?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background of study
Plants used for traditional medicine contain a wide range of substances which can be used to treat various infectious diseases.
Aim
The study evaluated the in vitro antioxidant, antinociceptive, and anti-inflammatory activities of the methanolic extract of Justicia secunda Vahl leaf.
Methods
The acute toxicity was performed with up and down method and the highest dose used was 2 g/kg. The anti-inflammatory activity was evaluated using the carrageenan and formalin-induced paw edema models, and antinociceptive activity was evaluated using acetic acid-induced writhing reflex and tail flick test models while the antioxidant activity was evaluated using 2,2-diphenyl-2-picryl hydrazyl (DPPH) and ferric reducing antioxidant power (FRAP) photometric assay.
Results
The extract was well tolerated as no signs of toxicity or death were noticed during the period of observation. The extract produced a concentration dependent increase in antioxidant activities in both DPPH and FRAP models. The extract produced its optimum activity at 400 μg/ml in both DPPH (54.07%) assay and FRAP (1.58 μM) assay. The extract produced significant (P < 0.05) dose-dependent increase in both anti-inflammatory and antinociceptive activities. The antinociceptive and anti-inflammatory activities of the extract (0.4 g/kg) were comparable with the reference drugs (aspirin and pentazocine) used in the study.
Conclusion
This study suggests that J. secunda possesses anti-inflammatory, antinociceptive and antioxidant activities and also provide the pharmacological basis for its uses in traditional medicine for these purposes.
1 Introduction
Medicinal plants are the most common sources of drugs used in traditional medicine.Citation1 Despite improvement in health care delivery system, medicinal plants play a vital role in both human and animal health care systems and about 60% of world population depend on herbs for their primary health care.Citation2,Citation3 The reason for this may be due to high cost of orthodox drugs, side effects and unavailability of orthodox medicine and personnel in remote areas. Poverty and cultural inclination may have contributed to the trend.Citation3,Citation4 One of the diseases that are usually treated with herbal medicine is inflammatory conditions.Citation5,Citation6 Inflammation is a vascular tissue response to injury which involves chemical and cellular infiltration of the affected areas.Citation7 The cardinal signs of inflammation are pain, edema, loss of function, redness and heat.Citation8 Some herbs used in ethnomedicine in the treatment of inflammation are Taraxacum officinale Linn., Magnifera indica Linn., Crocus sativus Linn., Ocimum sanctum Linn.Citation9–Citation12
Justicia secunda Vahl belonging to the family Acanthaceae is known as “Blood root” and “Sanguinaria” in Barbados and Venezuela respectively.Citation13 The folkloric uses of the plant include wound healing, anemia and abdominal pain.Citation14,Citation15 In South-Eastern Nigeria, Congo and South Côte-d’Ivoire the leaf decoction is consumed by Jehovah’s Witness believers in the management of anemia.Citation16 The anti-sickling, haematinic, antimicrobial and anti-hypertensive activities of J. secunda have been reported.Citation13,Citation16–Citation18 Phytochemical screening of the plant has shown the presences of tannins, flavonoids, alkaloids, quinines and anthocyanins.Citation19 Luteolin, aurantamide acetate, auranamide, quindoline and pyrrolidone derivatives; secundarellone A, B and C have been isolated from J. secunda.Citation19–Citation21 Notwithstanding the popular uses of J. secunda in folkloric medicine only few pharmacological studies have been done on the plant.Citation22 This study investigated the antioxidant, anti-inflammatory, and antinociceptive effects of J. secunda leaf methanolic extract (J. secunda).
2 Materials and methods
2.1 Plant collection and identification
The leaves of J. secunda were collected from uncultivated farmlands located at Eastern parts of Nigeria in June 2015 and its botanical identification was authenticated by Dr. Garuba Omosun of the Department of Plant Science and Biotechnology of Micheal Okpara University of Agriculture, Umudike. A voucher specimen catalogued MOUAU/VPP/2015/14 was deposited in the departmental herbarium for reference purposes.
2.2 Preparation of J. secunda extract
The plant material J. secunda were air dried under shed in the Veterinary Physiology, Pharmacology, Biochemistry, Animal and production Laboratory of Michael University of Agriculture. It was later ground with manual milling machine (Corona, China). Two hundred grams (200 g) of the fine powder of J. secunda was weighed, using weighing balance (Mettler, Germany). The plant material was soaked with 80% of methanol in a winchester bottle. This was shaken every 3 h interval and allowed to stand for 48 h (2 days) at room temperature and then filtered using Whatman No. 1 filter paper into a beaker. The filtrate was concentrated at temperature of 40 °C with the use of an electric oven and the extract was stored in a refrigerator at 4 °C as J. secunda until when needed.Citation4
The percentage yield was calculated using the formula below:
2.3 Antioxidant study
2.3.1 Assessment of 2,2-diphenyl-2-picryl hydrazyl (DPPH) free radical scavenging activities of J. secunda
The antioxidant activity of J. secunda was evaluated using DPPH photometric assay.Citation23 The test extract (2 ml) at different concentrations (25, 50, 1, 200 and 400 μg/ml) was mixed with 0.5 mM DPPH (in 1 ml of methanol) in a cuvette. The absorbance at 517 nm was taken after 30 min of incubation in the dark at room temperature. The concentrations were prepared in triplicates and the percentage antioxidant activity was calculated as follows:
A volume of 1 ml of methanol plus 2.0 ml of the extract was used as the blank while 1.0 ml of the 0.5 mM DPPH solution plus 2.0 ml of methanol was used as the negative control. Ascorbic acid (vitamin C) was used as reference standard.Citation24
2.3.2 Ferric reducing antioxidant power (FRAP) of test
The ferric reducing antioxidant power was carried out as described by Benzie and Strain.Citation25
2.3.2.1 Reagent composition
| 1. | Acetate buffer (300 mM), pH 3.6 (3.1 g sodium acetate. 3H2O and 16 ml glacial acetic acid in 1000 ml buffer solution). | ||||
| 2. | 2,4,6-Triphridyl-s-triazine (TPTZ) (10 mM) in 40 mM HCl. | ||||
| 3. | FeCl3⋅6H2O (20 mM) in distilled water. | ||||
The working solution was prepared by mixing solution 1, 2, and 3 in the ratio of 10:1:1 respectively. The working solution was freshly prepared in test. The aqueous solution of known amount of ascorbic acid was used for calibration.
FRAP reagent (3 ml) and 100 μl sample solution at concentrations of 25, 50, 100, 200 and 400 μg/ml were mixed and allowed to stand for 4 min. Colorimetric reading was recorded at 593 nm, at 37 °C. The ascorbic acid standard was tested in parallel process. Calculations were made by a calibration curve.
2.4 Experimental animals
One hundred and twenty-six (126) Wistar albino rats of both sexes weighing 90–105 g, obtained from the laboratory Animal Unit of the Faculty of Veterinary Medicine, University of Nigeria, Nsukka, Enugu State, were used for the study. The animals were housed in aluminum cages at room temperature and under natural light/darkness cycles. They were supplied with clean drinking water and fed ad libitum with standard commercial pelleted feed (Vital feed® Nigeria). They were maintained in accordance with the recommendations of the Guide for the care and use of laboratory animals.Citation26 The rats were acclimatized for two weeks prior to the study. The experimental protocol was approved by the University Animal Ethics Committee with reference MOUAU/CVM/EAEC/2013/210.
2.5 Acute toxicity test
This study was carried out using the up and down method of acute toxicity.Citation27 Six rats were randomly selected, weighed and placed in a cage. Three rats were treated with 2 g/kg of plant extract while three other rats were given equal volume of distilled water, orally by gastric gavage. The rats were observed for 48 h for signs of toxicity and mortality.
2.6 Anti-inflammatory study
2.6.1 Carrageenan – induced paw edema
This was done using the method of Owoyele et al.Citation6 The rats were fasted overnight and had free access to water prior to the day of the experiment but were denied access to feed and water during the experiment. Thirty albino rats were weighed and randomly divided into five groups (A–E) of 6 rats per each. Group A was given 10 ml/kg of distilled water (negative control), group B was treated with 0.2 g/kg of acetylsalicylic acid (aspirin) (positive control) and groups C, D and E were treated with 0.1, 0.2 and 0.4 g/kg of the plant extract respectively. One hour after treatment, paw edema was induced by injecting 0.1 ml of 0.6% solution of carrageenan into the sub-plantar surface of the hind right paw. Their left paw volumes were measured using the volume displacement method, as control. Thereafter, the right paw volume was determined at 1, 2, 3, and 24 h post treatment.
2.6.2 Formalin-induced paw edema
The method described by Ezeja et al.Citation28 was used for this experiment. The rats were fasted overnight and were given free access to water and feed prior to the experiment. Thirty albino rats were weighed and randomly divided into five groups (A–E) of 6 rats per group. Group A was given 10 ml/kg of distilled water (negative control), group B was treated with 0.2 g/kg of acetylsalicylic acid (aspirin) (positive control) and groups C, D and E were treated with 0.1, 0.2 and 0.4 g/kg of the plan extract respectively. One hour after treatment, paw edema was induced by injecting 0.1 ml of 1.0% solution of formalin into the sub-plantar surface of the hind right paw. Their left paw volumes were measured using the volume displacement method, as control. Therefore, the right paw volume was determined at 1, 2, 3 and 24 h post treatment. The increase in paw volume = .
2.7 Antinociceptive study
2.7.1 Acetic acid-induced abdominal writhing in rats
The method described by Hajhashemi et al.Citation5 was used. Thirty rats were assigned to five groups (A–E) of 6 rats each and were fasted for 12 h but free access to tap water was allowed. Group A served as negative control and received distilled water (10 ml/kg). Group B served as positive control and received aspirin (0.2 g/kg), while Groups C–E received 0.1, 0.2 and 0.4 g/kg of J. secunda, respectively. The drug and extract were administered orally. 45 min post treatment, the rats received 10 ml/kg of 0.7% acetic acid intraperitoneally. The number of writhing or abdominal stretches produced in each rat was counted for the next 30 min.
2.7.2 Tail flick test in rats
The experiment was carried out as described by Adzu et al.Citation29 Thirty rats were assigned to 5 Groups (A–E) of 6 rats each and fasted for 12 h with free access to drinking water. Group A received distilled water 10 ml/kg orally (negative control), Group B received pentazocine 0.003 g/kg intraperitoneally (positive control), while Group C–E received J. secunda 0.1, 0.2 and 0.4 g/kg orally, respectively. One hour post drug treatment about 3 cm of the tail of each rat was dipped into a water bath containing warm water maintained at a temperature of 55 ± 1 °C. The time taken for the mouse to flick the tail known as the pain reaction time (PRT) was recorded for all the rats.
2.8 Data analysis
The results were presented as mean ± standard error of mean (SEM). Data obtained were analyzed using one way analysis of variance (ANOVA) and the variant means were separated by Least Significant Difference (LSD) of the different groups. Significance was accepted at the level of p < 0.05.
3 Results
3.1 Plant extraction
The yield of J. secunda extract was 10.7% w/w dry matter.
3.2 Antioxidant study
3.2.1 DPPH free radical scavenging activities of J. secunda
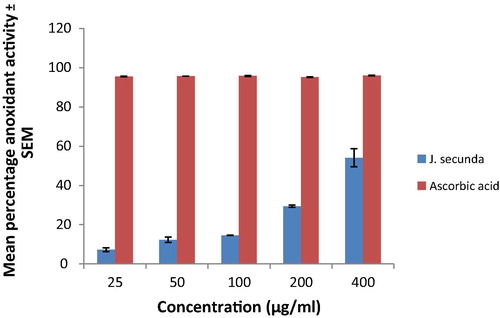
The result of the DPPH photometric assay of J. secunda is presented in . J. secunda extract caused a concentration dependent increase in percentage antioxidant activity increasing the antioxidant activity from 7.22% at 25 μg/ml concentrations to 54.07% at 400 μg/ml concentrations while ascorbic acid had 94.97% at 400 μg/ml concentrations.
3.2.2 Ferric reducing antioxidant power (FRAP) of J. secunda
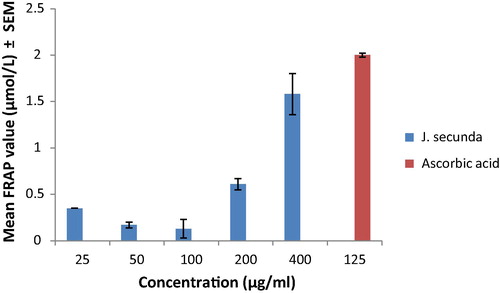
The result of the FRAP photometric assay of J. secunda is presented in . J. secunda extract caused a concentration dependent increase in FRAP value from 0.35 μM at the concentration of 25 μg/ml to 1.58 μM at the concentration of 400 μg/ml.
3.3 Acute toxicity test
Oral administration of 2 g/kg of J. secunda and an equal volume of distilled water produced no death or any sign of toxicity after 48 h.
3.4 Anti-inflammatory study
3.4.1 Formalin-induced paw edema
The results of J. secunda on the paw volume of formalin-induced paw edema in rats are presented in . The extract (0.1, 0.2 and 0.4 g/kg) and aspirin (0.2 g/kg) produced a significant (p < 0.05) dose-dependent decrease in the mean paw volume of the treated rats when compared to the negative control rats. The extract (0.4 g/kg) produced a significant (p < 0.05) time dependent decrease in the paw volume when compared to the negative control group. At the 3rd hour post treatment the Aspirin (0.2 g/kg), and extract 0.1, 0.2 and 0.4 g/kg produced 18%, 9%, 12% and 44% decrease in paw volume respectively, when compared to negative control.
Table 1 Effects of J. secunda on formalin induced paw edema.
3.4.2 Carrageenan-induced paw edema
The results of the J. secunda on the mean increase in paw volume of carrageenan induced paw edema in rats are presented in . The extract (0.1, 0.2 and 0.4 g/kg) and Aspirin (0.2 g/kg) produced a significant (P < 0.05) decrease in the mean paw volume of the treated rats when compared to the negative control rats. The extract (0.1 g/kg) produced a significant (P < 0.05) time dependent decrease in the paw volume when compared to the negative control group. At 24th hour post treatment the Aspirin (0.2 g/kg), and extract 0.1, 0.2 and 0.4 g/kg produced 43%, 45%, 57% and 60% decrease in paw volume respectively when compared to negative control.
Table 2 Effect of J. secunda on carrageenan-induced paw edema.
3.5 Antinociceptive study
3.5.1 Tail flick test
The results of the effects of J. secunda on tail flick test in rat are presented in . The extract produced dose-dependent increase in PRT of the treated rats. Pentazocine 0.003 g/kg and J. secunda 0.4 g/kg significantly (P < 0.05) increased the PRT of the treated rats when compared to negative control. Pentazocine 0.003 g/kg and J. secunda 0.4 g/kg caused 44.98% and 25.76% increase in PRT in treated rats respectively, when compared to the negative control group.
Table 3 Effects of J. secunda on tail flick test.
3.5.2 Acetic acid-induced writhing reflex
The results of the effects of J. secunda on acetic acid-induced writhing reflex in rats are presented in . The extract produced a significant (P < 0.05) dose-dependent reduction in the number of writhing reflex in the treated rats, when compared to the negative control group. Distilled water 10 ml/kg, aspirin and J. secunda 0.1, 0.2 and 0.4 g/kg caused 38.00, 22.00, 36.50, 29.00 and 13.75 mean number of writhing reflex in treated rats respectively.
Table 4 Effect of J. secunda on acetic acid-induced writhing reflex.
4 Discussion
The anti-inflammatory, antinociceptive and antioxidant capacity of methanolic extract of J. secunda were evaluated in this study. The extract was well tolerated by the rats as neither death nor signs of toxicity were observed in the rats dosed with 2 g/kg of J. secunda during the period of observation.Citation27 The LD50 of the extract is greater than 2 g/kg.
The extract demonstrated a potent anti-inflammatory, antinociceptive and antioxidant properties which may have been mediated by the phytochemical constituents.Citation19 Tannins have been documented to possess antinociceptive, anti-inflammatory and antioxidant activities.Citation9 The antinociceptive, anti-inflammatory activities and antioxidant capacity of flavonoids (luteolin) have been reported.Citation30–Citation32
The mechanism of the in vitro antioxidant effects of the extract may be through the scavenging of the reactive oxygen species (ROS) and in vivo may be via the inhibition of the ROS generating oxidases, enhancement of the endogenous antioxidant and/or direct inhibition of enzyme that catalyzes oxidation of cellular components.Citation32–Citation35 The antioxidant effects of the extract may be responsible for its anti-inflammatory and antinociceptive activities.Citation32
Carrageenan and formalin induce paw edema through the release of inflammatory mediators such as histamine, serotonin, protease, cytokine, lysosome and prostaglandins.Citation36,Citation37 The anti-inflammatory effect of the extract may be due to the inhibition of production and/or migration of these inflammatory mediators.Citation38–Citation40 Another possible mechanism of the anti-inflammatory and antinociceptive effects may be the inhibition of cyclooxygenase activities, just as the aspirin.Citation41 Cyclooxygenase catalyzes the biosynthesis of prostaglandins from arachidonic acid.Citation42 Again the mechanism of antinociceptive effects may be through the inhibition of pain perception, impulse transmission and/or elevation of pain threshold in the hypothalamus.Citation42 The antinociceptive activity of J. secunda is effective against both peripheral and deep pain sensation.
In conclusion, this study suggests that J. secunda possesses anti-inflammatory, antinociceptive and antioxidant activities and also provide the pharmacological basis for its uses in traditional medicine for these purposes. We recommend further studies to isolate and characterize the active compound(s) responsible for the activities.
Conflict of interest
The authors have to conflict of interest to declare.
Funding
No funding was provided for the study.
Acknowledgment
We appreciate the contribution of Dr. Garuba Omosun of the Department of Plant Science and Biotechnology of Micheal Okpara University of Agriculture, Umudike, for the identification of the plant material.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 1 July 2016
References
- E.A.SofoworaMedicinal plants and traditional medicine in Africa3rd ed.2008Spectrum Books Ltd.Nigeria
- U.F.EzuruikeJ.M.PrietoThe use of plants in the traditional management of diabetes in Nigeria: pharmacological and toxicological considerationsJ Ethnopharmacol1552014857924
- A.MiriJ.Sharifi-RadK.TabrizianA.A.NasiriAntinociceptive and anti-inflammatory activities of Teucrium persicum Boiss. extract in miceScientifica2015810.1155/2015/972827 Article ID 972827
- P.A.AkahC.E.OkoloA.C.EzikeThe haematinic activity of the methanol leaf extract of Brillantasia nitens Lindau (Acanthaceae) in ratsAfr J Biotechnol8200923892393
- V.HajhashemiS.E.SajjadiM.HeshmatiAnti-inflammatory and analgesic properties of Heracleum persicum essential oil and hydroalcoholic extract in animal modelsJ Ethnopharmacol1242009475480
- V.B.OwoyeleC.O.WuraolaA.O.SoladoyeS.B.OlaleyeStudies on the anti-inflammatory and analgesic properties of Tithonia diversifolia leaf extractJ Ethnopharmacol902004317321
- C.A.AnosikeO.ObidoaL.U.S.EzeanyikaThe anti-inflammatory activity of garden egg (Solanum aethiopicum) on egg albumin-induced oedema and granuloma tissue formation in ratsAsian Pac J Trop Med5120126266
- V.KumarA.K.AbbasN.FaustoRobbins and Cotran pathology basis of disease7th ed.2005Saunders ElseviersPhiladelphia
- H.HosseinzadehH.M.YounesiAntinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in miceBMC Pharmacol22002 <http://www.biomedcentral.com/1471-2210/2/7>
- H.JeonH.KangH.JungY.KangC.LimY.KimAnti-inflammatory activity of Taraxacum officinaleJ Ethnopharmacol11520088288
- G.GarridoD.GonzálezC.DelporteN.BackhouseG.QuinteroA.J.Núñez-SellésAnalgesic and anti-inflammatory effects of Mangifera indica L. extract (Vimang)Phytother Res1520011821
- R.RathoreS.JainAn experimental study of analgesic effect of medicinal plant Tulsi (Ocimum sanctum)Ethno Med7120132730
- S.CarringtonD.H.CohallM.Gossell-WilliamsJ.F.LindoThe antimicrobial screening of a Barbadian medicinal plant with indications for use in the treatment of diabetic wound infectionsWest Ind Med J6192012861864
- W.M.KonéA.G.KoffiE.L.BomissoF.H.TraBiEthnomedical study and iron content of some medicinal herbs used in traditional medicine in Cote D’ivoire for the treatment of anaemiaAfr J Tradit Complement Altern Med9120128187
- K.N’guessanK.H.KouassiD.OuattaraPlants used to treat anaemia, in traditional medicine, by Abbey and Krobou populations, in the South of Côte-d’IvoireJ Appl Sci Res20106201012911297
- P.T.MpianaK.N.N.NgboluaM.T.BokotaT.K.KasongaE.K.AtibuD.S.T.TshibanguIn vitro effects of anthocyanin extracts from Justicia secunda Vahl on the solubility of haemoglobin S and membrane stability of sickle erythrocytesBlood Transfus8201024825410.2450/2009.0120-09
- P.MandaD.P.AbrogouaC.BahiD.S.DanoG.GnahouiB.J.KablanEvaluation of the antihypertensive activity of total aqueous extract of Justicia secunda Valh (Acanthaceae)Afric J Pharm Pharmacol516201118381845
- P.T.MpianaM.T.BokotaM.B.L.NdjeleV.MudogoD.S.T.TshibanguK.N.NgboluaAntisickling activity of three species of Justicia from Kisangani (DR Congo): J. tenella, J. gendarussa and J. insularisInt J Biol Chem Sci46201019531961
- B.A.TheilerS.RevoltellaM.ZehlC.DanglL.O.E.CaisaJ.KoenigSecundarellone A, B, and C from the leaves of Justicia secunda VAHLPhytochem Lett102010cxxixcxxxii
- E.N.KoffiC.Le GuernevéP.R.LozanoaE.MeudecF.A.AdjéY.BekroPolyphenol extraction and characterization of Justicia secunda Vahl leaves for traditional medicinal usesInd Crops Prod492013682689
- A.I.CalderonA.HodelJ.WolfenderM.P.GuptaM.CorreaK.HostettmannLC–DAD–MS-based metabolite profiling of three species of Justicia (Acanthaceae)Nat Prod Res2715201313351342
- G.M.CorrêaA.F.C.AlcântaraChemical constituents and biological activities of species of Justicia – a reviewBrazil J Pharmacog2212012220238
- K.MishraH.OjhaN.K.ChaudhuryEstimation of antiradical properties of antioxidants using DPPH assay: a critical review and resultsFood Chem130201210361043
- E.O.IwalewaI.O.AdewaleB.J.AiwoT.ArogundabeA.OsinowoO.M.DaniyanEffects of Harungana madagascariensis stem bark extract on the antioxidant markers in alloxan induced diabetic and carrageenan induced inflammatory disorders in ratsJ Complement Integr Med512008118
- F.F.BenzieJ.J.StrainFerric reducing/antioxidant power assay: direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentrationMethods Enzymol29919991523
- J.W.WardJ.R.ElseaAnimal case and use in drug fate and metabolismR.G.EdwardL.H.JeanMethods and techniques1st ed.1997Markel DekkerNew York
- Organization for Economic Cooperation and Development. OECD guidelines for the testing of chemicals, acute oral toxicity – up-and down procedure. No. 425. Paris: Organization for Economic Cooperation and Development; 2008. [Online] Available from: <http://www.oecd-ilibrary.org/docserver/download/9742501e.pdf?expires=1437730561%26id=id%26accname=guest%26checksum=0B6C86B8F1B2E751997E5F54CFDF8F5E> [accessed on 5th May, 2015].
- M.I.EzejaY.N.OmehS.O.OnojaI.H.UkaonuAnti-inflammatory and antioxidant activities of the methanolic leaf extract of Cissus aralioidesAm J Pharmacol Sci31201516
- B.AdzuS.AmosC.WambebeK.GamanielAntinociceptive activity of Zizyphus spina-christi root bark extractFitoterapia722001344350
- M.BittarM.M.de SouzaR.A.YunesR.LentoM.F.DelleF.V.CechinelAntinociceptive activity of I3, II8-binaringenin, a biflavonoid present in plants of the guttiferaePlanta Med6620008486
- J.B.CalixtoA.BeirithJ.FerreiraA.R.SantosV.Cechinel FilhoR.A.YunesNaturally occurring antinociceptive substances from plantsPhytother Res142000401418
- Y.LinR.ShiX.WangShen H.LuteolinA flavonoid with potentials for cancer prevention and therapyCurr Cancer Drug Targets872008634646
- E.J.LienS.RenH.H.BuiR.WangQuantitative structure-activity relationship analysis of phenolic antioxidantsFree Radic Biol Med261999285294
- H.W.LeungC.L.KuoW.H.YangC.H.LinH.Z.LeeAntioxidant enzymes activity involvement in luteolin-induced human lung squamous carcinoma CH27 cell apoptosisEur J Pharmacol53420061218
- J.A.RossC.M.KasumDietary flavonoids: bioavailability, metabolic effects, and safetyAnnu Rev Nutr2220021934
- E.A.AsongalemH.S.FoyetS.EkooT.DimoP.KamtchouingAnti-inflammatory, lack of central analgesia and antipyretic properties of Acanthus montanus (Ness)J Ethnopharmacol9520046368
- G.N.SilvaF.R.MartinsM.E.MatheusInvestigation of anti-inflammatory and antinociceptive activities of Lantana trifoliaJ Ethnopharmacol1002005254259
- C.C.ChenM.P.ChowW.C.HuangY.C.LinY.J.ChangFlavonoids inhibit tumor necrosis factor-{alpha}-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-{kappa}B: structure–activity relationshipsMol Pharmacol662004683693
- Y.KumazawaK.KawaguchiH.TakimotoImmunomodulating effects of flavonoids on acute and chronic inflammatory responses caused by tumor necrosis factor alphaCurr Pharm Des12200642714279
- M.M.G.PinheiroS.B.O.FernandesC.E.FingoloF.BoylanP.D.FernandesAnti-inflammatory activity of ethanol extract and fractions from Couroupita guianensis AubletleavesJ Ethnopharmacol1462013324330
- M.S.BrandãoS.S.PereiraD.F.LimaJ.P.OliveiraE.L.FerreiraM.H.ChavesAntinociceptive effect of Lecythis pisonis camb. (lecythidaceae) in models of acute pain in miceJ Ethnopharmacol1462013180186
- B.MartinOpioid and non-opioid analgesicC.R.CharlesS.E.RobertModern pharmacology4th ed.1994Little Brown and CompanyBoston