Abstract
Objective
To evaluate the chondroprotective effect of l-carnitine in relation to glucosamine sulfate and in an experimental model of osteoarthritis (OA).
Materials and methods
Thirty-two adult male Wister albino rats weighing 150–210 g were assigned randomly into 4 groups: 8 rats in each group, group I (control group), group II (MIA induced OA group), group III (MIA induced OA + glucosamine sulfate treated group), and group IV (MIA induced OA + l-carnitine treated group). Weight, knee diameter, and knee bend score were recorded on days 0, 1, 7, 14 and 28. On day 28 all animals were sacrificed. Synovial fluid of left knee was collected, and the interleukin-1β (IL-1β), Cartilage oligomeric matrix protein (COMP) and matrix metalloproteinase-13 (MMP-13) levels were measured by ELISA. The knee joints were removed and stained with H&E for histological evaluation.
Results
The pathological abnormalities attributed to MIA induced arthritis was dramatically lowered in rats treated with glucosamine or l-carnitine. Synovial fluid levels of IL-1β, COMP and MMP-13 were increased in OA group, and significantly reduced with glucosamine or l-carnitine treated groups.
Conclusion
l-Carnitine has a potential chondroprotective effect in this animal model of OA.
1 Introduction
OsteoarthritisCitation1 is one of the most common forms of degenerative joint disease and a major cause of pain and disability affecting the aging population. Several factors including genetic susceptibility, obesity, injuries and inflammation of the joint have been long considered as important risk factors of the disease.Citation2
Figure 2 Histopathologic evaluation of knee cartilage from control (group 1), MIA-induced OA (group II), GS (group III) and LC (group IV). OARSI grading range from 1 to 6, where 1 is normal cartilage and 6 total loss of cartilage. Total mankin score is a sum of the scores for cartilage structure, cellular abnormalities, tideline and matrix Staining with toluidine blue, and total score of 15 means severe damage of cartilage while 0 means normal cartilage. According to OARSI and mankin score, both GS an LC groups show significant difference when compared to group II (p < 0.05) ∗ = significant difference compared to group II.
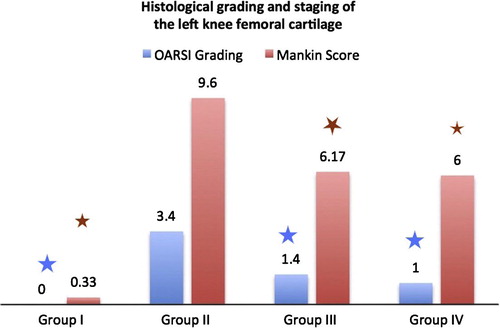
Table 1 Left knee diameter (mm), and knee bend score, for the studied groups.
Table 2 Levels of IL-1β (pg/ml), COMP (ng/ml), and MMP-13 (ng/ml) in the studied groups.
Under normal conditions, a dynamic equilibrium between synthesis and degradation of extracellular matrix [ECM] components is maintained.Citation3 In osteoarthritic states, however, a disruption of matrix equilibrium leads to cartilage degradation, induction of oxidative states, and eventually, apoptosis of chondrocytes.Citation4 The triggering events and exact pathologic mechanism that result in cartilage loss and degradation are not completely understood. It has been suggested that inflammatory mediators such as cytokines [IL-1β, TNFα, IL-6, IL-8, and IL-17], chemokine and reactive oxygen speciesCitation5 have a primarily destructive impact on articular cartilage.Citation3 These inflammatory mediators lead to increase synthesis and release of matrix metalloproteinases [MMPs] and cartilage degradation.Citation6
Analgesics and non-steroidal anti-inflammatory drugs [NSAIDs] are the main therapeutic treatment options for OA. Unfortunately, these medications are short-term and fail to adequately address pathophysiological and biochemical mechanisms involved with cartilage degeneration and the induction of pain in arthritic joints.Citation7 The search for effective treatment/dietary supplements able to slow the progression of disease seems warranted.
l-Carnitine [LC] [3-hydroxy-4-N-trimethylaminobutyrate], the bioactive form of carnitine, is an endogenous branched nonessential amino acid derivative that plays a critical role in energy production. It transports long-chain fatty acids from the cytoplasm to mitochondria so they can be oxidized to produce energy.Citation8 l-Carnitine bioavailability is 5–18%Citation9 and eliminated from the body mainly via urinary excretion.Citation10 l-Carnitine has been shown to offer a great therapeutic potential against several chronic conditions including cardiovascular, diabetes, neurodegenerative, and inflammatory diseases.Citation11 High dose can cause nausea, vomiting, abdominal cramps, diarrhea, a “fishy” body odor and rarely muscle weakness and seizures.Citation12
Glucosamine [GA] naturally occurring 6-carbon amino sugar, normally found in the body.Citation13 The oral bioavailability of glucosamine is about 26%. It is distributed to liver, kidney and other tissues including articular cartilage.Citation14 The mechanism of action of glucosamine is unknown. Glucosamine was demonstrated to reverse the deleterious effects of IL-1β, nuclear factor-κB and prostaglandin E2.Citation15 Glucosamine appears to be safe, with possibility of causing allergic reaction and possibly increasing the risk of developing diabetes.Citation16
Unilateral intra-articular [i.a.] injection of mono-iodoacetateCitation17, a chondrocyte glycolytic inhibitor, has been used to induce osteoarthritis-like changes in the articular cartilage of rodents. This minimally invasive model reproduces cartilage lesions with loss of proteoglycan matrix and functional joint impairment similar to human OA.Citation3
In this study, knee bend test, histological examination of knee joints, in addition to synovial fluid levels of interleukin-1β [IL-1β], and matrix metalloproteinase-13 [MMP-13] were estimated to evaluate the effect of l-carnitine, as compared to glucosamine sulfate,Citation18 in a rat model of MIA induced OA.
2 Materials and methods
2.1 Animals
Thirty-two adult male Wister rats weighing 150–210 g at the start of the experiment were purchased from Moassat Animal House [Faculty of Medicine, Alexandria University]. Animals were fed standard rat chow with free access to water, and were acclimatized for 2 weeks before the experiment. The study protocol was approved by the Ethics Committee, Faculty of Medicine; Alexandria University, Egypt.
2.2 MIA-induced OA
OA was induced in rats [pre-anesthetized with ether] by a single intra-articular injection of 2 mg of mono-iodoacetate [MIA, Sigma-Aldrich, St. Louis, MO, USA] through the infrapatellar ligament into the joint space of the left knee, in a total volume of 25 μl saline, via a 26.5-G needle.Citation19 Control rats were injected with an equivalent volume of saline.
2.3 Animal grouping
Rats were assigned randomly into four groups, 8 rats in each group, as follows: Group I [control group], group II [MIA induced OA group], group III [MIA induced OA/glucosamine sulfate, 250 mg/kg/day, treated group],Citation20 and group IV [MIA induced OA/l-carnitine, 100 mg/kg, treated group].Citation21
2.4 Knee diameter
Knee diameter was measured using calibrated digital caliper [World Precision Instruments, Stevenage, UK] in millimeter [mm] to assess the developmental stages of OA on days 0 [pre MIA injection], 1, 7, 14, 21, and 28 [post injection].Citation22
2.5 Knee bend test
The Knee-bend test was done at days 0, 1, 7, 14, 21 and 28 to evaluate the movement-induced pain caused by MIA. Briefly, we recorded the squeaks and/or struggle reactions in response to five alternate flexion and extensions of the knee joint [performed within the physiological limits of knee flexion/extension] for each rat. The score of the test was determined as follows: 0 – no responses; 0.5 – struggle to maximal flexion/extension; 1 – struggle to moderate flexion/extension or vocalizations to maximal flexion/extension; 2 – vocalizations to moderate flexion/extensions. The sum of the animal’s reactions, giving maximal values of 20, represents the Knee-Bend score, an indication of the animal’s movement-induced nociception. The contralateral knee was always tested first, in order to avoid an increase in the contralateral score arising from the manipulation of the injected knee. Results for both ipsilateral and contralateral knees were presented.Citation23
2.6 Biochemical analysis
On day 28, rats were anaesthetized with Thiopental sodium 40 mg/kgCitation24 intraperitoneally.Citation18 The left limb of the rat was flexed over a 20 ml glass vial, then 23-gauge needle was inserted, and the limb was secured in place with tape. Sterile saline was infused intra-articularly. 2 min after infusion of 100 μl of saline, the outflow fluid was aspirated. Synovial fluid was infused and withdrawn at a constant rate until a 400 μl basal sample was collected in a 1.5 ml centrifuge tube. Samples were immediately centrifuged, and the supernatants were collected and frozen at −20 °C.Citation5 The following parameters were estimated in the synovial fluid using ELISA kits: [IL-1β] [BMS630, eBioscience, Inc., Vienna, Austria], [MMP-13] [SCA099Ra Uscn Life Science Inc., Houston, USA], and Cartilage oligomeric matrix protein [COMP] [SEB197Ra, Uscn Life Science Inc., Houston, USA].Citation4,Citation25
Briefly, micro well strips for IL-1β, MMP-13, and COMP were washed with wash buffer. Standard curve solution was added to standard curve wells, 50 ml of sample serum +50 ml sample diluent and 50 ml of biotin conjugate were mixed together [mixture A] and added to the wells and incubated for 2 h at room temperature. Mixture A was removed, wells were washed and 100 ml of diluted Streptavidin-HRP [mixture B] was added and incubated for 1 h at room temperature with gentle agitation. Mixture B was removed, wells were washed and 100 ml of TMB substrate solution was added to the wells and incubated for 20 min at room temperature with gentle agitation until the highest standard curve point had developed [dark blue color]. Enzymatic reaction was stopped by the addition of 100 ml of stop solution. Plates were read on a spectrophotometer using 450 nm reference wavelengths, and cytokine concentration was determined from the standard curve. All samples and standards were run in triplicate.
2.7 Histopathological analysis
The knee joints of the rats were excised, and the soft tissues around it were removed. The knee joints were fixed in 10% buffered formalin, decalcified in hydrochloric acid, and embedded in paraffin. Sections [10 μm in thickness] were stained with hematoxylin and eosin [H&E].Citation26
2.8 Statistical analysis
Values are expressed as the mean ± SD. Statistical analyses were done using the Statistical Package of Social Sciences [SPSS] version 20. The IL-1β, COMP, MMP-13 levels were tabulated and analyzed statistically by one-way ANOVA, followed by a Post Hoc [Scheffe] test to compare variables between different groups. While the weight, knee diameter and knee bend score were tabulated and analyzed statistically using repeated measures ANOVA test. p values of less than 0.05 were considered significant.Citation27
3 Results
3.1 Weight
Rats in group II, III and IV had a decrease in weight gain compared to control group, in first week post-MIA induction. On day 28 all studied groups had almost same weight.
3.2 Knee diameter,
There was a significant increase in left knee diameter in groups II, III and IV as compared to normal control group [p < 0.05]. The maximal diameter was recorded on day 7 post-MIA injections. Treatment with GA or LC resulted in significant decrease in left knee diameter as compared to non-treated OA-induced group.
3.3 Knee bends score,
No sign of spontaneous nociceptive behavior or distress was found before MIA injection. Meanwhile, there was a significant increase in knee bend score in groups II, III and IV as compared to their baseline values, and to the normal control values [p < 0.05]. Treatment with GA or LC resulted in non significant decrease, in day 14, in knee bend score when compared to OA induced group.
3.4 Biochemical markers
3.4.1 IL-1β level,
IL-1β level was significantly increased in S.F from animals in group II as compared to group I [149.3 ± 3 pg/ml, 56.2 ± 19.7 pg/ml respectively, p < 0.05]. Meanwhile, statistically significant lower levels of IL-1β were obtained when MIA treated rats received either glucosamine or l-carnitine [65.1 ± 17.7 pg/ml, 76.2 ± 17 pg/ml respectively, p < 0.05 as compared to group II].
3.4.2 COMP level,
COMP, a marker of joint destruction, was dramatically increased in cartilage from group II rats [171.2 ± 26.6 ng/ml] as compared to group I [53.1 ± 14 ng/ml]. Treatment with glucosamine sulfate or l-carnitine caused a significant decrease in COMP levels [150.4 ± 9.6 ng/ml and 108.9 ± 15.2 ng/ml respectively p < 0.05 when compared to group II].
3.4.3 MMP-13 level,
There was a significant increase in MMP-13 level in cartilage in group II [9 ± 3.6 ng/ml] as compared to group I [2.4 ± 1 ng/ml], whereas treatment with glucosamine sulfate or l-carnitine significantly abrogated this increase [3.1 ± 1.1 and 3.4 ± 1.2 respectively, p < 0.05 vs group II].
3.5 Histological findings, and
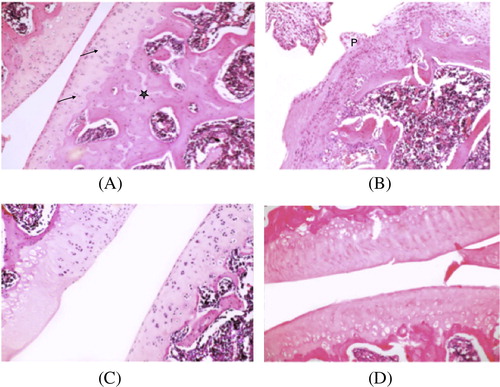
The isolated knee joints from the normal control group showed a smooth surface of the articular cartilage, with normal cellularity. In contrast, the joints from the MIA-induced OA rats showed joint space narrowing decrease in cartilage thickness, with irregular fibrillated surface, abnormal matrix intensity and chondrocytes.
Histological signs of cartilage degradation were reduced in GS, or LC treated groups. Preservation of cartilage smooth surface with almost normal thickness and matrix intensity was observed in both groups, and subchondral bone appeared with increased vascularization.
As shown in [A] Group I light photomicrograph of rat knee joint of control group shows cartilage with smooth non-fibrillated superficial region, matrix and scattered chondrocytes [↑]. Subchondral bone appears with more basophilic staining matrix [∗] enclosing bone trabeculae. [H&E stain. Mic. Mag. ×100.]
[B] Group II light photomicrograph of rat knee joint of induced osteoarthritis group reveals significant difference in articular cartilage thickness with irregular fibrillated surface, abnormal matrix intensity and chondrocytes. Subchondral bone shows irregularity in matrix and shape. Notice pannus formation [P] a hyperplastic synovial villus extends over the surface of the articular cartilage as a fibrous inflammatory membrane. [H&E stain. Mic. Mag. ×100.]
[C] Group III light photomicrograph of rat knee joint of glucosamine sulfate treated group depicts recovery of knee joint with preservation of cartilage smooth surface with normal thickness and matrix intensity. Subchondral bone appears with increased vascularization. [H&E stain. Mic. Mag. ×100.]
[D] Group IV light photomicrograph of rat knee joint of l-carnitine treated group depicts partial recovery of knee joint with preservation of cartilage smooth surface but decreases in its thickness and matrix intensity. Subchondral bone appears with increased vascularization. [H&E stain. Mic. Mag. ×100.]
4 Discussion
Osteoarthritis is a degenerative joint disease characterized by the progressive loss of cartilage, changes in subchondral bone, and chronic pain. Analgesics and non-steroidal anti-inflammatory drugs [NSAIDs] are the main therapeutic treatment options for OA. The search for treatments able to slow the progression of disease is a great challenge and would meet an important medical need for patients with OA.
The development and progression of OA are now believed to involve inflammation, even in the early stage.Citation1 Proinflammatory cytokines and chemokine play critical roles in disturbed homeostasis in the OA cartilage matrix. Interleukin-1β [IL-1β] among them plays a crucial role in the downregulation of the synthesis of major ECM components by inhibiting anabolic activities and increasing catabolic activities in chondrocytes.Citation28 Furthermore, IL-1β decreases the synthesis of a number of cytokines which contribute to inflammation, including IL-6 and IL-8.Citation6
The MIA-induced OA animal model used in the present study is a well-characterized model and mimics pain and biochemical/structural changes associated with human OA. In our study, we demonstrated that the synovial fluid level of IL-1β was increased in the untreated MIA induced OA group. On the other hand, synovial fluid levels of IL-1β were significantly reduced after treatment with GA or LC. These results demonstrated an anti-inflammatory effect of GA, and LC. The anti-inflammatory effects of LC are previously shown in different animal models of inflammation.Citation29
Parallel to acute and chronic inflammatory processes, the pathophysiology of OA development includes an active biological process of matrix degradation, leading to the release of MMPs and eventually cartilage loss. MMPs can degrade native fibrillar collagens of types I, II, III, V, and XI, being involved in various pathologic conditions.Citation30 Many studies have reported increased activities of MMP-3 and MMP-13 in human OA cartilage as well as experimental animal models of OA. IL-1β was shown to diminish the expression of type 2 collagen and aggrecan, and increases the expression of MMP-1, -3, and -13.Citation30
In accordance with previous studies,Citation31 we found that the synovial fluid level of MMP-13 was elevated in the untreated MIA induced OA group. Meanwhile, the levels of MMP-13 were significantly inhibited by GA, LC treatment; suggesting the potential of these agents as one of the DMOADs in OA treatment.
Cartilage oligomeric matrix protein is one of the essential components of the extracellular matrix of the cartilage. COMP regulates the cellular proliferation, apoptosis and cellular attachment in the cartilaginous tissue.Citation32 COMP has shown promise as a potential biomarker for monitoring progression of cartilage destruction, for evaluating cartilage effects of therapy, and as a prognostic tool reflecting cartilage damage.Citation33 Serum COMP levels can be detected quite early during the inflammatory process, in the synovial fluid and in the bloodstream, even before radiographic joint lesions and irreversible damage appear.Citation32
The elevation of COMP in MIA induced OA group was concomitant with that of other animal models of arthritis.Citation34 Joosten et al., previously demonstrated that therapeutic intervention which ameliorated cartilage destruction normalized serum COMP levels in murine CIA, whereas treatment which only reduced signs of inflammation did not affect COMP levels.Citation35
Glucosamine is one of the most frequently used alternatives worldwide due to their chondroprotective properties. Despite being considered effective by many research groups, controversy surrounds the use of glucosamine in OA. In long-term studies, no benefit of glucosamine for pain was found. The dose used in animal model was found to be much higher than that obtained through the oral ingestion of supplements.Citation36 Furthermore, in obese and diabetic patients glucosamine may cause alteration in glucose metabolism.Citation36
In conclusion, l-carnitine is shown to be as effective as glucosamine in lowering IL-1β [biochemical marker of inflammation], MMP-13 and COMP [markers of cartilage destruction] in OA induced rat model. These results suggest a promising use of l-carnitine as a potential DMOAD.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 28 March 2016
References
- B.J.de Lange-BrokaarA.Ioan-FacsinayG.J.van OschA.M.ZuurmondJ.SchoonesR.E.ToesSynovial inflammation, immune cells and their cytokines in osteoarthritis: a reviewOsteoarthr Cartil2012201214841499
- M.SowersEpidemiology of risk factors for osteoarthritis: systemic factorsCurr Opin Rheumatol1352001447451
- I.SulzbacherOsteoarthritis: histology and pathogenesisWien Med Wochenschr1639–102013212219
- M.MaldonadoJ.NamThe role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritisBiomed Res Int20132013284873
- N.J.BartonD.A.StevensJ.P.HughesA.G.RossiI.P.ChessellA.J.ReeveDemonstration of a novel technique to quantitatively assess inflammatory mediators and cells in rat knee jointsJ Inflamm4200713
- M.KapoorJ.Martel-PelletierD.LajeunesseJ.P.PelletierH.FahmiRole of proinflammatory cytokines in the pathophysiology of osteoarthritisNat Rev Rheumatol7120113342
- B.A.FoxM.M.StephensGlucosamine/chondroitin/primorine combination therapy for osteoarthritisDrugs Today45120092131
- C.J.ReboucheD.J.PaulsonCarnitine metabolism and function in humansAnnu Rev Nutr619864166
- Y.CaoY.X.WangC.J.LiuL.X.WangZ.W.HanC.B.WangComparison of pharmacokinetics of l-carnitine, acetyl-l-carnitine and propionyl-l-carnitine after single oral administration of l-carnitine in healthy volunteersClin Invest Med3212009 E13-9
- C.C.WagnerA.RuscaK.KletterM.TschurlovitsS.PaceA.LongoPlasma pharmacokinetics and gastrointestinal transit of a new propionyl-l-carnitine controlled release formulationXenobiotica41112011988995
- G.D’AntonaS.M.NabaviP.MichelettiA.Di LorenzoR.AquilaniE.NisoliCreatine, l-carnitine, and omega3 polyunsaturated fatty acid supplementation from healthy to diseased skeletal muscleBiomed Res Int20142014613890
- L.BonafeM.M.BergerY.A.QueJ.I.MechanickCarnitine deficiency in chronic critical illnessCurr Opin Clin Nutr Metab Care1722014200209
- S.G.AKirkhamR.K.SamarasingheReview article: GlucosamineJ Orthop Surg (Hong Kong)17120097276 B.BMalaekeh-Nikouei S.Golmohammadzadeh S.Salmani-Chamanabad N.Mosallaei K.JamialahmadiPreparation, characterization, and moisturizing effect of liposomes containing glucosamine and N-acetyl glucosamineJ Cosmet Dermatol122201396102
- S.QianQ.ZhangY.WangB.LeeG.V.BetageriM.S.ChowBioavailability enhancement of glucosamine hydrochloride by chitosanInt J Pharm4551–22013365373
- P.QvistA.C.Bay-JensenC.ChristiansenE.B.DamP.PastoureauM.A.KarsdalThe disease modifying osteoarthritis drug (DMOAD): Is it in the horizon?Pharmacol Res581200817
- Y.HenrotinC.LambertChondroitin and glucosamine in the management of osteoarthritis: an updateCurr Rheumatol Rep15102013361
- A.Scotto d’AbuscoV.CalamiaC.CicioneB.GrigoloL.PolitiR.ScandurraGlucosamine affects intracellular signalling through inhibition of mitogen-activated protein kinase phosphorylation in human chondrocytesArthritis Res Ther952007R104
- J.L.TremoledaA.KertonW.GsellAnaesthesia and physiological monitoring during in vivo imaging of laboratory rodents: considerations on experimental outcomes and animal welfareEJNMMI Res21201244
- R.E.GuzmanM.G.EvansS.BoveB.MorenkoK.KilgoreMono-iodoacetate-induced histologic changes in subchondral bone and articular cartilage of rat femorotibial joints: an animal model of osteoarthritisToxicol Pathol3162003619624
- M.U.RezendeH.M.GurgelP.R.Vilaca JuniorR.K.KurobaA.S.LopesR.Z.PhillipiDiacerhein versus glucosamine in a rat model of osteoarthritisClinics6152006461466
- E.BianchiMannelli L.Di CesareC.MenicacciP.LorenzoniM.AglianoC.GhelardiniProphylactic role of acetyl-l-carnitine on knee lesions and associated pain in a rat model of osteoarthritisLife Sci1061–220143239
- M.KhanM.AshrafA.HashmiM.D.AhmadA.A.AnjumClinical assessment of experimentally induced osteoarthritis rat model in relation to timeAnim Plant Sci2242012960965
- S.AdaesM.MendoncaT.N.SantosJ.M.Castro-LopesJ.Ferreira-GomesF.L.NetoIntra-articular injection of collagenase in the knee of rats as an alternative model to study nociception associated with osteoarthritisArthritis Res Ther1612014R10
- M.L.KaushikS.S.JalalpureEffect of Curcuma zedoaria Rosc root extracts on behavioral and radiology changes in arthritic ratsAdv Pharm Technol Res232011170176
- F.SongK.WisithphromJ.ZhouL.J.WindsorMatrix metalloproteinase dependent and independent collagen degradationFront Biosci11200631003120
- N.GerwinA.M.BendeleS.GlassonC.S.CarlsonThe OARSI histopathology initiative – recommendations for histological assessments of osteoarthritis in the ratOsteoarthr Cartil18Suppl. 32010S24S34
- J.R.LandisG.G.KochThe measurement of observer agreement for categorical dataBiometrics3311977159174
- C.ChadjichristosC.GhayorM.KypriotouG.MartinE.RenardL.Ala-KokkoSp1 and Sp3 transcription factors mediate interleukin-1 beta down-regulation of human type II collagen gene expression in articular chondrocytesBiol Chem2784120033976239772
- A.KocT.OzkanA.Z.KarabayA.SungurogluF.AktanEffect of l-carnitine on the synthesis of nitric oxide in RAW 264.7 murine macrophage cell lineCell Biochem Funct2982011679685
- C.AmalineiI.D.CaruntuS.E.GiuscaR.A.BalanMatrix metalloproteinases involvement in pathologic conditionsRom Morphol Embryol5122010215228
- J.LeeY.S.HongJ.H.JeongE.J.YangJ.Y.JhunM.K.ParkCoenzyme Q10 ameliorates pain and cartilage degradation in a rat model of osteoarthritis by regulating nitric oxide and inflammatory cytokinesPLoS One872013e69362
- B.R.DasA.RoyF.R.KhanCartilage oligomeric matrix protein in monitoring and prognostication of osteoarthritis and its utility in drug developmentPerspect Clin Res61201549
- P.VermaK.DalalSerum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: a novel diagnostic and prognostic biomarkerJ Orthop Res31720139991006
- Y.LaiX.P.YuY.ZhangQ.TianH.SongM.T.MucignatEnhanced COMP catabolism detected in serum of patients with arthritis and animal disease models through a novel capture ELISAOsteoarthr Cartil2082012854862
- L.A.JoostenM.M.HelsenT.SaxneF.A.van De LooD.HeinegardW.B.van Den BergIL-1 alpha beta blockade prevents cartilage and bone destruction in murine type II collagen-induced arthritis, whereas TNF-alpha blockade only ameliorates joint inflammationJ Immunol1639199950495055
- J.SalazarL.BelloM.ChavezR.AnezJ.RojasV.BermudezGlucosamine for osteoarthritis: biological effects, clinical efficacy, and safety on glucose metabolismArthritis20142014432463