDCT evaluation of the pancreas: nuts and boltsRadiol Clin North Am502012365377
- B.ChishtyRole of computed tomography in acute pancreatitis and its complications among age groupsJ Pak Med Assoc5520051017
- D.K.LenhartE.J.BalthazarMDCT of acute mild (non-necrotizing) pancreatitis, abdominal complications and fate of fluid collectionsAJR Am J Roentgenol1902008643649
- E.J.BalthazarAcute pancreatitis, assessment of severity with clinical and CT evaluationRadiology2232002603613
- J.D.CasasR.DiazG.ValderasA.MariscalP.CuadrasPrognostic value of CT in the early assessment of patients with acute pancreatitisAm J Roentgenol1822004569574
- E.Bradley3rdA clinically based classification system for on acute pancreatitis. Summary of the international symposium on acute pancreatitisArch Surg1281993586590
- E.J.SimchukL.W.TraversoY.NukuiComputed tomography severity index is a predictor of outcomes for severe pancreatitisAm J Surg1792000352355
- E.M.RemerM.E.BakerImaging of chronic pancreatitisRadiol Clin North Am40200212291242
- M.K.KalraM.M.MaherD.V.SahaniCurrent status of multidetector computed tomography urography in imaging of the urinary tractCurr Probl Diagn Radiol3152002210221
- G.CarbogninL.PinaliC.ProcacciPancreatic neoplasms and tumor like conditionsRadiologic-pathologic correlations from head to toeGourtsoyiannis NC & Ros PR2005Springer-VerlagBerlin Heidelberg409446 [chapter 4]
- S.DouglasM.DavidJ.JohnRelative accuracy of CT and MRI for characterization of cystic pancreatic massesAJR1892007657661
- K.JohnM.K.RobertP.S.UdoPancreas adenocarcinomaE Medicine020050
- M.ScaglioneA.PintoS.RomanoUsing multidetector row computed tomography to diagnose and stage pancreatic carcinoma: the problems and the possibilitiesJOP6200515
- J.MurfittThe pancreasA.SuttonText book of radiology and medical imaging2004Churchill LivingstoneLondon10791098
- G.KloppelMixed exocrine–endocrine tumors of the pancreasSem Diagn Pathol172000104108
- L.GrenacherM.KlaußComputed tomography of pancreatic tumorsRadiologe492009107123 [Zamboni et al., in (2007)]
- G.ZamboniJ.KruskalC.VollmerPancreatic adenocarcinoma, value of multidetector CT angiography in preoperative evaluationRadiology2452007770778
- D.DarrenA.GiuliaD.VassiliosComprehensive preoperative assessment of pancreatic adenocarcinoma with 64-section volumetric CTRadioGraphics27200716531666
- C.FrateR.ZanardiK.MorteleAdvances in imaging for pancreatic diseaseRos PR Curr Gastroenterol Rep422002140148
- A.McLeanEndoscopic ultrasound in the detection of pancreatic islet cell tumoursCancer Imag420048491
- R.AslamJ.YeeMDCT of pancreatic massesAppl Radiol3520061021
- C.DewhurstK.MorteleD.MorganCystic tumors of the pancreas: imaging and managementRadiol Clin North Am502012467486
- S.MechóS.QuirogaPancreatic metastasis of renal cell carcinoma: multidetector CT findingsAbdomen Imag10200810071015