?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Banana fruit is claimed to have antidiabetic effects despite its high calorie content, and its peels also contain vital phytoconstituents including gallocatechin. Previously banana pulp has been studied for antihyperglycemic effects, and in the present investigation antihyperglycemic effect of ethanolic extract of inner peels of Musa sapientum (EMS), Musa paradisiaca (EMP), Musa cavendish (EMC) and Musa acuminata (EMA) fruit was evaluated using oral glucose tolerance test in normoglycemic rats. In vitro antioxidant study was conducted using DPPH, H2O2 radical scavenging assay and ferric reducing power assay. Wistar rats were divided into fourteen groups and twelve groups received different doses of aforementioned extracts, while control group received gum acacia solution and remaining group received standard drug, glimepiride. All the rats received glucose load at a dose of 2 g/kg body weight. Groups treated with EMC and EMA showed significant decrease in glucose level (p < 0.01) at 150 min as compared to control group. In hypoglycemic study, only EMP 500 mg/kg, p.o. treated group revealed a significant decrease (p < 0.05) in glucose level at 120 min, while other groups did not show any sign of hypoglycemia. In glucose tolerance test, animals treated with EMC and EMA depicted dose dependent antihyperglycemic effect at 150 min while EMS and EMP showed significant reduction in plasma glucose at higher doses. In a similar fashion, EMA i.e. M. acuminata demonstrated highest antioxidant activity followed by EMC against DPPH radical. In ferric reducing power and H2O2 scavenging assay, EMA demonstrated maximal antioxidant activity when compared with other extracts.
1 Introduction
Diabetes mellitus (DM) ranks highly with the top ten disorders which cause mortality throughout the world and is affecting approximately 30% of the population worldwide.Citation1–Citation3 It is not a single disease entity, but a set of metabolic disorders with a common underlying feature of high blood glucose level. It is a systemic metabolic disease characterized by increased blood glucose, triglyceride and hypo insulinemia that may lead to decrease in both insulin action and insulin secretion.Citation4,Citation5 The increased blood glucose is associated with reduced quality of life and high risk factors for mortality and morbidity.
Normally, blood glucose levels are tightly controlled by insulin, a hormone produced by the pancreas. Insulin is released from the pancreas to normalize the glucose level.Citation6 It lowers the post-prandial blood glucose level when it is raised (e.g. after eating food). Hyperglycemia in DM, results from defects in insulin secretion, insulin signaling pathway or most commonly both.Citation7
Uncontrolled DM is often associated with complications which pose challenges to clinicians. These complications include development of micro- and macro-vascular complications such as neuropathy, nephropathy, retinopathy, cardiovascular and cerebrovascular diseases.Citation8,Citation9
Currently available antidiabetic agents possess potential side effects such as risk of hypoglycemia, anemia, cholestatic jaundice.Citation10 Many herbal constituents have varying degree of hypoglycemic and antihyperglycemic activity and until now no case of adverse effect is counted with herbal constituents. Among these are alkaloids, glycosides, galactomannan, gallocatechin, hypoglycans, guanidine, steroids, carbohydrates, terpenoids, glycopeptides, amino acids and inorganic ions.Citation11,Citation12 Musa commonly known as banana, reported to possess therapeutic potential in treatment of hyperglycemia.Citation13,Citation14
In Indian system of folk medicine, the peel of banana is also used to treat various diseases and disorders such as for treatment of wound, hyperglycemia, ulcer, dysentery.Citation15 Some people have a habit of eating inner peel, in addition to the pulp of banana fruit. Several flavonoids and related compounds (leucocyanidin, quercetin and its 3-O-glucoside, 3-O-galactoside, and 3-O-rhamnosyl glucoside, gallocatechin) were isolated from the unripe pulp and peel of plantain.Citation14,Citation16,Citation17 The various antioxidant components identified in bananas includes tocopherol, ascorbic acid, beta carotene, dopamine, phenolic groups and gallocatechin.Citation18 Banana is also reported to be a rich source of calcium, vitamins A, B1, B2, B3, B6, C and minerals such as potassium and phosphorus.Citation19 These phytochemicals have shown protective action against diseases which involve oxidative stress. On the basis of traditional claim, reported activities and chemical constituents, the present study was aimed to evaluate antihyperglycemic, hypoglycemic and in vitro antioxidant potential of peels of Musa species.
2 Materials and methods
2.1 Experimental animals
Albino rats of Wistar strain weighing 160–200 g were obtained from National Institute of Nutrition (NIN), Hyderabad. Animals of either sex were housed under standard laboratory conditions of 22 ± 3 °C temperature and relative humidity 30% and 12 h light and dark cycle maintained, free access to standard pellet diet and water ad libitum. The Institutional Animal Ethics Committee approved the experimental protocol (1613/PO/a/12/CPCSEA).
2.2 Acute toxicity study
The LD50 of the peel extract was tested to determine the safety of the agent according to the guidelines set by the OECD (Organization for Economic Cooperation and development) No. 423.Citation20 The study was carried out in two phases. In the first phase, nine mice were randomized into three groups of three mice per group and administered 100, 600 and 1000 mg/kg of the Musa species extract orally. The animals were observed for the first 4 h and 24 h for signs of toxicity and mortality. The results of this phase informed the choice of doses for the second phase, in which 2000, 3000 and 5000 mg/kg were administered to another set of three mice per group and these animals were observed for signs of toxicity and mortality for 72 h.
2.3 Collection and authentication of the plant material
Fresh unripe fruits of Musa sapientum, Musa paradisiaca, Musa cavendish, and Musa acuminata (Musa species) Linn. (Museaceae) were collected from Nanded, Satara of Maharashtra, Goa and Kerala states of India. The specimens were authenticated at Botanical Survey of India, Pune (MUPNAV2), and Science College Nanded (25-9/12.)
2.4 Preparation of extract
Banana pulp was removed and from the remaining peel inner fibrous part was removed by knife (we termed it as ‘inner peel’) and it was shed dried at room temperature and latter powdered using grinder. This powder was then defatted with petroleum ether. M. sapientum, M. paradisiaca, M. cavendish, and M. acuminata peel powder was extracted with ethanol using soxhlet extraction. It was then removed and the solvent was evaporated under vacuum and the residue was stored for further use.
The extract was not freely soluble in distilled water so the suspension of extract was prepared using 1% gum acacia (as a suspending agent).
2.5 Experimental design
The Wistar albino rats weighing 160–200 g were used. The overnight fasted animals were divided into fourteen groups (n = 6). Group 1 served as Control: The animals of this group received 1% gum acacia (1 ml/kg, p.o.). Group 2 served as Glim (Standard): The animals of this group received glimepiride (0.09 mg/kg, p.o.). Groups 3 to 5: Received ethanolic extract of M. sapientum (EMS) 50, 100 and 200 mg/kg, p.o. Groups 6 to 8: Received ethanolic extract of M. paradisiaca (EMP) 125, 250 and 500 mg/kg, p.o. Groups 9 to 11: Received ethanolic extract of M. cavendish (EMC) 250, 500 and 1000 mg/kg, p.o. Groups 12 to 14: Received ethanolic extract of M. acuminata (EMA) 100, 200 and 400 mg/kg, p.o.
2.5.1 Hypoglycemic study in non-diabetic ratsCitation21
The effect of various extracts of Musa species was studied in non-diabetic rats for the assessment of hypoglycemic effect if any. Animals were treated with ethanolic extract of different species of Musa and glimepiride was used as standard drug as per the experimental design. Blood samples were collected by puncturing retro-orbital plexus at the 0, 60, 120 and 180 min after drug administration.
2.5.2 Oral glucose tolerance test (OGTT) in non-diabetic ratsCitation21,Citation22
Oral glucose tolerance test of different extracts of Musa species was conducted in non-diabetic rats. In this study, glucose solution (2 g/kg, p.o.) was administered 30 min after vehicle/drug administration. Blood samples were collected at the 0, 30, 90 and 150 min after glucose load. The blood glucose level was estimated by using catalyst diagnostic kits.Citation22
2.6 In vitro antioxidant activity
2.6.1 DPPH· free radical scavenging activityCitation23,Citation24
The free radical scavenging activity of M. cavendish and M. acuminata was measured by DPPH· assay, wherein the bleaching rate of the stable free radical, (1, 1-Diphenyl-2-picryl-hydrazyl) DPPH· is monitored at a characteristic wavelength in the presence of the sample. In its radical form, DPPH· absorbs at 517 nm, but upon reduction by an antioxidant or a radical species, its absorbance decreases. Briefly, 0.1 mM solution of DPPH· in ethanol was prepared and 1 ml of this solution was added to 3 ml of M. cavendish and M. acuminata solution in water at various concentrations (25–250 μg/ml). Thirty minutes later, the absorbance was measured at 517 nm. Lower absorbance of the reaction mixture indicates higher free radical scavenging activity.Citation23,Citation25 IC50 value (concentration required to scavenge 50% free radicals) of the test samples was also determined.
The DPPH· radical scavenging activity was calculated according to the following equation:where
Ao is the absorbance of DPPH·,
A1 is the absorbance of DPPH· solution in the presence of the extract.
2.6.2 Hydrogen peroxide (H2O2) scavenging assayCitation26–Citation28
The hydrogen peroxide scavenging ability of M. cavendish and M. acuminata was determined according to the method of Ruch et al.Citation26 A solution of H2O2 (40 mM) was prepared in phosphate buffer (pH 7.4). The different concentrations of all M. cavendish and M. acuminata (10–50 μg/ml) in phosphate buffer were added to a H2O2 solution (0.6 ml, 40 mM). The absorbance value of the reaction mixture was recorded at 230 nm and phosphate buffer without H2O2 was used as a blank.Citation23 The percentage of H2O2 scavenging of M. cavendish, M. acuminata and standard compound (Ascorbic acid) was calculated aswhere Ao is the absorbance of H2O2 and A1 is the absorbance of H2O2 solution in the presence of the extract.
2.6.3 Ferric reducing antioxidant power (FRAP) assay
Depending upon the reducing power (changes in brown color of peel extract) of each antioxidant sample of M. cavendish and M. acuminata, the reduction potential was determined. The reducing capacity of compound may serve as significant indicator of its potential antioxidant activity. The presence of reductant such as antioxidant substance causes the reduction in the Fe3+/ferricyanide complex to the ferrous form. Therefore, the Fe2+ can be monitored by measuring the formation of Perl’s Prussian blue at 700 nm.Citation26,Citation29,Citation30 Different concentrations of M. cavendish and M. acuminata (25–250 μg/ml) in 1 ml of distilled water were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1%). The mixture was then centrifuged for 10 min at 1000 rpm. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%), and the absorbance was measured at 700 nm in a spectrophotometer. Increased absorbance of the reaction mixture indicates increased reducing power.where
Ao is the absorbance of FeCl3,
A1 is the absorbance of FeCl3 solution in the presence of the extract.
2.7 Statistical analysis
The data obtained were treated statistically by using analysis of variance (ANOVA) followed by Dunnett’s test to detect any significant difference among different means, with level of significance set at p < 0.05. The results were expressed as mean ± S.E.M.
3 Results
3.1 Acute toxicity
The acute oral toxicity test showed normal behavior of the treated animals. There was no mortality observed at a high dose of 2000 and 5000 mg/kg. Hence the 1/10th of the safer dose was selected as a therapeutic dose.
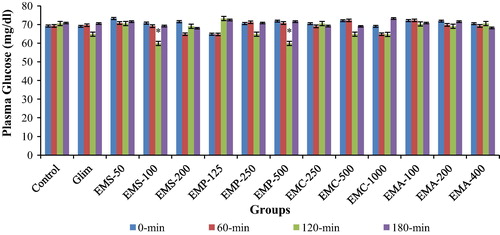
3.2 Effect of various extracts of Musa species on blood glucose in normoglycemic rats
As like glimepiride-treated group, groups treated with different extracts of Musa species did not show any significant change in blood glucose level, when compared with control group (). However, marginal reduction in blood glucose level below normal was observed at higher dose of EMS-100 mg/kg, p.o. at 120 min. But, this effect of smaller decrease in blood glucose disappeared at 180 min. Group treated with EMP-500 mg/kg, p.o. showed minor reduction (p < 0.05) in blood glucose level at 120 min when compared with the control group. Apart from above mentioned groups, no other treated groups depicted any sign of hypoglycemia.
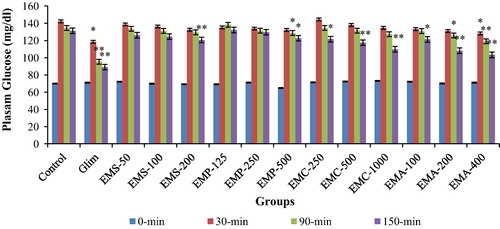
3.3 Effect of various extracts of Musa species on blood glucose in glucose loaded rats
Effect of Musa species on glucose loaded normoglycemic rats is presented in . From these studies it was evident that out of four species, M. acuminata (EMA-200 and 400) depicted greater decrease (p < 0.01) in blood glucose level, followed by M. cavendish (EMC 500 and 1000) i.e. (p < 0.01) at 150 min. Hyperglycemic animals treated with EMS-200 mg/kg, p.o. showed significant reduction in blood glucose level at 150 min (p < 0.01). EMP at the dose of 500 mg/kg also demonstrated noteworthy anti-hyperglycemic effect from 90 min onward. All the groups treated with various extracts of Musa species showed dose dependant reduction in the blood glucose level.
3.4 In vitro antioxidant study
As depicted in M. acuminata and M. cavendish demonstrated significant DPPH· radical scavenging activity in a dose-dependent manner in a range of 25–250 μg/ml. Ethanolic extract of M. acuminata revealed highest inhibition considering the IC50 value (139.50 μg/ml). Similarly, in ferric reducing power activity, dose-dependent iron chelating activity of both extracts was observed and M. acuminata demonstrated greater reducing power than M. cavendish with IC50 value (198.80 μg/ml). Extracts were also screened for H2O2 scavenging activity (). In conformity with the earlier results, both extracts revealed potential H2O2 scavenging activity. In comparison with M. cavendish, the ethanolic extract of M. acuminata demonstrated greater H2O2 scavenging activity.
Table 1 In vitro antioxidant activity of ethanolic extracts of Musa cavendish and Musa acuminata fruit peel by DPPH and ferric reducing power assay.
Table 2 In vitro antioxidant activity of ethanolic extracts of Musa cavendish (EMC) and Musa acuminata (EMA) fruit peel using H2O2 scavenging activity.
4 Discussion
Earlier studies have demonstrated anti-hyperglycemic activity of various parts of different Musa species.Citation13,Citation31,Citation32 Mostly its pulp has been explored for antidiabetic potential. The inner peel that is consumed by some local population for their belief has not been fully explored for antidiabetic activity. Moreover, comparative evaluation of different Musa species is yet to be explored. In the present study evaluation of Musa species for possible antidiabetic effect was performed by glucose tolerance test and hypoglycemic study. Significant anti-hyperglycemic effect was seen with all four Musa species viz. M. cavendish, M. acuminata, M. sapientum, and M. paradisiaca. Anti-hyperglycemic effect was more prominent with M. acuminata and M. cavendish species. However, extract of other two species also demonstrated significant effect at higher doses. The result confirmed earlier findings of Musa species.Citation33,Citation34 Anti-hyperglycemic effect of Musa species may be due to the presence of phyto-constituents such as tannins, saponins and flavonoids.Citation16,Citation17 The phytochemical investigation of various extracts of Musa species showed the presence of tannins, alkaloids and flavonoids (e.g. gallocatechin).Citation14,Citation35 These substances are frequently implicated for its anti-hyperglycemic effects.Citation36,Citation37 Furthermore, banana peels are rich in phytochemical composites, principally antioxidants. Developed banana peels, which hold the anthocyanins, delphinidin and cyanidin, and catecholamine may also contribute to this effect.Citation38 Inhibition of intestinal absorption of glucose or increased secretion of insulin by pancreatic beta cells may be one of the probable mechanisms for this anti-hyperglycemic effect as gallocatechin is one of the active constituents of banana peel which increases peripheral glucose utilization.Citation14,Citation39
Marginal hypoglycemia observed with the administration of extract of M. sapientum could be due to increased utilization of glucose in the liver for glycogen synthesis and decreased degradation of glycogen and due to reduced gluconeogenesis.Citation40
On the basis of hypoglycemic and anti-hyperglycemic activity, most active Musa species i.e. Musa cavendish and M. acuminata were further evaluated for its in vitro antioxidant activity, using DPPH radical scavenging assay, Ferric reducing power assay and H2O2 scavenging activity. The present work has shown that the extracts of both Musa species exhibited a marked DPPH scavenging activity (). As a result, the new findings showed dose dependent decrease in the concentration of DPPH due to the free radical scavenging effect of M. acuminata. In the presence of hydrogen donors, DPPH gets oxidized and a stable free radical is formed from the scavenger.Citation41 Since the hydrogen donating ability of Musa species was comparable to ascorbic acid, it was evident that the M. acuminata could serve as hydrogen donors, and thus consequently terminating the radical chain reaction.
Phenolic compounds are good electron donors, and they reveal reducing power and have ability to reduce ferric ion to ferrous ion by donating electron.Citation42 This may explain a current interest in the applicability of the reducing power assay in determining the antioxidant capacity of plant extracts. The reducing capacity of a compound may serve as a significant indicator of its potential antioxidant activity. The present study indicates the reducing power of M. acuminata as a potential source of antioxidants. This study, therefore, suggests that the reducing power of M. acuminata might be due to the presence of its phenolic content.Citation38
Hydrogen peroxide itself is not very reactive because of its weak oxidizing and reducing capabilities but it can inactivate few enzymes by oxidizing essential thiol (–SH) groups and it also can cross the cell membrane rapidly and once inside the cell, in the presence of metal ions it is converted into highly toxic hydroxyl radical which may originate from many of its toxic effects.Citation43 Therefore it is biologically important for cells to scavenge hydrogen peroxide that gets entered into the cell. Hence in vitro testing for H2O2 scavenging activity of extracts is one of the valuable tools to discover new natural antioxidants to control diseases induced by H2O2 and its products. From the result (), it appeared that M. acuminata possesses marked H2O2 scavenging ability.
5 Conclusion
In the present study, inner peels of various Musa species demonstrated significant antihyperglycemic and in vitro antioxidant activity. In particular ethanolic extracts of M. acuminata demonstrated marked antihyperglycemic activity in Wistar rats. Similarly antioxidant assays viz., DPPH, ferric reducing power and H2O2 scavenging assay also confirmed noteworthy free radical scavenging activity of M. acuminata peels. Hence, it can be concluded that eating of inner peel of banana fruit would be beneficial considering its potential antioxidant and antihyperglycemic property.
Conflict of interest
We have no conflict of interest to declare.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 25 July 2016
References
- G.KaushikS.SatyaR.K.KhandelwalS.N.NaikCommonly consumed Indian plant food materials in the management of diabetes mellitusDiabetes Metab Syndrome. Clin Res Rev420102140
- J.S.SkylerDiabetes mellitus: pathogenesis and treatment strategiesChemistry47200441134117
- M.S.MirM.M.DarziH.M.KhanS.A.KamilA.H.SofiA.WaniPathomorphological effects of alloxan induced acute hypoglycaemia in rabbitsAlexandria J Med492013343353
- R.MaitiD.JanaU.K.DasD.GhoshAntidiabetic effect of aqueous extract of seed of Tamarindus indica in streptozotocin induced diabetic ratsJ Ethnopharmacol9220048591
- K.A.WadkarC.S.MagdumS.S.PatilN.S.NaikwadeAntidiabetic potential and Indian medicinal plantsJ Herbal Med Toxicol220084550
- I.M.LiuT.F.TzengS.S.LiouT.W.LanImprovement of insulin sensitivity in obese Zucker rats by myricetin extracted from Abelmoschus moschatusPlanta Med73200710541060
- RobbinsCotranThe endocrine pancreas, pathologic basis of disease2004ElsevierIndia
- M.S.AkhtarJ.IqbalEvaluation of the hypoglycaemic effect of Achyranthes aspera in normal and alloxan-diabetic rabbitsJ Ethnopharmacol3119914957
- M.J.RuffaG.FerraroM.L.WagnerM.L.CalcagnoR.H.CamposL.CavallaroCytotoxic effect of Argentine medicinal plant extracts on human hepatocellular carcinoma cell lineJ Ethnopharmacol792002335339
- B.P.SchimmerK.L.ParkerAdrenocorticotropic hormone; adrenocortical steroids and their synthetic analogs; inhibitors of synthesis and actions of adrenocortical hormonesJ.G.HardmanL.E.LimbardA.G.GilmanA.Goodman-GilmanGoodman & Gilman’s the pharmacological basis of therapeutics2001Mc Graw-Hill16581659
- J.K.GroverS.YadavV.VatsMedicinal plants of India with anti-diabetic potentialJ Ethnopharmacol81200281100
- R.K.GoyalA.A.MehtaS.G.MahajanClassification of herbal antidiabetic based on mechanism of action and chemical constituentsJ.N.GovilV.R.SinghS.K.MisharaRecent progress in medicinal plants vol. 20, Phytopharmacology and Therapeutic values II2008Stadium Press LLCUSA65110
- L.PariU.J.MaheswariHypoglycemic effect of Musa sapientum L. in alloxan-induced diabetic ratsJ Ethnopharmacol681999321325
- S.SomeyaY.YoshikiK.OkuboAntioxidant compounds from bananas (Musa cavendish)Food Chem792002351354
- Deshpande AP, Jawalgekar RR, Ranade S. Dravyagun Vigayan. Anmol prakashan, Pune; 2002.
- S.GhosalSteryl glycoside and acylsteryl glycoside from Musa paradisiacaPhytochem18071985
- D.A.LewisG.P.ShawA natural flavonoid and synthetic analogues protect the gastric mucosa from aspirin-induced erosionsJ Nutr Biochem12200195100
- S.Y.QustiA.N.Abo-KhatwaM.A.LahwaFree radical scavenger enzymes of fruit plant species cited in holy QuranWorld Appl Sci J92010338344
- O.T.OsundahunsiScanning electron microscope study and pasting properties of ripe and unripe plantainJ Food Agric Environ73&42009182186
- OECD Guidelines for the testing of chemicals (No. 423) “Acute oral toxicity acute toxic class method” (Adopted on 17 December 2011); 2012. p. 1–14.
- Z.A.BhatS.H.AnsariH.M.MukhtarT.NavedN.A.KhanI.A.ChashooAnti-hyperglycemic activity of the alcoholic extracts of Aralia cachemirica DecnerootaJ Nat Rem52005160164
- A.ShirwaikarK.RajendranR.BarikEffect of aqueous bark extract of Garuga pinnata Roxb. in streptozotocin-nicotinamide induced type-II diabetes mellitusJ Ethnopharmacol1072006285290
- S.A.ShodehindeG.ObohAntioxidant properties of aqueous extracts of unripe Musa paradisiaca on sodium nitroprusside induced lipid peroxidation in rat pancreas in vitroAsian Pac J Trop Biomed32013449457
- A.L.ChewJ.J.A.JessicaS.SasidharanAntioxidant and antibacterial activity of different parts of Leucas asperaAsian Pacific J Trop Biomed2012176180
- M.S.BloisAntioxidant determination by the use of a stable free radicalNature26195811991200
- R.J.RuchS.J.ChengJ.E.KlaunigPrevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green teaCarcinogenesis10198910031008
- I.GulcinV.MshvidadzeA.GepdiremenR.EliasScreening of antiradical and antioxidant activity of monodesmosides and crude extract from Leontice smirnowii tuberPhytomedicine132006343351
- P.NainA.KumarS.SharmaJ.NainIn vitro evaluation of antimicrobial and antioxidant activities of methanolic extract of Jasminum humile leavesAsian Pacific J Trop Med2011804807
- Y.C.ChungC.T.ChungW.W.ChaoC.F.LinS.T.ChouAntioxidant activity and safety of 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1J Agric Food Chem50200224542458
- S.A.BabaS.A.MalikEvaluation of antioxidant and antibacterial activity of methanolic extracts of Gentiana kurroo royleSaudi J Biol Sci212014493498
- B.A.SalauE.O.AjaniA.A.AkinloluM.N.EkorM.O.SoladoyeMethanolic extract of Musa sapientum sucker moderates fasting blood glucose and body weight of alloxan induced diabetic ratsAsian J Exp Biol Sci120103035
- S.K.SinghA.N.KesariP.K.RaiG.WatalAssessment of glycemic potential of Musa paradisiaca stem juiceIndian J Clin Biochem2220074852
- I.B.FarjouM.Al-AniS.V.GuirgosLowering of blood glucose in diabetic rabbits by Artemisia extractJ Faculty Med Univ Baghdad291987137141
- A.Al-LamiI.B.FarjouEffect of feeding Artemisia herba-alba on glucokinase and ATPase activity in normal and diabetic rabbitsJ Faculty Med Univ Baghdad3219901320
- V.V.NavghareS.C.DhawaleM.A.PhanseP.G.IngoleS.S.PawaleP.P.SonwaneFree radical scavenging property of some commonly known Musa speciesIndo Am J Pharm Res3201360276034
- H.MatsudhaT.MorikawaM.YoshikawaAntidiabetogenic constituents from several natural medicinePure Appl Chem74200213011308
- K.GuyK.JaekyungH.KlausC.YanyanC.XiaozhuoAntidiabetes and antiobesity activity of Laserstroemia speciosaeCAM10200717
- A.PereiraM.MaraschinBanana (Musa spp.) from peel to pulp: ethnopharmacology, source of bioactive compounds and its relevance for human healthJ Ethnopharmacol1602015149163
- R.A.AndersonM.M.PolanskyTea enhances insulin activityJ Agric Food Chem50200271827186
- R.GomathyN.R.VijayalekshmiP.A.KurupHypoglycemic action of the pectin present in the juice of the inflorescence stalk of plantain (Musa sapientum) – mechanism of actionJ Biosci151990297303
- MohammedAl-MamaryMolhamAl-HaboriAdel S.Al-ZubairiThe in-vitro antioxidant activity of different types of palm dates (Phoenix dactylifera) syrupsArabian J Chem72014964971
- M.Y.ShonS.D.ChoiG.G.KahngS.H.NamN.J.SungAntimutagenic, antioxidant and free radical scavenging activity of ethyl acetate extracts from white, yellow and red onionsFood Chem Toxicol422004659666
- M.C.LeeH.ShojiH.MiyazakiF.YoshinoN.HoriM.ToyodaAssessment of oxidative stress in the spontaneously hypertensive rat brain using electron spin resonance (ESR) imaging and in Vivo L-Band ESRHypertens Res272004485492