 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Introduction
The decreased ovarian estrogen production that occurs at menopause, results in osteoporosis and climacteric manifestations, and decreases women’s quality of life. The hormone replacement therapy (HRT) is the primary treatment options but has been associated with increased oncogenic potential. The tissue selective estrogen complex (TSEC) is a novel therapy, partnering a selective estrogen receptor modulator (SERM) with one or more estrogens.
Aim
Our study was done to evaluate the potential relative estrogenic agonist activities of a SERM, raloxifene (RLX), when dosed alone and its antagonist activities when paired with conjugated estrogen (CE), as a TSEC and its potential use for the postmenopausal osteoporosis, vulvar/vaginal atrophy (VVA) in VCD induced menopausal rat model.
Material and methods
Female rats were dosed daily with 4-vinylcyclohexene diepoxide (VCD) (80 mg/kg/d, IP) for 15 days to induce ovarian failure, followed by one month free drug. VCD injected rats received 12 weeks of RLX, CE, or combined RLX/CE with 17β-estradiol (E2), vehicle treated groups used as positive and negative controls, respectively. The bone turnover markers (BTM) were measured. The uterotropic activity was assessed by the uterine index and peroxidase assay. Vaginal wet weight (wt.) and glycogen were measured to evaluate the vaginotropic effects. Uterine and vaginal (ER) protein levels were assayed.
Results
Our findings showed that the appropriate RLX/CE dose combination exhibits significant bone sparing with minimal vaginal stimulation and neutral uterine effect.
Conclusion
We can conclude that appropriate RLX/CE combination could effectively be a promising alternative for the prevention of postmenopausal osteoporosis and VVA with no oncogenic risk.
1 Introduction
The menopausal transition is characterized by gradual ovarian follicle depletion, with dramatic decrease in the ovarian-derived estradiol, results in multiple undesirable side effects including vasomotor instability, VVA, mood changes and a substantial increase in bone turnover, eventually osteoporosis and bone fractures with increased risk of cardiovascular and cerebrovascular complications.Citation1
Under menopausal hypoestrogenism, the tissues of the vulva and the lining of the vagina become thinner, drier, and less elastic, a condition known as VVA. Unlike vasomotor symptoms, VVA typically worsen without treatment and can significantly impact the quality of life.Citation2
Unlike the ovariectomy (OVX) model, which parallels with surgically-induced menopause in humans, VCD induced ovarian failure rat model provides a more physiologically relevant model, that resembles the natural and transitional menopause, in which there is a gradual rather than abrupt decline of sex hormones.Citation3
Although hormone replacement therapy (HRT) with exogenous estrogen may effectively offset the menopausal symptoms, some women are susceptible to hormone-dependent neoplasias.Citation4
CE is the most widely used estrogen for HT, comprised of estrone sulfate and at least ten other hormones. Estradiol is the most potent and natural human form of estrogen in premenopausal women, comprised of 17β-estradiol. The estrone (ER) binding potency is approximately ⅔ the affinity of estradiol to the ERα and about ⅓ the potency of estradiol for ERβ. Additionally, estrone and estradiol likely have differential potencies for membrane mediated actions.Citation4
Continued efforts to provide women with efficacious menopausal therapies have generated interest in the development of SERMs. Similar to estrogens, SERMs have been shown to bind to ERs with high affinity, despite lacking the estrogen steroid moiety and to regulate transcriptional events in a variety of target tissues.Citation5
Whereas estrogens typically exhibit ER agonist effects in all tissues, SERMs demonstrate mixed functional activity (ER agonist/antagonist) depending on the target tissue. Unfortunately, they do not provide relief from climacteric symptoms.Citation5
RLX is a second generation SERM. It is the only SERM actually approved internationally by the Food and Drug Administration (FDA) for the prevention and treatment of postmenopausal osteoporosis, with added benefit of preventing breast cancer.Citation6
Currently RLX is undergoing clinical investigation for the management of postmenopausal conditions associated with estrogen deficiency, as the most attractive SERMs with acceptable safety and tolerability.Citation7
To date, no SERM alone has been able to achieve an ideal balance of ER agonist and antagonist activity for an optimal menopausal therapy that would act as an ER agonist by preserving the positive estrogenic effects on bone, CNS and lipid metabolism, while acting as an ER antagonist by maintaining breast and endometrial safety. However, it may be possible to achieve optimal results based on the blended tissue-selective activities of a SERM and estrogens in a novel approach termed (TSEC).Citation8
The goal of the present study was to investigate the effects of combined RLX/CE, as TSEC, on the bone and reproductive organs in VCD-treated rats, after selection of the appropriate dose combination depending upon uterine weight.
2 Material and methods
Three-months-old albino female rats (weight: 250 ± 10 g) were purchased from the Faculty of Science, Tanta University. The rats were acclimatized to the controlled laboratory conditions for one week and appropriately housed, four per cage, at a constant temperature of 22 ± 2 °C and 12 h artificial light/dark cycle. All rats had free access to food pellets and water throughout the study period. All the animal experiments were conducted according to the guidelines established by the Research Advisory Ethical Committee of Faculty of Medicine, Tanta University, Egypt.
All experimental animals were injected with VCD, except control normal group. All treatments (obtained from Sigma, St Louis, MO, USA) were started, one month after induction of ovarian failure, continued for 12 week and were administered orally (by gavage) in a vehicle of 2%Tween 80/0.5% methylcellulose with the exception of E2, which was delivered I.P., dissolved in distilled water with 1% dimethyl sulfoxide (DMSO) and 0.1% Tween 20.Citation1
2.1 Chemical induction of ovarian failure
After acclimation, the occupational chemical 4-vinylcyclohexene diepoxide (VCD) (96% purity, Sigma–Aldrich, St Louis, MO) was administered at a dose of (80 mg/kg/day, IP; 5 times per week; for 15 days) dissolved in sesame oil (2.5 mL/kg), followed by a drug-free period of 30 days. The control group dosed with sesame oil (2.5 mL/kg). Body weights were checked twice weekly throughout the period of induction to adjust the VCD dose. VCD specifically causes gradual apoptotic cell death of primordial and primary follicles resulting in induced ovarian failure without evidence of systemic toxicity, resulting in an endocrine state that closely mimics the natural modeling the transition to menopause in women.Citation9
2.2 Vaginal smear
After the injection period was completed, the estrous cycles were monitored daily by obtaining vaginal smears and evaluating samples microscopically using a standard light microscope at 100× magnitude to ensure loss of cycling and validating ovarian failure. The rats are considered acyclic with persistent diestrus phase, which is dominated by leukocytes, while control animals showed regular estrous cycles.Citation9
2.3 Experiment I: determination of minimally efficacious CE dose
An initial study was conducted on, 80 female albino rats, to determine the appropriate CE dose to elicit an increase in uterine wet wt., a surrogate measure for an estrogenic stimulatory response. The VCD treated rats were randomly assigned into seven groups, 10 rats each, dosed with vehicle, 0.05, 0.5, 2.5, 5 or 10 mg/kg/d of CE and E2 (5 μg/kg/d) was included as a positive control, for 2 weeks. The minimal officious CE dose would then be used in the second experiment.
2.4 Experiment II: determination of the uterine minimum fully antagonist dose of RLX
This subsequent study was conducted on, 70 female albino rats, to determine the minimum fully effective antagonist dose on the uterine wt. for RLX. Three VCD treated groups, 10 rats each, were dosed with three doses of RLX (0.3, 3 and 10 mg/kg/d), combined with 0.5 mg/kg/d of CE for 2 weeks. The minimum fully antagonist RLX dose would then be tested in subsequent study for assessment of the effect on bone and climacteric symptoms.
2.5 Experiment III: to determine the relative estrogenic and antiestrogenic effects of RLX, on the bone and climacteric symptoms and its potential therapeutic effect, as a TSEC, when combined with CE
After establishment of the ovarian failure, fifty VCD treated female rats, divided into 5 groups, 10 rats each, Vehicle treated VCD, E2 treated VCD: E2 was given in a dose of 5 μg/kg/d, CE treated VCD: CE was given in a dose of 0.5 mg/kg/d, RLX treated VCD: RLX was given in a dose of 10 mg/kg/d and combined RLX and CE treated VCD groups, received RLX (10 mg/kg/d) and CE (0.5 mg/kg/d). All treatments continued for 12 weeks.
At the end of experiment III, all rats were weighed and their body weights were recorded. Each rat was individually housed in a metabolic cage without food for 24 h. A urine sample was collected and acidified with 2 mL of 1 mol/L HCl, stored at −20 °C until further analysis, and then all rats were sacrificed. Blood sample was collected, and then centrifuged at 2000g for 10 min. Serum samples were stored at −80 °C until further analysis.
2.5.1 Biochemical markers of bone metabolism
Serum osteocalcin (OC) level and Alkaline phosphatase (ALP) activity, sensitive biochemical markers of bone formation,Citation10 were measured using the rat osteocalcin ELISA kit (Biomedical Technologies Inc., Stoughton, USA) and Quanti-Chrome ALP assay kits (DALP-250, BioAssay Systems, CA, USA), according to the manufacturers’ instructions, respectively.
2.6 Biochemical markers of bone resorptionCitation10
2.6.1 Serum C-terminal telopeptide fragment of type I collagen C-terminus (CTX) level
It was determined using commercial Rat Laps ELISA kits (Nordic Bioscience Diagnostics, Herlev, Denmark).
2.6.2 Serum tartrate-resistant acid phosphatase 5b (TRACP 5b) level
It was determined using rat ELISA kits (IDS Ltd., Boldon, UK).
2.6.3 Urinary deoxypyridinoline (DPD) cross-links
DPD content as a bone absorption marker was determined by ELISA using a commercial kit (Metra, San Diego, CA, USA), and corrected for urinary creatinine measured by quantitative, colorimetric assay, and was expressed as nanomole (nmol) per milli mole (mmol) of urinary creatinine.
2.7 Utero- and vagino trophic assay, measured by
2.7.1 Uterine and vaginal wet weights
At the end of our study, all animals were sacrificed. The uterine and vaginal tissues were quickly dissected and weighed. Wet wts. were recorded in grams after removal of associated fat and luminal fluids. The standardized uterine index was calculated based on uterine wet wt. (mg)-to-body weight (g) ratios and multiplied by 100.Citation11
2.7.2 Uterine Eosinophil peroxidase assay
A marker enzyme for uterine growth, was extracted and assayed as described by Farley et al.Citation12
2.7.3 Estrogen receptor (ER)–binding assay in uterine and vaginal tissues
Uterine and vaginal tissues were minced and homogenized in 10 volumes of buffer A [25 mm Tris–HCl, 1.5 mm EDTA, 10 mm α-monothioglycerol, 10% glycerol, 10 mM sodium molybdate (pH 7.4)], and the homogenates were centrifuged at 105,000g for 60 min. The steroid-binding assay was performed with freshly prepared cytosol. [3H]E2 binding was measured using the dextran-coated charcoal absorption technique as described by Martel et al.Citation13 and Asselin et al.Citation14 The protein concentration of cytosol was determined using the method of Bradford with BSA as standard.Citation15
2.7.4 Vaginal glycogen assay
Glycogen content in the female reproductive tract is important for energy-consuming events, so it is a critical biomarker in normal and disease conditions.Citation16 Vaginal glycogen was estimated by the method of Hassid and Abraham,Citation17 as modified by Morales et al.Citation18
3 Statistical analysis
The results were expressed as mean ± standard deviation (SD). All experimental data were analyzed using one-way analysis of variance (ANOVA). Probability values (P) of <0.05 were considered to be statistically significant. Data were analyzed by GraphPad Prism software, release 4.0 (GraphPad Software, San Diego, CA).
4 The results
4.1 Experiment I: selection of the appropriate CE dose
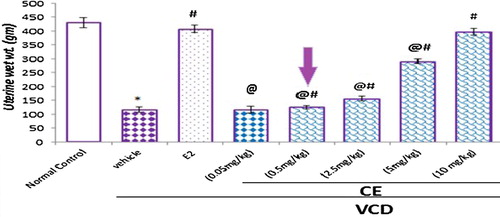
Using the lowest effective CE dose, that is better tolerated with fewer adverse effects, it remains a fundamental tenet of clinical practice and a valuable goal in the treatment of postmenopausal women. Based on the uterine wet wt., 0.05 mg/kg fails to increase the uterine wt., and demonstrated wt. statistically similar to the VCD group. Higher CE doses (0.5, 2.5, 5 and 10 mg/kg) used in the present study, resulted in dose dependent significant increase in uterine wet wt., compared to the VCD treated group. Minimal uterine stimulation was observed with CE 0.5 mg/kg and maximal was observed with CE 10 mg/kg, which was similar to that seen with E25 μg/kg. We chose to proceed with 0.5 mg/kg/d of CE given for 2 weeks as our estrogenic dose for future combination studies with RLX.
Figure 1 Selection of the appropriate CE dose. Effect of different doses of CE on uterine wet weight in VCD-treated rats. Data are represented as mean ± SD, of 10 rats in each group. Significance was considered at level of P < 0.05. *Significantly different from normal control group. #Significantly different from vehicle – treated VCD group. @Significantly different from E2 treated group.
4.2 Experiment II: selection of the minimum fully antagonist RLX dose
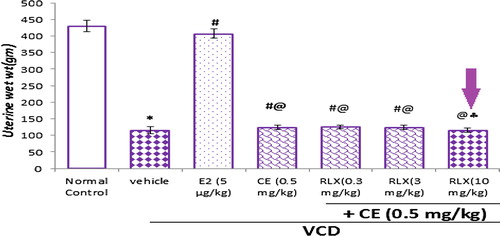
According to our results, RLX at doses, 0.3 and 3 mg/kg/d, failed to completely antagonize the CE induced uterine effect, and uterine wet wt. recorded significant increase compared to the VCD treated group, while 10 mg/kg/d RLX fully antagonized the CE-induced increase in uterine wet wt., lowering uterine wet wt. to level statistically similar to the vehicle. As a result, 10 mg/kg/d RLX was chosen for future studies.
Figure 2 Selection of the minimum fully antagonist RLX dose. Effect of different RLX doses on estrogen stimulated uterine weight. Data are represented as mean ± SD, of 10 rats in each group. Significance was considered at level of P < 0.05. *Significantly different from normal control group. #Significantly different from vehicle – treated VCD group. @Significantly different from E2 – treated VCD group. Significantly different from CE- treated VCD group.
4.3 Experiment III
4.3.1 Effect of the CE/RLX TSEC on bone turnover markers in VCD treated rats and
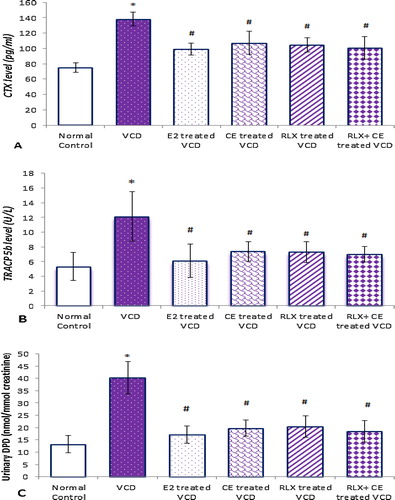
As in postmenopausal women, the VCD induced estrogen deficiency in rats produced a high turnover state, with accelerated bone loss. All bone resorping parameters, increased significantly in VCD-induced state, where serum CTX and TRACP 5b levels increased by about 1.9- and 2.3-folds respectively, along with increased urinary DPD by about 3.1-fold, in VCD -treated group compared to the control normal group.
Figure 3 Serum markers of bone formation in all studied groups. Data are represented as mean ± SD, of 10 rats in each group. Significance was considered at level of P < 0.05. *Significantly different from normal control group. #Significantly different from vehicle – treated VCD group. °Significantly different from E2 – treated VCD group.
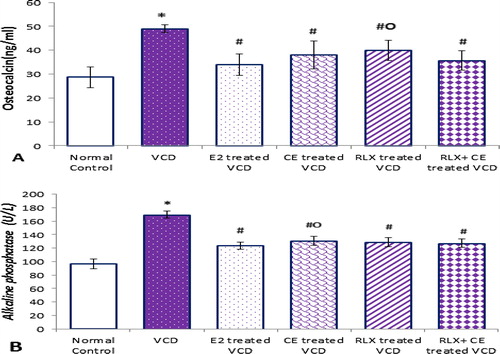
Figure 4 Bone resorping parameters in all studied groups. Data are represented as mean ± SD, of 10 rats in each group. Significance was considered at level of P < 0.05. *Significantly different from normal control group. #Significantly different from vehicle – treated VCD group.
The increased bone resorption observed in our model, was associated with significant elevation in the sensitive biochemical markers of bone formation, where serum OC and ALP levels, were increased by 1.7- and 1.8-folds, respectively, in VCD treated rats compared to the control normal group. It is obvious from our results that bone loss in the VCD treated rats parallels with the early skeletal changes observed immediately in postmenopausal women, who typically have a high rate of bone remodeling with bone resorption exceeding bone formation.Citation19
To evaluate the bone sparing effects, the CE (0.5 mg/kg/d) and RLX (10 mg/kg/d) treatments, were continued for 12 weeks, either alone or combined as TSEC, to VCD-treated rats.
The results of the current work showed that both CE and RLX treatments, either alone or combined, were associated with suppression of BTM to values statistically comparable to that observed in the positive control, which was suggestive of a full estrogen agonist effect of RLX on bone, an effect that was preserved when combined with CE.
4.3.2 The uterine effects of the CE/RLX TSEC in VCD treated rats
The additional important target site to investigate the potential application of appropriate RLX/CE dose combination for the postmenopausal women is the endometrial safety, so we investigated the effect of RLX and CE on estrogen sensitive uterine parameters.
Figure 5 The uterine parameters in all studied groups. Data are represented as mean ± SD, of 10 rats in each group. Significance was considered at level of P < 0.05. *Significantly different from normal control group. #Significantly different from vehicle – treated VCD group. °Significantly different from E2 – treated VCD group.
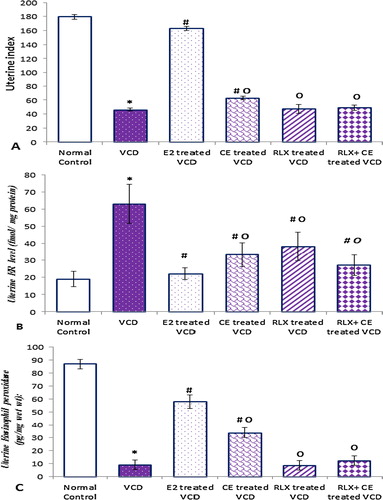
As obvious from our results, the VCD-induced estrogen deficiency, resulted in reduction in uterine index by about 74.1%, along with 89.4% reduction in uterine eosinophil peroxidase activity that was associated with compensatory increase in ERs protein level, compared to the control normal group.
In contrast to the full estrogen agonist activity of CE on endometrial profile in CE-treated VCD group, which recorded significant increase in estrogen sensitive uterine parameters, RLX alone resulted in distinct neutral uterine effects, with uterine index, eosinophil Peroxidase activity, statistically similar to the vehicle-treated VCD group. Uterine ER protein levels were significantly down regulated with both CE and RLX treated VCD groups.
Our findings characterize a novel phenotype of the endometrial markers of estrogen agonist activity, when RLX and CE combined appropriately, the RLX completely obliterated the CE – induced increase in uterine index and eosinophil Peroxidase activity, to levels statistically similar to the VCD group, with reduction in ER protein level, that remained statistically elevated relative to E2 treated VCD group.
4.3.3 The vaginal effects of the CE/RLX TSEC in VCD treated rats
The question to be addressed was whether the bone sparing effect of the combined RLX and CE could prevent the postmenopausal associated vaginal atrophy or could aggravate the condition, so we evaluated the effect of the RLX/CE TSEC on vaginal tissue.
Figure 6 The vaginal parameters in all studied groups. Data are represented as mean ± SD, of 10 rats in each group. Significance was considered at level of P < 0.05. *Significantly different from normal control group. #Significantly different from vehicle – treated VCD group. °Significantly different from E2 – treated VCD group.
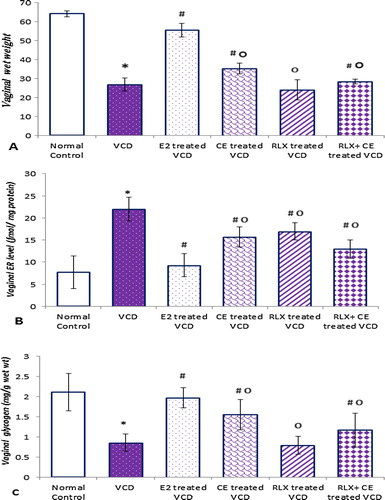
The VCD induced estrogen depletion, resulted in significant reduction in vaginal wet wt. and glycogen content of 26.89 ± 3.356, and 0.857 ± 0.2099 compared to 64.16 ± 1.567, and 2.11 ± 0.4675, in the control normal group respectively, associated with significant increase in vaginal ER (21.982 ± 2.721 versus 9.301 ± 2.627), compared to the control normal group.
In contrast to the estrogen agonist activity of CE on vaginal parameters in VCD group, presented in significant increase in vaginal wet wt., glycogen content, RLX alone resulted in obvious neutral vaginal effects, with vaginal wet wt., glycogen content, statistically similar to the VCD group. Both CE and RLX were accompanied by significant reduction in the vaginal ER levels compared to VCD group.
The RLX/CE TSEC preserved slight vaginotropic activity with significant increase in vaginal wet wt. and glycogen content (28.490 ± 1.247 and 1.171 ± 0.4202), compared to 26.89 ± 3.356 and 0.857 ± 0.2099, in VCD group respectively with significant decrease in vaginal ER level of 16.938 ± 1.929, compared to 21.982 ± 2.721, in VCD group.
5 Discussion
The TSEC approach is a new therapeutic strategy of the postmenopausal manifestations, based on the simultaneous but differential effects of each compound on ER activity.
This study highlighted the potential for an optimal RLX/CE combination that may provide the benefits of HRT with eliminated or reduced oncogenic potential, in an attempt to gain the benefits of each with better overall tolerability.
The high bone turnover, observed in VCD group, after estrogen withdrawal is responsible for bone loss, and has been claimed to be a risk factor in bone fractures.Citation10
Our study revealed drastic decrease in bone turnover in VCD treated groups, with CE, RLX, or both, reflecting an important reduction in bone remodeling and increased bone mineral density.Citation1
The mechanisms underlying the bone sparing effect of RLX/CE TSEC are still poorly understood. Recent reports reveal increasing complexity of these mechanisms that imply direct and indirect activities on bone cells.Citation20
The bone anabolic effect of both RLX and estrogen, through direct action on bone cells was previously confirmed in a concentration-dependent manner, and was blocked by the ER antagonist, ICI 164,384.Citation21
Emerging evidence has shown that inhibition of osteoclastogenesis is the main mechanism by which estrogen and RLX prevent bone loss, directly by potently suppressing osteoclast differentiation induced by the ligand of receptor activator of NF-кB (RANKL) and macrophage-colony stimulating factor (M-CSF), through the classical ER – and in dose-dependent manner.Citation22 These effects are mediated, at least in part, through down regulation and repression in the level and functional activity of c-fos and c-jun and osteoclast adhesion through modulating their mode of interaction with the bone microenvironment,Citation23 possibly by decreasing the expression of mRNA and protein of β3-integrin, in a time-dependent manner, which has been identified as major functional adhesion receptor in osteoclast.Citation24
Preclinical data, either in vivoCitation25 or in vitroCitation19 showed that RLX, similar to E2 could affect osteoclastic differentiation and activity, indirectly through reducing level of proinflammatory cytokines, especially IL-6 and TNF-α, and TGF-β1, and increased insulin-like growth factor-1 that originate from osteoblastic cells and have been implicated in the etiology of bone resorption. This explanation has been defined in a series of clinical studies in postmenopausal women.Citation26
Beside the anti-resorptive action of RLX/CE, it has osteoblast stimulatory role through increased osteoblast-specific Core binding factor 1 (Cbfa1)/runt-related transcription factor 2 (Runx2) and alpha2 procollagen type I chain mRNAs, that have been identified as “master genes” in osteoblastic differentiation.Citation27
It has also been shown that RLX and estrogen enhance osteoprotegerin (OPG) production from osteoblasts, as demonstrated in vitroCitation19 and in vivo.Citation28 OPG is a post-translationally glycolized protein that binds (RANKL) in competitive inhibition manner. Lowering RANKL to OPG ratio by RLX and estrogen is associated with enhanced osteoblastic activity, preserved bone mineral density, blocked osteoclastic differentiation and modulation of osteoclastic apoptosis, improving osteoporosis.Citation19
Gallant et al.,Citation29 have shown that RLX, similar to estrogen acts directly on the bone matrix to improve material properties, specifically the modulus of toughness, a measure of the ability of the tissue to absorb energy prior to fracture, independently of bone mass and architecture, elucidating another possible mechanism of RLX bone anabolic effect.
Another indirect delayed mechanism of the bone-restoring activity of RLXCitation26 and estrogenCitation28 could be attributed to change of calcium metabolism at the level of the intestine, kidneys and parathyroid, through suppression of parathyroid hormone and induction of 25-OH vitamin D.
Our results are consistent with series of earlier in vivo studies in both OVX animalsCitation30 and postmenopausal women withCitation31 and without osteoporosis,Citation25 where RLX, similar to estrogen has demonstrated its ability to increase bone density and decrease BTM and inhibit accelerated bone resorption.
Whereas RLX produced effects very similar to those of E2 and CE on bone, there is a striking difference in the effects of these compounds on uterine tissue, as RLX lacked uterotropic activity of estrogen, with neutral effects, when administered alone compared to the VCD control group.
The mechanisms of the tissue-selective action of RLX, although still only partly understood, can be explained based on the mounting evidence that the ER does not act in the same way in all target tissues and RLX has different affinities for different ERs subtypes,Citation28 leading to different conformations, different coactivator associations, different affinities for different gene promoters, yielding peculiar gene regulation, including estrogen metabolizing genes.Citation32
Our results provide an interesting initial step in profiling uterine effects of the RLX/CE TSEC, that protect the endometrium from the unopposed estrogenic stimulation, and would therefore provide endometrial protection without counteracting the positive effects of estrogens on bone, and vagina.
The mechanisms of action of RLX/CE TSEC are not clear and resolution of a specific mechanism to describe its activities is difficult as both components of the TSEC compete for the same ligand binding pocket on the ERs.
The favorable endometrial safety profile of RLX/CE TSEC, would be attributed to blocking of the estrogen-induced DNA transcription, through competitive binding of RLX to the ER,Citation20 that has been essentially attributed to the peculiar orientation and interaction of the critical alkylaminoethoxy RLX side-chain with the amino acid aspartate at position 351, present within the ligand binding domain of ER.Citation28 Moreover crystallographic studies have confirmed that the RLX-ER complex demonstrates that Helix 12 becomes reoriented and cannot seal the pocket containing the ligand, because RLX side-chain is large and inflexible, which blocks its possible interaction with activation factors-2 (AF-2) region, so that coactivators cannot form a transcription complex, and estrogen signal transduction is blocked. It appears that RLX more strongly recruits co-repressor proteins and consequently is still an antagonist in the uterus despite the higher concentration of co-activators relative to co-repressors.Citation33
RLX competitive antagonistic effect has been proved previously by Wardell et al.Citation34 who stated that E2 – induced transcriptional response is completely reversed by RLX, and that the expression level of genes in the presence of E2 together with RLX is not distinguishable from the basal level. Giner et al.Citation19 claimed that RLX may act, directly or indirectly at the post-transcriptional level, blocking the estrogen effects.
RLX component of TSEC could mediate its effects, like E2, through nongenomic actions, that are frequently associated with the activation of various protein-kinase cascades, independent of the ER-ligand complex induced nuclear transcription properties.Citation35
Although several data revealed that RLX’s action is ER mediated, non-ER-based mechanisms, cannot be ruled out as suspected by Bjornstrom et al.Citation35
To further demonstrate the underlying mechanism, through which the RLX or estrogen mediates their effects, uterine and vaginal ERs were assayed.
In the current study, ER protein levels in uterine tissue were downregulated in the presence of its cognate ligand, E2, and were significantly lower with RLX and RLX/CE compared to control VCD group. This finding suggests that RLX actions, similar to estrogen, are mediated through high affinity to ERs and may facilitate ubiquitarian and protea some-mediated ER degradation.Citation33
ERs have emerged as ligand-dependent transcriptional activator, whose expression is regulated by native E2, in a reciprocal manner. Estrogen-deficient animals express higher amounts of functional ER that may serve as a homeostatic mechanism to buffer against decreased estrogen levels. Moreover ERα mRNA increases in postmenopausal women who are not receiving hormone therapy, and decreases to levels similar to those found in premenopausal women, with systemic HRT.Citation11
Our results support a small body of prior evidence from cell culture,Citation20 experimental and clinical studiesCitation33 conducted on RLX and estrogen, demonstrating that RLX shows either neutrality or an estrogen antagonist, blocking the estradiol-induced effects in the uterus. Moreover RLX wasn’t associated with, and may even be effective at, preventing endometrial cancer.Citation33
Despite the fact that the vagina is a classic estrogen-responsive organ, it has received little attention compared to the adjacent uterus.
In our study, unlike the agonist effect of estrogen or neutral effect of RLX, the RLX/CE TSEC had unique vaginal profile, which supports its potential use for treating VVA in postmenopausal women.
Our results are in accordance with those obtained from preclinical experiments and clinical data showing that RLX has neutral effect on vagina.Citation2
Together with the slight but significant increase in vaginal wet wt., the RLX/CE TSEC treatment also resulted in an increase in vaginal glycogen content. Glycogen-consuming Lactobacilli can then colonize the vagina and lower the vaginal pH by catabolism of glycogen into lactic acid, generating an acidic environment, that plays an important role in maintaining the health of the lower genital tract in women.Citation2 The significant reduction in vaginal ER protein level in all treated VCD groups, indicating that RLX, similar to estrogen exerted ERs mediated effects.
Indeed, data obtained from randomized, double-blind clinical trials, support the possibility of safely use of RLX together with estrogen in postmenopausal women with vaginal atrophy,Citation5 where RLX did not alter the effects of the estrogen in alleviating genitourinary atrophy, and had no negative effects on sexual function.Citation6
6 Conclusion and recommendation
Data from our study demonstrated that a combined RLX/CE TSEC given at an appropriate dose may dominate the estrogen phenotype in bone with more favorable profiles in the endometrium and vagina and may be used as alternatives in future postmenopausal therapies. Further studies directly comparing different RLX doses and estrogen combinations are needed to identify the safest combination dosage for this purpose, that would demonstrate efficacy with minimal to no stimulation of the breast or endometrium, and elucidate the possible long term clinical advantages and side effects over presently available treatment modalities.
Conflict of interest
The authors declare that there are no conflict of interests.
Acknowledgments
We wish to acknowledge the contributions of Prof Dr/Karima El Disoky, for her great help in histopathological assessment of ovarian failure.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 9 July 2016
References
- D.W.LimY.T.KimAnti-osteoporotic effects of angelica sinensis (Oliv.) Diels extract on ovariectomized rats and its oral toxicity in ratsNutrients610201443624372
- A.DelmantoJ.Nahas-NetoE.A.NahasM.L.de OliveiraC.E.FernandesP.TraimanEffect of raloxifene on the vaginal epithelium of postmenopausal womenEur J Obstet Gynecol Reprod Biol1392008187192
- F.H.PottooM.BhowmikD.VohoraRaloxifene protects against seizures and neurodegeneration in a mouse model mimicking epilepsy in postmenopausal womanEur J Pharm Sci652014167173
- H.OhtaJ.SolankiIncorporating bazedoxifene into the treatment paradigm for postmenopausal osteoporosis in JapanOsteoporos Int262015849863
- J.V.PinkertonF.Z.StanczykClinical effects of selective estrogen receptor modulators on vulvar and vaginal atrophyMenopause2132014309319
- B.KesselL.NachtigallL.PlouffeS.SiddhantiA.RosenA.ParsonsEffect of raloxifene on sexual function in postmenopausal womenClimacteric632003248256
- S.FujiwaraE.HamayaM.SatoJ.A.FlynnR.BurgeSystematic review of raloxifene in postmenopausal Japanese women with osteoporosis or low bone mass (osteopenia)Clin Interv Aging9201418791893
- T.GoldbergB.FidlerConjugated estrogens/bazedoxifene (Duavee): a novel agent for the treatment of moderate-to-severe vasomotor symptoms associated with menopause and the prevention of postmenopausal osteoporosisPharm Ther4032015178182
- P.GeN.XingY.RenL.ZhuD.HanH.KuangPreventive effect of American ginseng against premature ovarian failure in a rat modelDrug Dev Res7582014521528
- G.WheaterM.ElshahalyS.P.TuckV.LaarThe clinical utility of bone marker measurements in osteoporosisJ Transl Med112013201
- H.R.MaJ.WangH.X.QiY.H.GaoAssessment of the estrogenic activities of chickpea (Cicer arietinum L) sprout isoflavone extract in ovariectomized ratsActa Pharmacol Sin342013380386
- D.B.FarleyS.P.FordJ.P.N.RosazzaIncrease in uterine peroxidase activity in the rat uterus during oestrogen hyperaemiaJ Reprod Fertil951992551558
- C.MartelL.ProvencherX.LiG.LeblancS.GauthierF.LabrieBinding characteristics of novel non-steroidal antiestrogens to the rat uterine estrogen receptorsJ Steroid Biochem Mol Biol641998199205
- J.AsselinP.A.KellyM.G.CaronF.LabrieControl of hormone receptor levels and growth of 7, 12-dimethylbenz(a)anthracene-induced mammary tumors by estrogens, progesterone and prolactinEndocrinology1011977666671
- M.M.BradfordA rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye bindingAnal Biochem721976248254
- J.AndersonA.PolitchJ.PudneyI.MarquezC.MauckA quantitative glycogen assay to verify use of self-administered vaginal swabsSex Transm Dis39122015949953
- W.Z.HassidS.I.AbrahamDetermination of glycogen and starchSPaKColowickNOMethods in enzymologyvol. 81957Academic PressNew York
- M.A.MoralesA.J.A.JabbagyH.P.TerenziMutations affecting accumulation of glycogenNeurospora News Lett20197324
- M.GinerM.J.RiosM.J.MontoyaM.A.VázquezC.MirandaAlendronate and raloxifene affect the osteoprotegerin/RANKL system in human osteoblast primary cultures from patients with osteoporosis and osteoarthritisEur J Pharmacol6502–32011682687
- M.S.Movérare-SkrticA.E.BörjessonH.H.FarmanK.SjögrenS.H.WindahM.K.LagerquistA.AnderssonC.OhlssonThe estrogen receptor antagonist ICI 182,780 can act both as an agonist and an inverse agonist when estrogen receptor α AF-2 is modified.Proc Natl Acad Sci USA113201411801185
- A.TarantaM.BramaA.TetiG.SperaD.AgnusdeiJ.D.TermineThe selective estrogen receptor modulator raloxifene regulates osteoclast and osteoblast activity in vitroBone3022002368376
- E.M.MessalliC.ScaffaLong-term safety and efficacy of raloxifene in the prevention and treatment of postmenopausal osteoporosis: an updateInt J Womens Health120101120
- D.SaintierM.A.BurdeJ.M.ReyT.MaudelondeM.E.Cohen-Solal17 beta-estradiol downregulates beta3-integrin expression in differentiating and mature human osteoclastsJ Cell Physiol19822004269276
- S.SchmidtI.NakchbandiR.RuppertN.KawelkeM.W.HessK.PfallerKindlin-3-mediated signaling from classes is required for osteoclast-mediated bone resorptionJ Cell Biol19252011883 multiple integrin 97
- R.JoseC.ReyE.V.CervinoM.L.RenteroM.CasillasRaloxifene: mechanism of action, effects on bone tissue, and applicability in clinical traumatology practiceOpen Orthop J320091421
- B.OzmenC.KirmazK.AydinS.O.KafescilerF.GucluInfluence of the selective oestrogen receptor modulator (raloxifene hydrochloride) on IL-6, TNF-alpha, TGF-beta1 and bone turnover markers in the treatment of postmenopausal osteoporosisEur Cytokine Netw1832007148153
- S.TakedaS.SakaiA.ShiraishiN.KoikeM.MiharaK.EndoCombination treatment with eldecalcitol (ED-71) and raloxifene improves bone mechanical strength by suppressing bone turnover and increasing bone mineral density in ovariectomized ratsBone5312013167173
- E.M.MessalliC.ScaffaG.MaininiA.CafieroP.L.SalzilloA.RagucciRaloxifene therapy interacts with serum osteoprotegerin in postmenopausal womenMaturitas56120073844
- M.A.GallantD.M.BrownM.HammondD.BlackD.JonathanStock Bone cell-independent benefits of raloxifene on the skeleton: a novel mechanism for improving bone material propertiesBone612014191200
- E.K.StuermerS.F.DaubM.KomrakovaM.TezvalK.M.StuermerRaloxifene supports early fracture healing more than estrogen in ovariectomized ratsOsteologie42013249328
- E.M.MessalliC.ScaffaG.MaininiA.CafieroP.L.SalzilloA.RagucciRaloxifene therapy interacts with serum osteoprotegerin in postmenopausal womenMaturitas56120073844
- P.HadjiTheevolutionofselectiveestrogenreceptormodulatorsinosteoporosistherapyClimactericv1562012513523
- S.MartinkovichD.ShahS.L.PlaneyA.JohnJ.A.ArnottSelective estrogen receptor modulators: tissue specificity and clinical utilityClin Interv Aging9201414371452
- S.E.WardellD.KazminD.P.Mc DonnellResearch resource: transcriptional profiling in a cellular model of breast cancer reveals functional and mechanistic differences between clinically relevant SERM and between SERM/estrogen complexesMol Endocrinol267201212351248
- L.BjornstromM.SjobergMechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genesMol Endocrinol192005833
