Abstract
Objective
To evaluate the potential role of sphingosine-1-phosphate receptor agonist, fingolimod (FTY-720), in rat I/R induced testicular injury and its possible underlying protective mechanisms.
Experimental protocol
Thirty male albino rats were categorized into three groups, 10 rats each: sham operated control group, Ischemia/Reperfusion (I/R) group: underwent torsion/detorsion procedures of the left testes and FTY720 treated (I/R+FTY720) group: received single FTY720 injection (at a dose of 4 mg/kg i.p) 30 min. before detorsion. At the end of the ischemia and reperfusion period (4 h) left testes were dissected carefully in all rats for histological and biochemical investigations. Testicular malondialdehyde (MDA), testicular antioxidant enzymes and testicular sphingolipids were assessed.
Results
FTY720 treatment of I/R group resulted in significant reduction in I/R induced elevation in testicular GSH-Px, SOD, MDA and total ceramide level, along with significant increase in testicular catalase, sphingomyelin and sphingosine-1-phosphate. Also, FTY720 treatment of I/R group improved severe testicular injury changes in I/R group.
Conclusion
FTY treatment may be used as potential therapeutic treatment for I/R induced testicular injury.
1 Introduction
Testicular torsion (TT) is a serious medical emergency that is mostly encountered in newborn and adolescent males but it can occur in any age and needs urgent surgical intervention.Citation1 TT is defined as a rotation of the longitudinal axis of the spermatic cord resulting in obstruction of testicular blood flow.Citation2
Torsion/detorsion is a typical condition of ischemia reperfusion in which torsion comprises the ischemic period whereas detorsion occupies the reperfusion period which is the main cause of testicular damage.Citation3 TT causes testicular injury, leading to potential serious sequelae of infertility and subfertility; thus, immediate diagnosis is important for testicular salvage to avoid testicular atrophy, infarction and infertility.Citation4
Although the main pathological mechanisms of testicular injury following TT are not completely understood, the overproduction of reactive oxygen species (ROS) generated during the ischemia reperfusion (I/R) process has been implicated as one of the main factors in cellular and tissue damage.Citation5
Fingolimod (FTY720) (2-amino-2-[2-(4-octylphenyl) ethyl]-1, 3-propanediol hydrochloride) is synthetically derived from myriocin, a metabolite isolated from ascomycete, Isaria sinclarii.Citation6 FTY720 was demonstrated to prolong allograft survival in several solid organ transplant animal models.Citation7 The possible mechanism of its immunomodulation was related to sequestration of peripheral blood lymphocytes to secondary lymphoid organs and selective induction of infiltrated lymphocytes apoptosis.Citation8 FTY720 has been found to be able to prevent I/R injury in liver and kidney models under a similar mechanism.Citation9 In vivo, FTY-720 is phosphorylated (FTY-720-P) by sphingosine kinase to form a potent sphingosine 1-phosphate (S1P) analog. S1P, found in high levels in blood, is produced by phosphorylation of sphingosine and is a pleiotropic lysophospholipid mediator of a wide variety of biological processes through binding to a family of G protein-coupled receptors: S1P1–5.Citation10 S1P is released by mononuclear phagocytes and platelets and binds to S1P1–5 in nanomolar affinities. FTY-720 interacts with S1P1, S1P3, S1P4, and S1P5, but not S1P2 receptorsCitation11; thus, the receptor subtype-mediating tissue protection following FTY-720 administration is not known.
Ceramide accumulation has been demonstrated in various in vivo models of IR and it has been implicated as an important mediator of apoptosis in the injured tissue, but the mechanisms of ceramide generation are not well-defined and the downstream targets of ceramide remain unresolved. The identification and characterization of key proteins of ceramide synthesis are expected to contribute to further understanding of molecular mechanisms of ceramide involvement in tissue damage in IR.Citation12
Continued research efforts are required to better understand the pathophysiologic mechanisms of IR injury, to identify and test new protective agents. Further studies of the molecular basis of ceramide’s role in the ischemic organs are warranted. This will allow the discovery of novel and groundbreaking therapeutic approaches to mitigate diseases that may result from an elevation in ceramide and its metabolites. Therefore the aim of our study was to investigate the protective effect of FTY720 on testicular damage induced by unilateral testicular ischemia reperfusion and also to elucidate the role of FTY720 on the level of testicular sphingolipids.
2 Material and methods
2.1 Animals
Thirty male albino rats (weight: 250 ± 10 g) were purchased from the Faculty of Science, Tanta University. The rats were acclimatized to the controlled laboratory conditions for one week and appropriately housed, four per cage, at a constant temperature of 22 ± 2 °C and 12 h artificial light/dark cycle. All rats had free access to food pellets and water throughout the study period. All rats were fasted overnight before the experiment. All the animal experiments were conducted according to the guidelines established by the Research Advisory Ethical Committee of Faculty of Medicine, Tanta University, Egypt.
2.2 Experimental design
The animals were anaesthetized with intraperitoneal ketamine injection (50 mg/kg). All of the surgical procedures were performed under sterile conditions through standard left inguinoscrotal incisions. After entrance to the scrotum, tunica vaginalis was opened and the left testis was delivered to the surgical field. The left testis was rotated 720° in a clockwise direction and maintained torsed by fixing it to the scrotum by atraumatic silk suture (4/0) through the tunica albuginea for 2 h with the closure of inguinoscrotal incision. Afterward the spermatic cord was detorsed and the testis was reperfused for additional 2 h according to the previously described method.Citation13,Citation14 Interruption of blood supply to the testis can be seen by the naked eyes from the changes in testicular color as testicular torsion (720°) caused dusky appearance of the testis within 5 min. After 1 h torsion the ipsilateral testis vasculature becomes dark red to dark purple and returned to normal 5–10 min after detorsion.Citation15 The rats were randomly divided into three experimental groups as follows: Group 1 (n = 10), sham operated control group (sham), Sham operations were performed through standard ilioinguinal incisions to the control rats and the left testicle was fixed to the scrotum by a silk suture through the tunica albuginea; then, rats in this group underwent excision of the left testis after sham operation. Group 2, (n = 10), ischemia-reperfusion group (I/R); this group of animals underwent torsion/detorsion procedures as explained before. Group 3, (n = 10), comprised the FTY720 treatment group (I/R+FTY720) in addition to I/R process and the animals in this group received FTY720 (4 mg/kg, i.p.) 30 min. before detorsion.Citation16 FTY720 (molecular weight 343.94 Da) was kindly provided by Novartis Pharmaceuticals Ltd (Basel, Switzerland). FTY720 was dissolved in injection water at a concentration of 1 mg/mL. All studies were performed at the same time of day to obviate circadian influences. At the end of the ischemia and reperfusion period (4 h) left testes were dissected carefully in all rats to avoid mechanical trauma for biochemical and histological investigations and the animals were sacrificed by decapitation.
The harvested testicular tissues were longitudinally bisected. The halves of the testicular tissues were immediately stored at −80 °C pending for biochemical analysis.
2.3 Biochemical assays
All of the testicular tissues were washed three times in cold isotonic saline (0.9% [v/w]) solution and wet tissue weights were obtained. The tissues were then homogenized in ice-cold Tris-HCl buffer solution (pH 7.4, 0.2mmoL/L and 50/39.9 [v/v]), within a homogenizer (Ultra Turrax Type T25-B; IKA Labortechnic, Staufen, Germany) for 2 min at 11,200g. The homogenate was centrifuged at 3500g for 60 min. and a supernatant was obtained for measurement of the following parameters:
| 1. | SOD activity was analyzed by colorimetric assay kit (Cayman, MI, USA) and performed according to the manufacturer’s instructions. SOD was expressed as U/mg protein. | ||||
| 2. | The GSH-Px activity was measured by colorimetric assay kit (Randox Laboratories, UK, catalog number CPTK0316). GPx activity results were expressed as U/gm protein. | ||||
| 3. | Catalase activity was determined according to Aebi’s method.Citation17 | ||||
| 4. | MDA as described by Tatum et al.,Citation18 MDA level was expressed as nmol/gm protein. The protein content in the tissue was measured by the method of Lowry et al.Citation19 | ||||
| 5. | Testicular Sphingomyelin (ng/μg tissue) according to method described by Hojjati and Jiang.Citation20 | ||||
| 6. | Testicular total ceramide (pmol/n mol phosphate) according to method described by Sugita et al.Citation21 | ||||
| 7. | Testicular sphingosine-1-phosphate (nmols/mg protein) according to method described by Kirby et al.Citation22 | ||||
2.4 Histopathological evaluation
The specimens were prepared for histological study, according to the method described by Drury and Wallington,Citation23 where the remainder halves of testes were fixed in 10% formalin for 3 days. Afterward, the tissues were processed for paraffin embedding and 5 μm thick paraffin sections were obtained and stained with hematoxylin and eosin staining for light microscopic analysis. Histological findings in seminiferous tubuli were evaluated according to the Johnsen’s scoring system.Citation24 A score of 0–10 was given to each tubule according to epithelial maturation: 10: complete spermatogenesis and perfect tubules; 9: many spermatozoa present and disorganized spermatogenesis; 8: only a few spermatozoa present; 7: no spermatozoa but many spermatids present; 6: only a few spermatids present; 5: no spermatozoa or spermatids but many spermatocytes present; 4: only a few spermatocytes present; 3: only spermatogonia present; 2: no germ cells but only Sertoli cells present; 1: no germ cells and no Sertoli cells present. The same pathologist who was unaware of the experimental procedures examined the samples histopathologically.
2.5 Statistical analysis
Data were analyzed using the one-way analysis of variance (ANOVA) test. Probability values (P) less than 0.05 were considered significant. All the analyses were performed using Graph Pad Prism software release 6.0 (Graph Pad Software, San Diego, CA).
3 Results
3.1 Effect of FTY720 treatment on antioxidant enzymes activities in testicular tissue
Our results showed that SOD and GSH-Px activities were increased in the I/R group (0.139 ± 0.008 and 10.69 ± 1.823 respectively) when compared with sham group (0.04 ± 0.006 and 3.62 ± 0.9 respectively), but FTY720 treatment caused significant decrease in the SOD and GSH-Px activities in I/R+FTY720 treated group (0.069 ± 0.01 and 3.56 ± 0.506 respectively) when compared with I/R group. CAT activity is decreased significantly in I/R group (0.180 ± 0.003) compared to sham group (0.192 ± 0.005), and FTY720 caused significant increase in its level in I/R+FTY720 treated group (0.194 ± 0.005) when compared with I/R group (, ).
Figure 1 Effect of FTY720 treatment on antioxidant enzyme activities in testicular tissue (A) testicular GSH-Px, (B) testicular SOD, and (C) testicular catalase. The mean (horizontal line) and individual values are shown in each experimental group. Values are mean ± SD. *P < 0.05 vs. sham group, **P < 0.05 vs. I/R group.
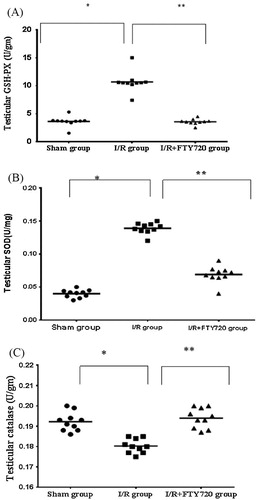
Table 1 Effect of FTY720 treatment on antioxidant enzyme activities in testicular tissue and testicular MDA.
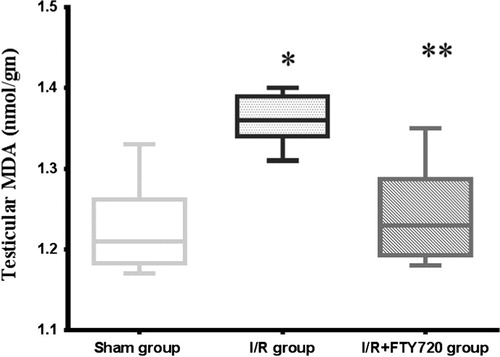
3.2 Effect of FTY720 treatment on testicular lipid peroxidation
Compared with sham group (1.22 ± 0.052), testicular T/D was associated with significant increase in testicular MDA level (1.36 ± 0.0299), and FTY720 treatment induced significant decrease in testicular MDA (1.24 ± 0.056) compared with I/R group (, ).
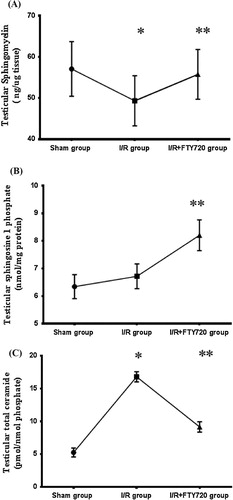
3.3 Effect of FTY720 treatment on testicular sphingolipids
It is obvious from our results that testicular T/D was associated with significant decrease (P < 0.05) in testicular sphingomyelin (49.335 ± 6.084) as compared with sham group (57.08 ± 6.64), while FTY720 treatment caused significant increase (P < 0.05) in its level in I/R+FTY720 treated group (55.756 ± 6.036) when compared with I/R group. On the other hand, testicular T/D caused non-significant increase (P > 0.05) in sphingosine 1 phosphate in I/R group (6.717 ± 0.4501) when compared with sham group (6.341 ± 0.4338), but it is significantly increased (P < 0.05) in I/R+FTY720 treated group (8.203 ± 0.559) in comparison with I/R group. As regards total ceramide level in testicular tissue, it is significantly increased in I/R group (16.806 ± 0.79) in comparison with sham group (5.257 ± 0.6831), and it is significantly decreased in I/R+FTY720 treated group (9.157 ± 0.7865) when compared with I/R group ().
3.4 Histological examination
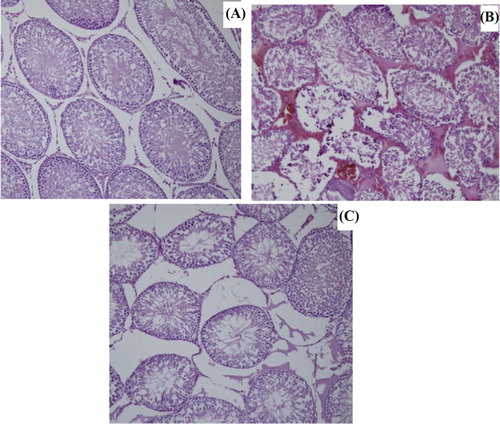
In sham group, animals demonstrated a normal testicular architecture of the seminiferous tubules morphology and interstitium and had intact germinal epithelium (a). In I/R group, seminiferous tubular disorganization, degenerative changes and loss of maturation in the germinal cells, interstitial edema and interstitial hemorrhage were observed (b). FTY720 pretreated animals showed an improved histological appearance in testes compared with I/R group (c).
Figure 4 Testicular histopathology (A): Normal testicular tissue in sham group (B):Testicular tissue in I/R group (C): Testicular tissue in I/R+FTY720 group.
In I/R, there was significant decrease (P < 0.05) in Johnsen’s score (6.77 ± 0.71) when compared to sham group (9.37 ± 0.37). On the other hand, FTY720 treatment increased Johnsen’s score significantly (8.83 ± 0.62) when compared to I/R group ().
Table 2 Effect of FTY720 treatment on Johnsen’s score.
4 Discussion
Testicular torsion is still an important cause of male infertility. Mammalian testes are highly sensitive to oxidative insult, and the most important component of post-torsion testicular damage is oxidative injury.Citation25
Mechanisms associated with testicular ischemia, such as free radical generation and lipid peroxidation, are contributing factors. Ischemia occurs due to the torsion of the testis and the reperfusion related to the detorsioning of the twisted testis can cause various biochemical and morphological changes in the testicular tissue. Moreover, reperfusion after ischemia causes an increase in the damage. Therefore, testis ischemia and consecutive reperfusion result in testicular cell damages.Citation26
The underlying pathophysiologic mechanisms in testicular I-R damage are most likely multifactorial, and interdependent involving hypoxia, inflammatory responses and oxidative stress. This oxidative stress is characterized by an imbalance between ROS and the antioxidative defense system.Citation27
Novel therapeutic interventions for preventing or attenuating testicular I/R induced injury remain a focus of significant interest. Although the exact pathogenic mechanisms remain poorly defined, accumulating evidence supports the potential role of sphingolipids in the pathogenesis of ischemia reperfusion induced injury in various organs.Citation12 The immunosuppressant “FTY720” that has recently been approved by FDA for the treatment of multiple sclerosis has become the gold standard for S1P-centric drugs.Citation28
The mechanisms involved in testicular protection by FTY720 in I/R injury are complex, and it is not known whether the tissue-protective effects of the FTY720 are mediated by the canonical effect to induce lymphopenia or by stimulating S1P1Rs on testicular tissue or both.
As a result of biochemical and statistical analyses, changes in the tissue antioxidant enzyme activities (CAT, SOD and GSH-Px) and changes in the tissue levels of MDA in the I/R group demonstrated evidences of I/R injury in the ipsilateral torted testis. The most important indicator of tissue injury due to I/R was the MDA level. Our results revealed increased MDA level, with induction of testicular I/R injury, implicating the role of oxidative stress in testicular lesion with I/R injury. This oxidative stress is caused mainly by excessive generation of ROS that are formed early during reperfusion period. These ROS are difficult to quantify directly in tissues due to their high reactivity and short half-life. The ROS causes chain reactions of lipid peroxidation in the cell membranes, which eventually leads to the generation of the major lipid peroxidation product MDA.Citation29
In many studies, the activities of the antioxidant enzymes in many tissues were reported to change either positively or negatively with the respect to the intensity of lipid peroxidation. In some of these studies, long-term I/R led to increase in the intensity of lipid peroxidation and inactivation of antioxidant enzymes in testicular tissues. On the other hand, other studies showed compensatory enhancement in enzyme activities.Citation30 This discrepancy in the changes of antioxidant enzyme activities was based on the specific responses of the tissues and the tissue specific activities of antioxidant enzyme system.Citation30 So, we observed in our study that there were diverse activities of antioxidant enzymes, where in spite of significant increase in SOD and GSH-Px levels, there is significant decrease in CAT level in I/R group.
Our results regarding the role of oxidative stress in pathophysiology of testicular I/R are in accordance with others such as Tamamura and coworkersCitation31 who reported that edaravone, a free radical scavenger, significantly ameliorated I-R induced testicular damage by reducing the oxidative stress.
ROS signaling may be mediated through sphingolipid metabolites where there is growing evidence that the sphingostat plays a role in cellular response to oxidative stress.Citation32 Ceramide generation has been implicated in mediating I/R induced ROS generation and apoptotic cell death. I/R rapidly activate neutral sphingomyelinases in a ROS-dependent manner. This is consistent with an earlier finding that the enzymatic activity of neutral sphingomyelinase is inhibited by glutathione and increased when glutathione scavenges cellular ROS.Citation33
The role of ceramide in I/R is seen also in other organs as the ceramide and S1P pathways are inversely related in I/R injured hearts, as ROS not only increases ceramide, but also induces the degradation of SphK1 isoenzyme known to synthesize S1P.Citation29 Also, the role of ceramide has been studied in renal tissues where ceramide directly inhibits mitochondrial respiratory chain. So ceramide not only enhances ROS production but also decreases antioxidant redox system in various cells, aggravating tissue injury.Citation34
It is evident from our experiment that fingolimod displayed an anti-oxidative activity in testicular injury induced by I/R, as reflected from the changes in antioxidant enzyme activities where there is significant decrease in SOD and GSH-Px activity together with significant increase in catalase activity compared to I/R group. The protective role of fingolimod observed in FTY720 treated I/R group could be explained partially through mitigating oxidative stress. Antioxidant effect of fingolimod could be explained by its activity in adjusting the dynamic balance between intracellular S1P and ceramide, the “sphingolipid rheostat (sphingostat)”, and the consequent regulation of opposing signaling pathways.Citation32
To further characterize the mechanisms responsible for I/R induced testicular injury in this model, we analyzed the sphingolipids in testicular tissue, namely SM, and its bioactive metabolites, ceramide, and S1P. With the induction of I/R induced testicular injury, there was a significant reduction in SM with significant increase in ceramide level associated with non-significant increase in S1P levels, supporting the concept that sphingolipids could potentially contribute to the evolution of testicular damage.
Increased ceramide level may be attributed to ROS that caused sphingomyelinase activation with subsequent turnover of SM either at cell membrane or at endosomal/lysosomal compartment and de novo synthesis in endoplasmic reticulum by the activity of ceramide synthase.Citation32 So the antioxidant effect of fingolimod could be achieved indirectly through reduction in ceramide and increase in S1P levels.
Our results regarding ceramide level in I/R group are in accordance with results in other organs where there is dramatic increase in ceramide content in renal I/R rat model.Citation12
Activation of sphingomyelinases and the de novo synthesis of the ceramide pathway have already been reported to contribute to ceramide accumulation in different stress situations, including I/R. While others implicated the I/R induced free radicals in ceramide production, where hypoxia/reoxygenation activates a neutral SMase and ceramide accumulation, through activation of the c-Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) pathway, and this effect was blocked with the use of antioxidants that prevented both ceramide release and JNK activation.Citation35
Our results revealed that there is non-significant increase in S1P level in I/R group that may be explained by that some of I/R induced increase in ceramide is metabolized to S1P initiating a protective response. This occurs as follows: at the onset of hypoxia, ROS tend to activate neutral sphingomyelinase generating ceramide. Also, these ROS induce activation of SphK1. Thus, some of this ceramide is metabolized to S1P.Citation36
However, as hypoxia proceeds, SphK1 is degraded that will prevent conversion of ceramide to S1P resulting in further increase in ceramide level and removal of the protective effect of S1P Citation37, decreasing S1P/ceramide ratio to about 0.4 in I/R group compared to 1.2 in sham group.
Because the sphingolipid metabolites ceramide and S1P are interconvertible, it has been proposed that it is not the absolute amounts of these metabolites but rather their relative levels that determine cell fate. The relevance of this “sphingolipid rheostat” and its role in regulating cell fate has been borne out by work in many laboratories using many different cell types and experimental manipulations.Citation38
FTY720 treatment is associated with significant increase in S1P level which is an indication of increased flux of ceramide to S1P which is then irreversibly degraded by S1P lyase. A second possibility is that S1P feedback inhibits one or more steps of ceramide synthesis.Citation39
S1P is interconvertible with ceramide. I/R play a crucial role in regulation of the sphingolipids balance. Conversely, S1P modulator, FTY720 tilted the balance from ceramide to S1P, raising S1P/ceramide ratio to about 0.9 in FTY720 treated I/R group compared to 0.4 in I/R group, contributing to improvement of testicular injury.
The potential functional contributions of the intracellular effects of FTY720 through the modulation of the balance of signaling sphingolipids have been considered as part of its mechanism of action. It appears to interrupt this circle by adjusting S1P/ceramide rheostat, exerting antioxidant and anti apoptotic effect, so abrogating I/R induced testicular injury.
It appears that the functional protection of FTY720 in our model could be attributed to its ability to adjust the decisive signaling for cell fate, through controlling the levels of both ceramide and S1P.Citation37
Besides the new mechanistic insight concerning the protective signaling pathway in the I/R induced testicular injury, this study corroborates the finding that the sphingolipid mediator, FTY720 has a strong protective effect in an I/R testicular injury through anti-inflammatory, antioxidant and modulation in sphingolipid signals, providing a new therapeutic intervention for treating I/R induced testicular injury.
Conflict of interest
We have no conflict of interest to declare.
Acknowledgment
Great thanks to Prof. Dr. Karima El Dosoky for her help in histopathological examination of testes.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 25 July 2016
References
- N.L.FarringtonM.A.LuckyT.BarnesR.CalvertConfirmed testicular torsion in 67 years oldJ Surg Case Rep1201412
- C.YangB.SongX.LiuG.H.WeiT.LinD.W.HeAcute scrotum in children: an 18 year retrospective studyPediatr Emerg Care2742011270274
- T.LiangP.MetcalfeW.SevicikM.NogaRetrospective review of diagnosis and treatment in children presenting to the pediatric department with acute scrotumAm J Roent20052013444449
- A.K.SaxenaC.CastellaniE.M.RuttenstockM.E.HollwarthTesticular torsion: a 15 year single center clinical and histological analysisActa Pediatr10172012282286
- B.AltunolukH.SöylemezV.BakanH.CiralikF.I.TolunProtective effects of zofenopril on testicular torsion and detorsion injury in ratsUrol J82011313319
- M.KiuchiK.AdachiT.KoharaM.MinoguchiT.HananoY.AokiSynthesis and immunosuppressive activity of 2-substituted 2-aminopropane-1,3-diols and 2-aminoethanolsJ Med Chem43200029462961
- T.KimuraT.HasegawaH.NakaiT.AzumaN.UsuiT.SasakiFTY720 reduces T-cell recruitment into murine intestinal allograft and prevents activation of graft-infiltrating cellsTransplantation75200314691474
- V.BrinkmannK.R.LynchFTY720: targeting G-protein-coupled receptors for sphingosine 1-phosphate in transplantation and autoimmunityCurr Opin Immunol142002569575
- D.M.AnselmoF.F.AmersiX.D.ShenF.GaoM.KatoriC.LassmanFTY720 pretreatment reduces warm hepatic ischemia reperfusion injury through inhibition of T-lymphocyte infiltrationAm J Transplant22002843849
- T.HlaM.J.LeeN.AncellinJ.H.PaikM.J.KlukLysophospholipids–receptor revelationsScience294200118751878
- V.BrinkmannM.D.DavisC.E.HeiseR.AlbertS.CottensR.HofThe immune modulator FTY-720 targets sphingosine 1-phosphate receptorsJ Biol Chem27720022145321457
- S.A.NovgorodovT.I.GudzCeramide and mitochondria in ischemia/reperfusionJ Cardiovasc Pharmacol5332009198208
- T.T.TurnerKenneth S.TungHiroshiTomomasaLeigh W.WilsonAcute testicular ischemia results in germ cell specific apoptosis in the ratBiol Reprod57199712671274
- T.T.TurnerK.J.BrownSpermatic cord torsion: loss of spermatogenesis despite return of blood flowBiol Reprod491993401407
- M.SabaCr.MoralesE.De LamirandeC.GagnonMorphological and biochemical changes following acute unilateral testicular torsion in prepubertal ratsJ Urol157199711491154
- H.J.ShihJ.C.YenA.W.ChiuY.C.ChowW.H.PanT.Y.WangFTY720 mitigates torsion/detorsion-induced testicular injury in ratsJ Surg Res19622015325331
- Aebi H.CatalaseH.U.BergmeyerMethods of enzymatic analysis1974Academic PressNew York673677
- V.L.TatumC.ChangchitC.K.ChowMeasurement of malondialdehyde by high performance liquid chromatography with fluorescence detectionLipids2541990226229
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin-phenol reagentJ Biol Chem1931951265275
- M.R.HojjatiX.C.JiangRapid, specific, and sensitive measurements of plasma sphingomyelin and phosphatedylcholineJ Lipid Res4732006673676
- M.SugitaM.IwamoriJ.EvansR.H.McCluerJ.T.DulaneyH.W.MoserHigh performance liquid chromate-graphy of ceramides: application to analysis in human tissues and demonstration of ceramide excess in Farber’s diseaseJ Lipid Res1531974223226
- R.J.KirbyY.JinJ.FuJ.CubillosD.SwertfegerL.J.ArendDynamic regulation of sphingosine-1-phosphate homeostasis during development of mouse metanephric kidneyAm J Renal Physiol29632009634641
- R.A.DruryE.A.WallingtonCarleton’s histological techniques5th ed.1980Oxford University
- S.G.JohnsenTesticular biopsy score count – a method for registration of spermatogenesis in human testes: normal values and results of 335 hypogonadal malesHormones11970225
- A.BeheshtianA.H.SalmasiS.PayabvashS.KiumehrB.GhazinezamiProtective effects of sildenafil administration on testicular torsion/detorsion damage in ratsWorld J Urol262008197202
- S.OzbalB.U.ErgurG.ErbilI.TekmenA.BagrıyanıkZ.CavdarThe effects of α-lipoic acid against testicular ischemia-reperfusion injury in ratsSci World J2012489248
- F.IsikdemirZ.KurcerG.O.DengizE.Y.SipahiZ.N.BanogluF.BabaEffects of montelukast and zileuton on testicular torsion/detorsion injury in ratsAndrologia46120145964
- R.TotaroC.Di CarmineG.CostantinoR.FantozziP.BellantonioA.FuianiFingolimod treatment in relapsing multiple sclerosis patients: a prospective observational multicancer postmarketing studyMult Scler Int20152015763418
- F.I.DuruC.C.NoronhaA.I.AkinwandeA.O.OkanlawonEffects of torsion, detorsion and melatonin on testicular malondialdehyde levelWest Afr J Med262007312315
- A.CayA.AlverM.KucukO.IşikM.S.EminağaoğluThe effects of N-acetylcysteine on antioxidant enzyme activities in experimental testicular torsionJ Surg Res1312006199203
- M.TamamuraM.SaitoY.KinoshitaProtective effect of edaravone, a freeradical scavenger, on ischaemia-reperfusion injury in the rat testisBJU Int1052010870876
- M.MaceykaS.MilstienS.SpiegelShooting the messenger: oxidative stress regulates sphingosine-1-phosphateCirc Res1001200779
- M.M.YoungM.KesterH.G.WangSphingolipids: regulators of crosstalk between apoptosis and autophagyJ Lipid Res5412013519
- N.UedaCeramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phoshateInt J Mol Sci16201550765124
- M.MatsuyamaK.FunaoK.KuratsukuriT.TanakaY.KawahitoH.SanoExpression of sphingosine-1 phosphate receptor in rat renal ischemia reperfusion injuryMol Med Rep322010233236
- Z.Q.JinH.Z.ZhouP.ZhuN.HonboD.Mochly-RosenR.O.MessingCardioprotection mediated by sphingosine-1-phosphate and ganglioside GM-1 in wild-type and PKC epsilon knockout mouse heartsAm J Physiol Heart Circ Physiol2822002H1970H1977
- M.MaceykaS.MilstienS.SpiegelShooting the messenger oxidative stress regulates sphingosine-1-phosphateCirc Res1001200779
- M.KawaboriR.KacimiJ.S.KarlinerM.A.YenariSphingolipids in cardiovascular and cerebrovascular systems: pathological implications and potential therapeutic targetsWorld J Cardiol5420137586
- M.MaceykaH.SankalaN.C.HaitH.Le StunffH.LiuR.TomanSphk1 and Sphk2: sphingosine kinase isoenzymes with opposing functions in sphingolipid metabolismJ Biol Chem28020053711837129