?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
The present study was designed to investigate the in vitro anti-inflammatory, antimicrobial, cytotoxic and antioxidant effects of Naproxen metal complexes.
Methodology
The anti-inflammatory activity was evaluated by HRBC membrane stabilization method while antimicrobial activity by disk diffusion method. The cytotoxicity was evaluated by brine shrimp lethality bioassay and compared with vincristine sulfate. Antioxidant potential was evaluated in terms of DPPH radical scavenging potential, ABTS scavenging potential, reducing power assay, superoxide dismutase assay and total antioxidant capacity by specific standard procedures.
Results
The Naproxen metal chelates showed significant anti-inflammatory effects in dose dependent manner. Naproxen standard showed maximum inhibition occurred 73.21% at the dose of 2000 μg/ml. Among Naproxen metal chelates, Naproxen silver complex showed potent antimicrobial activity against most of the tested microorganisms while Naproxen zinc complex showed better activity against gram positive strains than gram negative. In brine shrimp lethality bioassay, varying degree of lethality to Naproxen metal chelates was observed showing Naproxen iron complex surprisingly very potent cytotoxic activity compared to vincristine sulfate where other metal complexes displayed reduced cytotoxicity than parent Naproxen while Naproxen exhibited the lowest antioxidant assay among all the metal complexes compared to the standard ascorbic acid.
Conclusion
The present study demonstrated that Naproxen and its complexes possess in vitro anti-inflammatory activity while silver, zinc and iron complexes possess higher antimicrobial and cytotoxic properties than the parent ligand and possess very mild antioxidant activity.
1 Introduction
Recent findings on the chemical and biochemical activity of metal complexes play an essential role in agriculture, pharmaceutical and industrial chemistry.Citation1 In therapeutics, the use of metal complexes with traditional drugs as therapeutic agents for treatment of different diseases has been extensively studied.Citation2–Citation6 As they generally possess different mechanisms of activity from the organic compounds, the development of metal complexes provides an alternative route of novel drug delivery system.Citation7 Very recent works on metal complexes have proved that binding of a drug to metalloelement enhances its activity and in many cases the complex possesses even such activity that the parent compound does not have.Citation8 Thus we have motivated to study metal binding properties of Naproxen derivatives with different transition metal ions and analyzed its different biological properties for the sake of getting any new possibilities of using Naproxen metal complexes for different therapeutic purposes.
Our whole study was designed for understanding the most potential therapeutic activities achieved by formation of metal complexation in the most important biological fields such as inflammation, cytotoxicity, antimicrobial efficacy or efficiency and anti-oxidation reactions. We studied anti-inflammatory activity as there are many modes of anti-inflammatory actions of different transition metals when complexes with organic ligands.Citation9 Anti-inflammatory activity is measured in HRBC or erythrocyte membrane which is analogous to the lysosomal membrane and its stabilization implies that the synthetic compound may stabilize lysosomal membranes.Citation10 Stabilization of human red blood cell membrane (HRBC) by hypotonicity induced membrane lysis can be taken as an in vitro measurement of anti-inflammatory activity of the drugs. Cytotoxic properties are studied for the complexes which are considered as valuable tool for the screening of anti-tumor or anti-neoplastic agents under controlled in vitro conditions. We used brine shrimp lethality bioassay for cytotoxic studies as a preliminary screening technique to find out the potential toxicity profiles.
Multi-Drug Resistant (MDR) microorganisms are the great risk of health hazards in many countries for the today’s world. There is no doubt that metal complexes are highly well known for their anti-microbial activities for both resistant and non-resistant species. It is also clearly observed from many studies that anti-microbial activities of metal complexes sometimes possess enhanced activity than their conventional organic drugs itself because metal complexation may lead to varying degree of synergistic effect for either metal ion or ligand or for both.Citation11–Citation13 Thus, we used a vast range spectrum of microorganisms for determining the anti-microbial properties of synthesized compounds by disk diffusion method. Antioxidant activities are related to those compounds which are capable of protecting a biological system against the potential harmful effects of oxidative processes, therefore, making it important in medicine for the prevention and treatment of free radical pathologies.Citation9 It has received increased attention in the last years from nutritionists and medical researchers due to their potential chemical and molecular mechanisms in oxidative stress, DNA damage, protein modification, and enzyme activity with emphasis on the chemical and cell-free biological system.Citation14–Citation17
To the best of our knowledge and available literature on the subject no detailed research works have been done on all of these properties of Naproxen metal complexes. In the present study we report Naproxen metal complexes with there in vitro anti-inflammatory, cytotoxic, anti-microbial and antioxidant properties.
2 Material and methods
2.1 General procedure for synthesis of transition metal complexes of Naproxen
Equimolar metal salts dissolved in water were added to the sodium salt of Naproxen so that the ratios n(metal):n(ligand) of monovalent, divalent and trivalent ions used were 1:1, 1:2 and 1:3 respectively in each case and immediate precipitation was occurred. Then the solid complexes were isolated by filtration, washed with the corresponding solvent (water) and finally dried at room temperature.Citation18 The synthesized samples were freely soluble in different coordination solvents such as DMF, DMSO, THF and moderately soluble in chloroform, CCl4. However, they were insoluble in water, ethanol, and acetone.
2.2 Chemicals and reagents
All chemicals were obtained commercially and were of analytical grade. Sodium phosphate was collected from the Department of Clinical Pharmacy and Pharmacology, Faculty of Pharmacy, University of Dhaka, Bangladesh. All the solutions, reagents and buffers were prepared with distilled water. Vincristine sulfate, used as a standard drug in cytotoxicity assay was collected from the Techno Drugs Limited, Bangladesh. Dimethyl sulfoxide (DMSO) was purchased from Sigma-Aldrich, India. Sodium Chloride Crystal GR from Merck Ltd, Mumbai, India, was used to prepare seawater in brine shrimp lethality bioassay. 1,1-Diphenyl-2-picrylhydrazyl (DPPH), l-ascorbic acid, and gallic acid were purchased from Sigma Chemical Co. (St. Louis, USA). Naproxen was used as a standard drug.
2.3 In vitro anti inflammatory activity
2.3.1 Preparation of red blood cells
Human blood was collected from a donor not consuming any NSAIDS drugs for past two weeks. The blood was subjected to centrifugation and the supernatant part was carefully pipetted out with sterile pipettes. The packed cells were resuspended with equal volume of normal physiological saline (pH 7.4) and centrifuged again. The process was repeated five times until the supernatants were clear. A 10% HRBC suspension was then prepared with normal physiological saline and used immediately.Citation19
2.3.2 Membrane stabilizing activity assay
4.5 ml of reaction mixture consisting of 2 ml hypotonic saline (0.25% w/v NaCl), 1 ml of sodium phosphate buffer (0.15 M, pH 7.4) and 1 ml of metal chelates was dissolved in normal physiological saline. Then 0.5 ml of 10% HRBC was also added. Two controls were used, one with 1.0 ml of isotonic saline instead of metal chelates, and the second control with 0.5 ml of isotonic saline instead of red blood cells. The mixture was incubated at 56 °C for 30 min. The tubes were cooled under running water for 20 min and the mixture was centrifuged at 3000 rpm. The supernatants were separated and the absorbance of the supernatants was noted at 560 nm. The percentage membrane stabilizing activity was determined using the equation shown below.Citation20 Naproxen was used as standard.
Blood control represented 100% lysis or zero percent stability.
2.4 Collection of microorganisms
The microbial species used in the present study were Bacillus cereus, Bacillus subtilis, Sarcina lutea, Staphylococcus aureus, Bacillus megaterium, Escherichia coli, Salmonella paratyphi, Salmonella typhi, Shigella boydii, Shigella dysenteriae, Pseudomonas aeruginosa, Vibrio mimicus, Vibrio parahaemolyticus, Candida albicans, Aspergillus niger and Saccharomyces cerevisiae. These were collected as pure cultures from the Institute of Nutrition and Food Sciences, Dhaka University, Dhaka, Bangladesh.
2.5 Antimicrobial activity
A total of 16 reference microbial strains (five Gram-positive, eight Gram-negative and three fungi) were used as the test organism for the antimicrobial screening. The antimicrobial activity of metal chelates against the test organisms was performed by disk diffusion method using standard disk (5 μg/disk) for comparison.Citation21 Ciprofloxacin was used as the standard disk for comparing antibacterial and miconazole for antifungal activity. The test organisms were inoculated on 10 ml previously sterilized nutrient agar media, mixed thoroughly and transferred immediately to the sterile Petri dish under an aseptic condition using a sterile loop. The paper disks containing the samples and standard disk were placed to the corresponding Petri dish and were incubated at concentration of 106 CFU/ml for overnight at 37 °C. Clear zone of inhibition around the disks represented the presence of antimicrobial activity which was measured in millimeter (mm).
2.6 Determination of cytotoxicity
The cytotoxic potentiality of all Naproxen metal chelates was performed on brine shrimp nauplii using Mayer’s method.Citation22,Citation23 The eggs of brine shrimp (Artemia salina Leach) were collected from the Department of Clinical Pharmacy and Pharmacology, University of Dhaka and hatched in a tank containing 1 l of simulated seawater at a temperature around 37 °C and pH 8.4 with constant oxygen supply. Two days were allowed to hatch and mature the nauplii. About 4 mg of each sample was dissolved in DMSO and solutions with varying concentrations (400, 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.563, 0.781 μg/ml) obtained by serial dilution technique. The prepared test solutions were added to the pre-marked vials containing 10 live brine shrimp nauplii in 5 ml simulated seawater and incubated for 24 h. After incubation period, the vials were examined using a magnifying glass in order to count the number of survived nauplii in each vial. From this data, the lethality percent of the brine shrimp nauplii was calculated for each concentration. The median lethal concentration LC50 of each tested sample was calculated from the plotted graph of percentage of the shrimp mortality vs logarithm of the sample concentration, which was defined as the amount of sample required to kill 50% of brine shrimps within 24 h of exposure respectively.
2.7 Antioxidant activity
2.7.1 DPPH free radical scavenging activity
The method of KirubhaCitation24 was used for performing the DPPH radical scavenging activity. Serial dilutions from the stock solutions were carried out to obtain concentrations of 5, 10, 20, 40, 60, 80, 100, 250 and 500 μg/ml. An equal amount of the sample solution and 0.1 mM of solution of DPPH were mixed. The mixture was vortexed and allowed to stand in the dark at 25 °C for 30 min. After incubation, the absorbance of the mixture was read against a blank at 517 nm using a double beam Analykjena UV/Visible spectrophotometer (Model 205, Jena, Germany).
The radical scavenging activity was expressed as the inhibition percentage (I %) and calculated as per the following equation:where Ablank is the absorbance of the control (containing all the reagents except the testing compound) and Asample is the absorbance of the experimental sample with all reagents.
The IC50 value (the concentration of a sample required to scavenge 50% DPPH radical) was calculated from the plot of inhibition (%) against the concentration of the metal chelates. All determinations were carried out in triplicate and the average was noted. Ascorbic acid was used as the standard antioxidant.
2.7.2 ABTS radical scavenging activity
The antioxidant capacity of Naproxen and its derived complexes was determined by ABTS radical cation as described by Luo and coworkersCitation25, with some modifications. The ABTS radical cation was produced by reacting 7 mM ABTS solution with 2.45 mM potassium persulfate and the mixture was allowed to stand in the dark at room temperature for 16 h. The ABTS solution was diluted with sample 01 to an absorbance of 0.70 ± 0.02 at 734 nm. 1 ml of sample at different concentrations 5, 10, 20, 40, 60, 80, 100, 250, and 500 μg/ml was added to 1 ml of the ABTS solution and mixed vigorously. The reaction mixture was allowed to stand at room temperature for 6 min before the absorbance at 734 nm was recorded.
The ABTS scavenging effect was calculated as per the following equation:where
Ao = absorbance of control.
As = absorbance of sample.
The IC50 value (the concentration of a sample required to scavenge 50% ABTS radical) was calculated from the plot of inhibition (%) against the concentration of the metal chelates. All determinations were carried out in triplicate and the average was noted. Ascorbic acid was used as the standard antioxidant.
2.7.3 Reducing power assay
The reducing power of Naproxen and its derived complexes was determined according to the method followed by Fathi and co-workers.Citation26 Different concentrations of Naproxen and its derived complexes at 5, 10, 20, 40, 60, 80, 100, 250 and 500 μg/ml in 1 ml of distilled water were mixed with phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide [K3Fe(CN)6] (2.5 ml, 1%). The mixture was incubated at 50 °C for 20 min. A 10% solution of trichloroacetic acid (2.5 ml) was added to the mixture, which was then centrifuged at 3000 rpm for 10 min. The upper layer of the solution (2.5 ml) was mixed with distilled water (2.5 ml) and FeCl3 (0.5 ml, 0.1%) and the absorbance of the mixture was measured at 700 nm with spectrophotometer. Increased absorbance of the reaction mixture indicated increased reducing power. All the tests were carried out in triplicate and average of the absorptions was recorded. Ascorbic acid was used as the standard reference compound.
2.7.4 Superoxide radical scavenging activity
The superoxide anion scavenging activity was measured as described by Omwamba and coworkers.Citation27 The superoxide anion radicals were generated in 3.0 ml of Tris-HCl buffer (16 mM, pH 8.0), containing 0.5 ml of nitro blue tetrazolium (NBT) (0.3 mM), 0.5 ml reduced nicotinamide adenine dinucleotide (NADH) (0.936 mM) solution, 1.0 ml Naproxen and its derived complexes of different concentrations 5, 10, 20, 40, 60, 80, 100, 250 and 500 μg/ml and 0.5 ml Tris-HCl buffer (16 mM, pH 8.0). The reaction was started by adding 0.5 ml PMS solution (0.12 mM) to the mixture. The mixture was then incubated at 25 °C for 5 min before the absorbance was measured at 560 nm against a blank sample.
The percent inhibition was calculated by using the following equation:where
Ao is the absorbance of the control reaction.
A1 is the absorbance presence in Naproxen, its derived complexes and reference.
2.7.5 Total antioxidant capacity
The total antioxidant capacity of Naproxen and its derived complexes was evaluated by the phosphomolybdenum assay methodCitation28 which is based on the reduction of Mo (VI) to Mo (V) and the subsequent formation of a green phosphate-Mo (V) complex in acidic condition. The metal chelates were allowed to mix with 3.0 ml of the reagent solution (0.6 M H2SO4, 28 mM Na3PO4, 4 mM ammonium molybdate). The reaction mixture was incubated at 95 °C for 90 min. After letting the solution cool back to room temperature, the absorbance was measured at 695 nm using a UV-Visible spectrophotometer against a blank solution. The antioxidant activity was expressed as the number of gram equivalents of ascorbic acid.
2.8 Statistical analysis
All results were expressed as mean ± SEM of three parallel measurements. The differences between experimental groups were compared by one way ANOVA followed by Student’s t-test and were considered statistically significant when P < 0.001. Regression analysis was carried out for analyzing the data obtained from brine shrimp lethality bioassay.
3 Results
3.1 Characterization of metal complexes of Naproxen
Physical, analytical and thermal properties, NMR spectra, FTIR spectra, scanning electron microscopy and HPLC study of Naproxen metal complexes were described by Hasan.Citation18
3.2 In vitro membrane stability activity
The results of in vitro membrane stability activity of Naproxen metal complexes are shown in . The metal chelates showed significant anti-inflammatory activity in a concentration dependent manner. Incubation of the erythrocyte suspension with different concentrations of naproxen metal chelates gave significant anti-inflammatory properties. Naproxen standard showed 68.75% of membrane protection at the concentration of 100 μg/ml and maximum inhibition occurred 73.21% at the dose of 2000 μg/ml. On the other hand, among Naproxen metal chelates, Cobalt-Naproxen complex showed highest protection of RBC membrane stabilization i.e. 71.43%, 82.14% and 85.71% in the concentration of 100 μg/ml, 1000 μg/ml and 2000 μg/ml respectively. Almost same anti-inflammatory activity was shown by Iron-Naproxen complex where Zinc-Naproxen complex showed RBC membrane stability up to 76.79%.
Table 1 Results of in vitro anti-inflammatory activity of Naproxen metal complexes.
3.3 Antimicrobial activity
The results of different metal chelates of Naproxen with disk diffusion method are shown in . The antimicrobial activity of all test metal chelates was tested using concentration 400 μg/disk. Naproxen cobalt and silver complexes showed considerable antimicrobial activity on various microorganisms. Other test samples did not possess any antimicrobial activity against any of the microorganisms.
Table 2 Antimicrobial activity of Naproxen and metal complexes.
3.4 Cytotoxic activity
In brine shrimp lethality bioassay, percentage of mortality increased gradually with the increase in concentration of the test samples. The lethal concentration (LC50) of the test samples after 24 h was obtained by a plot of percentage of the shrimps died against the logarithm of the sample concentration (toxicant concentration) and the best-fit line was obtained from the curve data by means of regression analysis. Vincristine Sulfate (VS) was used as positive control and the LC50 for VS was found as 0.544 μg/ml. Compared with the negative control, VS (positive control) gave significant mortality.
In brine shrimp lethality bioassay, varying degree of lethality to Naproxen metal chelates was observed with exposure to different dose levels of the test samples ( and ). Copper, cobalt, silver and zinc complexes showed reduced cytotoxicity compared to parent Naproxen whereas Naproxen iron complex with the LC50 value of 0.205 μg/ml displayed greater activity compared to the Vincristine Sulfate (LC50 value 0.544 μg/ml). Naproxen iron complex showed very potent cytotoxic activity than standard and thus further other ways of cytotoxicity study would be performed in future.
Table 3 Percent of mortality of the nauplii of Naproxen metal complexes.
Table 4 LC50 values of the test samples of Naproxen metal chelates.
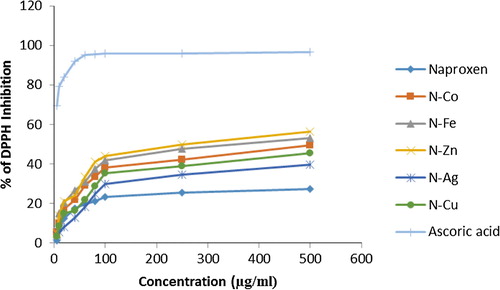
3.5 DPPH free radical scavenging activity
The percentage of DPPH neutralization of Naproxen metal and its complexes were found to be concentration dependent. Naproxen Iron complex produced the maximum free radical scavenging activity and Naproxen was found to produce weakest free radical scavenging activity while compared to that of reference antioxidant of the test. represents the percentage of DPPH neutralization activity of all complexes considered in the study.
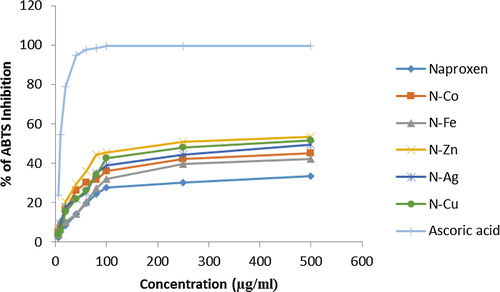
3.6 ABTS radicals scavenging activity
The percentage of ABTS neutralization of Naproxen metal and its derived complex were found to be concentration dependent. Naproxen Iron complex produced the maximum free radical while Naproxen metal produced weakest free radical scavenging activity. represents the percentage of ABTS neutralization activity of all complexes considered in the study.
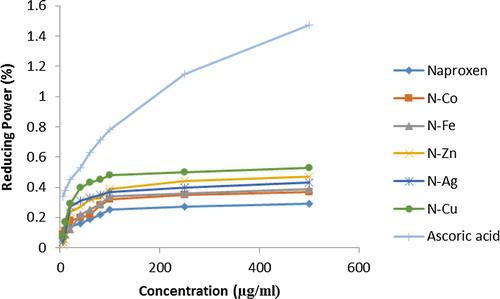
3.7 Reducing power assay
The reducing power was also found to be concentration dependent. Here, Naproxen Copper complex provided the most intense reducing power with value of 0.33 ± 0.005 at 500 μg/ml concentration, and Naproxen metal produced the least reducing power assay. represents the reducing power assay of all complexes in the study.
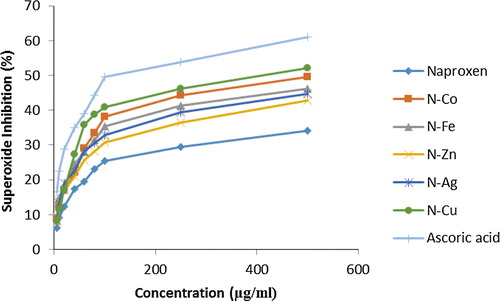
3.8 Superoxide dismutase radical scavenging activity
The superoxide dismutase radical scavenging activity of Naproxen metal and its complex were found to be concentration dependent. Like percentage of DPPH neutralization activity, Naproxen iron complex provided most superoxide scavenging activity with the value of 53.21 ± 0.19 μg/ml concentration and Naproxen metal produced least superoxide scavenging activity. represents the reducing power assay of all complexes in the study.
3.9 Total antioxidant capacity
Total antioxidant capacity of all complexes is expressed in . The most powerful antioxidant activity was recorded in Naproxen iron complex which amounted to 1.54 ± 0.04 mg AAE equivalents/g of dry weight while Naproxen metal was found to provide the least antioxidant activity which amounted to 0.81 ± 0.01 mg AAE equivalents/g of dry weight.
Table 5 Total antioxidant capacity of Naproxen metal and its derived complexes.
4 Discussion
Almost all Naproxen metal complexes showed prominent anti-inflammatory activity. However, in case of Naproxen Copper complex and Naproxen Silver complex, there was a decrease in stability of RBC membrane. It was due to the fact that Copper and Silver made complex with the released hemoglobin thus altering the absorbance value. The reaction of hemoglobin (Hb) with copper (II) complex was studied in phosphate buffer by ultraviolet-visible spectrophotometry.Citation29 The result showed that the interaction of Hb and Cu(II) complex produced complex with its maximum absorption peak at 537 nm. At the maximum absorption, the composition of the complex was determined to be n(Hb):n(Cu(II)) = 1:4. A preliminary investigation elucidated the reaction mechanism that the Hb and Cu(II) complex was combined mainly by electrostatic attraction.
As the present study also carried out at 560 nm, it was predicted that Cu(II) ions present in the Naproxen copper complex were combined by electrostatic attraction with hemoglobin thus providing significantly higher absorbance than any other samples.
The interaction as well as the formation of a bioconjugate of human hemoglobin (Hb) with silver (Ag) was also reported recently. The UV-Vis study demonstrated the perturbation of the heme band and generated conformational heterogeneity within the heme group in the presence of silver FTIR spectra indicated alpha-helix to beta-sheet conversion, and unfolding of Hb was also responsible for the bioconjugate formation.Citation30 The overall data showed that there was a change in the secondary as well as the tertiary structure of Hb after conjugation with silver. For these reasons, Naproxen copper and silver complex showed no feasible anti-inflammatory activity in vitro test.
Naproxen silver complex showed potent antimicrobial activity against most of the tested microorganisms compared to other complexes. Naproxen zinc complex showed better activity against gram positive strains than gram negative. Naproxen iron complex showed potent antimicrobial activity against gram negative E. coli than other strains. Naproxen copper and cobalt complexes had variable moderate activity against few microorganisms. According to Hard–soft acid base theory (HSAB theory) soft acids such as Ag(I) and borderline acids (such as Co(II), Cu(II) and Zn(II)) tend to associate tightly with soft bases, such as the sulhydryl (R-SH) groups that are found in proteins. Consequently, the antibacterial toxicity of these metals is approximately proportional to their affinity for S atom.Citation31–Citation33
Naproxen iron complex showed surprisingly very potent cytotoxic activity compared to vincristine sulfate while other metal complexes displayed reduced cytotoxicity than parent Naproxen. Antimicrobial and cytotoxic activity of iron obtained here demonstrated that iron had inhibitory effects on bacterial and cellular growth. These findings in agreement with results obtained from other studies of electron microscopy suggested that the integrity of the cytoplasmic membrane was severely compromised by exposure to toxic doses of iron.Citation34,Citation35 Based on these results we predicted that there might be any antioxidant activity of these compounds; however, there was nothing at all.
As Naproxen exhibited the lowest antioxidant property, the metal complexes had better antioxidant activity than Naproxen, however, still lower compared to the standard ascorbic acid. As a result, this study provides evidence that the chelation of different metal complexes with Naproxen had significant influence on the antioxidant activities in different in vitro model systems. Similar activity was also observed in other works related to transition metal complexes where significant antioxidant activity was found. This was due to the reaction capability of the metal complexes with the free radicals or ions present in the system. In was observed that almost all metal complexes showed the same plateau and the inhibition level of DPPH, ABTS and superoxides was around 40%. As we mentioned before that the metal complexes had better antioxidant activity than Naproxen, we assumed that the antioxidant activity solely came from the metal portion of the complexes. Naproxen structure might not participate in such activity. Furthermore, their antioxidant activity was almost same indicating that there was no any significant difference in mechanism of antioxidant activity because all metals were surrounded by the same ligand making a similar coordination sphere. The extent of inhibition activity was similar to all metals probably due to the same level of reaction with the free radicals or ions regardless of different metal complexes and coordination numbers. Moreover, in vitro anti-oxidation methods are not suitable for determining anti-oxidant properties of Naproxen metal complexes as the samples were too much difficult to dissolve. This is why our results might not be the proper indication for the anti-oxidant properties of these metal complexes. We would strongly suggest to perform in vivo experiment for antioxidant activity determination for Naproxen metal complexes. Hence further works on in vivo antioxidant activity can be done using different transition metals in search of new metal complexes with potent antioxidant activity in future.
5 Conclusion
From these investigations, it may be concluded that Naproxen metal chelates showed significant in vitro anti-inflammatory activity while Naproxen cobalt and silver complexes showed moderate antimicrobial activity against some tested microorganisms and Naproxen iron complex showed potent antitumor activity compared to standard but displayed no antioxidant properties. Therefore, for these compounds in vivo tests should be performed to study the anti-inflammatory activity of these samples as well as further research is essential to find out the possible side effects that may provide because of central metal of chelation and its principles responsible for such activity.
Conflict of interest
We declare that we have no conflict of interest.
Acknowledgment
The authors would like to acknowledge Head of the Department of Clinical Pharmacy and Pharmacology, Faculty of Pharmacy, University of Dhaka, Dhaka, Bangladesh, for providing chemicals, laboratory facility and moral support to carry out this research.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 1 July 2016
References
- I.P.KostovaI.I.ManolovI.N.NicolovaN.D.DanchevNew metal complexes of 4-methyl-7-hydroxycoumarin sodium salt and their pharmacological activityIL Farmaco562001707713
- I.SheikhshoaieA.BadieiM.GhazizadehSynthesis and spectroscopic studies of two new complexes containing Fe (III) and Mo (VI) of two tridentate ONO donor sets ligandsDer Chemical Sinica31201224
- S.BorhadeSynthesis, characterisation and spectrophotometric determination of Fe(II) complex of 2,4-dihydroxybenzaldehyde isonicotinoyl hydrazone {(E)-N′-(2,4-dihydroxybenzylidene) isonicotinohydrazide}, it’s application & biological activityDer Chemical Sinica2420116471
- S.I.HabibM.A.BaseerP.A.KulkarniSynthesis and antimicrobial activity of cobalt (II), nickel (II), and copper(II) complexes of some 2′-hydroxychalconesDer Chemical Sinica2120112732
- A.S.MundeV.A.ShelkeS.M.JadhavA.S.KirdantS.R.VaidyaS.G.ShankarwarSynthesis, characterization and antimicrobial activities of some transition metal complexes of biologically active asymmetrical tetradentate ligandsAdv Appl Sci Res312012175182
- A.SabastiyanM.Y.SuvaikinSynthesis, characterization and antimicrobial activity of 2-(dimethylaminomethyl)isoindoline-1,3-dione and its cobalt(II) and nickel(II) complexesAdv Appl Sci Res3120124550
- I.KostovaG.MomekovNew zirconium (IV) complexes of coumarins with cytotoxic activityEur J Med Chem4162006717726
- F.A.AdekunleJ.A.O.WoodsO.O.E.OnawumiO.A.OdunolaSynthesis and characterization of nickel(II) complexes of various substituted acid hydrazidesAsian J Chem227201055435550
- S.K.BhartiS.K.SinghMetal based drugs: current use and future potentialScholars Res Libr1220093951
- C.T.ChouThe antiinflammatory effect of an extract of Tripterygium wilfordii Hook F on adjuvant-induced paw oedema in rats and inflammatory mediators releasePhytother Res111997152154
- A.A.El-SheriffT.M.EldebssSynthesis, spectral characterization, solution equilibria, in vitro antibacterial and cytotoxic activities of Cu(II), Ni(II), Mn(II), Co(II) and Zn(II) complexes with Schiff base derived from 5-bromosalicylaldehyde and 2-aminomethylthiopheneSpectrochim Acta Part A Mol Biomol Spectrosc795201118031884
- A.R.ShaikhR.GiridharM.R.YadavBismuth-norfloxacin complex: synthesis, physicochemical and antimicrobial evaluationInt J Pharm3321–220072430
- W.GuerraE.de A. AzevedoA.R.de S. MonteiroM.B.RodriguezE.C.SouzaA.M.A.NascimentoSynthesis, characterization, and antibacterial activity of three palladium(II) complexes of tetracyclinesJ Inorg Biochem9912200523482354
- B.N.AmesM.K.ShigenagaT.M.HagenOxidants, antioxidants, and the degenerative diseases of agingProc Natl Acad Sci USA9017199379157922
- M.G.HertogE.J.FeskensP.C.HollmanM.B.KatanD.KromhoutDietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly StudyLancet3428878199310071011
- N.J.TempleK.K.GladwinFruit, vegetables, and the prevention of cancer: research challengesNutrition1952003467470
- U.PetersM.F.LeitzmannN.ChatterjeeY.WangD.AlbanesE.P.GelmannSerum lycopene, other carotenoids, and prostate cancer risk: a nested case-control study in the prostate, lung, colorectal, and ovarian cancer screening trialCancer Epidemiol Biomarkers Prev1652007962968
- M.S.HasanR.KayeshF.BegumS.M.A.RahmanTransition metal complexes of naproxen: synthesis, characterization, forced degradation studies, and analytical method verificationJ Anal Method Chem201620153560695
- O.O.OyedapoA.J.FamurewaAntiprotease and membrane stabilizing activities of extracts of Fagara zanthoxyloides, Olax subscorpioides and Tetrapleura tetrapteraInt J Pharmacogn3319956569
- N.DasD.GoshwamiM.S.HasanZ.A.MahmudS.Z.RaihanM.Z.SultanEvaluation of antinociceptive, anti-inflammatory and anxiolytic activities of methanolic extract of Terminalia citrina leavesAsian Pac J Trop Dis5Suppl. 12015S137S141
- A.W.BauerW.M.KirbyJ.C.SherriesM.TruckAntibiotic susceptibility testing by a standardized single disk methodAm J Clin Pathol4541966493496
- M.S.HossainM.A.HossainR.IslamA.H.M.K.AlamK.ZahanS.SarkarAntimicrobial and cytotoxic activities of 2-aminobenzoic acid and 2-aminophenol and their coordination complexes with Magnesium (Mg-II)Pak J Biol Sci7120042527
- M.A.IslamM.A.SayeedM.A.IslamG.R.KhanM.A.MosaddikM.S.BhuyanTerpenes from bark of Zanthoxylum budrunga and their cytotoxic activitiesRev Latinoamer Quim30120022428
- T.S.V.KirubhaM.JegadeesanS.KavimaniEvaluation of antioxidant property of Desmodium gangeticum and Pseudarthria viscida rootsJ Chem Pharma Res512013367370
- A.LuoA.LuoJ.HuangY.FanPurification, characterization and antioxidant activities in Vitro and in Vivo of the polysaccharides from Boletus edulis BullMolecules17201280798090
- H.FathiM.A.EbrahimzadehAntioxidant and free radical scavenging activities of Hypericum perforatum L. (St. John’s wort)Int J For Soil Erosion3220136872
- M.OmwambaF.LiG.SunQ.HuAntioxidant effect of roasted barley (Hordeum vulgare L.) grain extract towards oxidative stress in Vitro and in VivoFood Nutr Sci42013139146
- K.L.RaghuC.K.RameshT.R.SrinivasaK.S.JamunaTotal antioxidant capacity in aqueous extracts of some common vegetablesAsian J Exp Biol Sci2120115862
- X.H.WuJ.G.MiaoY.Q.MiaoJ.R.ChenStudy on the interaction of hemoglobin and Cu(II)-ARS complexGuang Pu Xue Yu Guang Pu Fen Xi276200711681171
- M.MahatoP.PalT.KamilyaR.SarkarA.ChaudhuriG.B.TalapatraHemoglobin-silver interaction and bioconjugate formation: a spectroscopic studyJ Phys Chem B114201070627070
- M.L.WorkentineJ.J.HarrisonP.U.StenroosH.CeriR.J.TurnerPseudomonas fluorescens’ view of the periodic tableEnviron Microbiol102008238250
- D.H.NiesEfflux-mediated heavy metal resistance in prokaryotesFEMS Microbiol Rev272003313339
- J.J.HarrisonH.CeriR.J.TurnerMultimetal resistance and tolerance in microbial biofilmsNat Rev Microbiol52007928938
- G.A.HarrisonK.A.DawsonR.W.HemkenmEffects of high iron and sulfate ion concentrations on dry matter digestion and volatile fatty acid production by ruminal microorganismsJ Anim Sci704199211881194
- S.ChamnogpolW.DodsonM.J.CromieZ.L.HarrisE.A.GroismanFe (III) mediated cellular toxicityMol Microbiol4532002711719