?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The leaves of Spondias mombin L. (Anacardiaceae) when chewed with Cola acuminata (P. Beauv.) Schott & Endl. (Sterculiaceae) seeds have memory enhancing and anti-ageing properties. This study sought to investigate the protective effect of hydroethanolic leaf extract of Spondias mombin (SM) and Cola acuminata seed extract (CA) against scopolamine-induced cognitive dysfunction. SM or CA (50, 100 or 200 mg/kg, p.o.) or SM + CA (50 mg/kg, p.o.) was administered to rats for 3 consecutive days. One hour post-treatment on day 3, scopolamine (3 mg/kg i.p) was administered and 5 min later, the Y-maze test or Morris water maze test (MWM; days 3–7) was conducted. The rat’s brains were isolated for the estimation of oxidative-nitritive stress status following the MWM task. The antioxidant capacity of SM and CA was also evaluated in vitro using the 1,1-diphenyl-2-picrylhydrazyl (DPPH), nitric oxide (NO) and ferric ion reducing power (FRAP) assays. Pretreatment of rats with SM, CA or SM + CA significantly ameliorated the learning and memory impairment induced with scopolamine as evidenced in Y-maze and MWM paradigms. Moreover, SM, CA or SM + CA significantly attenuated the oxidative-nitritive stress induced by scopolamine, evidenced in the decrease in malondialdehyde and nitrite levels and restoration of glutathione, catalase and superoxide dismutase levels. Furthermore, SM and CA showed promising free radical scavenging effect against DPPH and moderate antioxidant activity in NO and FRAP tests. This study showed that Spondias mombin and Cola acuminata have significant protective effect against scopolamine-induced memory deficit that could be attributed to their antioxidant properties.
1 Introduction
Dementia is a clinical syndrome caused by neurodegeneration and Alzheimer’s disease (AD) being the most common, characterized by inexorably progressive deterioration in cognitive ability and capacity for independent living. AD is associated with the presence of intracellular neurofibrillary tangles and extracellular amyloid-β plaques, loss of neuronal subpopulations, synaptophysin immunoreactivity of presynaptic terminals, loss of cholinergic fibres, mitochondrial dysfunction and proliferation of reactive astrocytes in the entorhinal cortex, hippocampus, prefrontal cortex and amygdala.Citation1,Citation2 Extensive evidence indicates that disruption of cholinergic function is characteristic of ageing and AD, and experimental manipulation of the cholinergic system in laboratory animals suggests that age-related cholinergic dysfunction may play an important role in cognitive deterioration associated with ageing and AD.Citation3 Impaired cortical cholinergic neurotransmission may also contribute to β-amyloid plaque pathology and increase phosphorylation of tau protein, the main component of neurofibrillary tangles in ADCitation4. The restoration of cholinergic function through prolongation of the availability of acetylcholine (ACh) released into the neuronal synaptic cleft by inhibiting acetylcholinesterase (AChE) activity, remains a rational target in the treatment of AD. Acetylcholinesterase inhibitors (tacrine, donepezil, and rivastigmine) are the mainstay in the treatment of AD, though effective but not without adverse effects.Citation5,Citation6
Scopolamine (muscarinic cholinergic receptor antagonist) impaired learning and memory, thus, used extensively to screen for potential antidementic drugs.Citation7,Citation8 Several post-mortem and in vivo studies have demonstrated an accumulation of the products of reactive oxygen species (ROS) or reactive nitrogen species (RNS) damage in AD and scopolamine treated subjects; these substances can be considered biomarkers of oxidative and nitrosative damage, respectively.Citation9–Citation11 Increased level of malondialdehyde (MDA) is a very reliable index of in vivo lipid peroxidation.Citation12 On the other hand, glutathione (GSH) (redox regulator in the maintenance of oxidant homeostasis and cellular detoxification of ROS in brain cells) depletion has been shown to affect mitochondrial function.Citation13 Thus, enhanced expression/activity of the endogenous antioxidant enzymes such as superoxide dismutase (SOD), catalase and GSH have been used as an index of brain oxidative stress.Citation12,Citation14 Therefore, supplementation with antioxidants may delay the development of AD, and attenuate neuronal cell death induced by oxidative stress.Citation8,Citation15
Spondias mombin L. (Anacardiaceae) is a fructiferous tree, native to Nigeria, Brazil and several other tropical forests in the world and can reach a height of 15–22 m. A decoction of the mashed leaves with lemon is effective for worms in children and believed to expel calcifications from the bladder.Citation16 A tea made from the flowers and leaves is taken to relieve stomach ache, biliousness, urethritis, cystitis and eye and throat inflammations.Citation17 In Belize, a decoction of the young leaves is a remedy for diarrhoea and dysentery.Citation18 Interestingly, the antimicrobial,Citation19 sedative, antiepileptic, and antipsychotic propertiesCitation20 of Spondias mombin leaf extract have been reported. In an ethnobotanical survey by Elufioye et al.Citation21 they showed that the leaves of Spondias mombin when chewed with Cola acuminata (P. Beauv.) Schott & Endl. (Sterculiaceae) seeds have memory enhancing and anti-ageing properties. C. acuminata is a bitter brown seed found in the pod of evergreen trees native to Africa, commonly used stimulant in Nigeria, and has been reportedly used for the management of memory loss and other neurodegenerative diseases in folklore.Citation22 Niemenak et al.Citation23 reported that caffeine and theobromine were the major purine alkaloids in C. acuminata seeds while catechin and epicatechin were the predominant polyphenols. Oboh et al.Citation24 also reported anticholinesterase (anti-AChE) and anti-butyrylcholinesterase (anti-BuChE) activities of C. acuminata seed extract with median inhibitory concentrations (IC50) of 14.60 and 96.20 μg/mL, respectively, in a dose-dependent manner. In this study, we examined the effect of S. mombin and C. acuminata on memory processes and oxidative stress status in the prefrontal cortex, striatum and hippocampus of scopolamine-treated animals.
2 Materials and methods
2.1 Plant material
The leaves of S. mombin were collected from Abatadu, Osun state, Nigeria, in May 2015 and seeds of C. acuminata were purchased from Mushin Herbal Market, Lagos state, Nigeria. The Botanical identification and authentication were done by Mr. Oyebanji, Department of Botany and Microbiology, University of Lagos, Akoka, Lagos state, Nigeria. The Voucher Specimens: LUH 6511 (Spondias mombin) and LUH 6905 (Cola acuminata) were deposited in the Herbarium of the Department for reference purposes.
2.2 Laboratory animals
Male Sprague-Dawley rats (150–170 g) and female Swiss albino mice (18–22 g) used in this study were obtained from the Laboratory Animal Centre, College of Medicine, University of Lagos, Lagos, Nigeria. The animals were housed six per cage, allowed access to water and food (Livestock Feeds, Lagos, Nigeria) ad libitum, and maintained at a constant temperature (23 °C ± 1 °C) and humidity (60% ± 10%) under a 12-h light/dark cycle (light on 07:00–19:00 h). The animals were maintained under laboratory conditions for an acclimatization period of 7 days before performing the experiment. Animal maintenance and treatment were carried out in accordance with the United States, National Institutes of Health Guidelines for Care and Use of Laboratory Animals in Biomedical Research (2011).
2.3 Preparation of the extract
The air-dried leaves of S. mombin were pulverized into powder (356 g). The powdered leaf was soaked in 6.25 L of 70% ethanol for 72 h at room temperature with intermittent agitation. The extract was filtered and the filtrate obtained was concentrated by Heidolph® Rotavapor (Switzerland) at 40 °C, and further oven-dried at 40 °C to give a deep green extract with a yield of 19 g (5.34% w/w).
The seeds of C. acuminata (2870 g) were chopped into small pieces and air-dried for 7 days. The dried seeds were pulverized into fine powder. The powdered seeds (1441 g) were soaked in 3.25 L of 70% ethanol for 72 h. The extract was filtered and the filtrate obtained was concentrated by Heidolph® Rotavapor (Switzerland) at 40 °C, and further oven-dried at 40 °C to give a deep brown extract with a yield of 149 g (10.34% w/w).
2.4 Drugs and chemicals
Ethanol, chloral hydrate, tacrine, glacial acetic acid, Folin-Ciocalteu reagent, scopolamine hydrobromide, phosphate buffer solution, thiobarbituric acid, sodium chloride, sodium hydroxide, potassium ferricyanide, trichloroacetic acid, napthylethylenediamine dihydrochloride, ferric chloride, DPPH solution, dithio-bis-nitrobenzoic acid (DTNB), and bovine serum albumin were purchased from Sigma Aldrich, St. Louis MO, USA, while normal saline was purchased from Unique Pharmaceutical Ltd, Lagos, Nigeria.
2.5 Quantitative phytochemical estimation and antioxidant capacity
2.5.1 Determination of total phenolic content
Total phenolic content of CA or SM was determined using Folin-Ciocalteu reagent as described by Velioglu et al.Citation25 with slight modifications. The total phenolic content of CA or SM was estimated using Folin-Ciocalteu reagent with gallic acid as a standard. Briefly, 12.5 μL of CA, SM or gallic acid (0–100 mg/ml) was mixed with 12.5 μL of Folin-Ciocalteu reagent. Five minutes later, 12.5 μL of saturated sodium carbonate (NaCO3) solution (7%) was added to the reaction mixture. The reaction mixtures were incubated at room temperature for 90 min and the absorbance was recorded at 725 nm. Ethanol was used as a blank. A dose response linear regression was generated by using the gallic acid standard absorbance and the levels in the samples were expressed as gallic acid equivalents (mg of GAEs/mg dry weight).
2.5.2 Assay for total flavonoid content
The total flavonoid content was determined spectrophotometrically according to the method previously described by Kumaran and Karunakaran.Citation26 One millilitre of the extracts in ethanol (200 μg/ml) was mixed with 1 ml of aluminium trichloride in ethanol (20 mg/ml). The absorbance values of the reaction mixtures were read at 415 nm after 10 min against a blank. Rutin was used as the standard and the total flavonoid content of the extracts was expressed as mg of rutin equivalent per gram of dry extract (mg RE/g of dry extract).
2.5.3 Assay for total antioxidant capacity
The antioxidant activity of CA or SM was evaluated by the phosphomolybdenum method in accordance with the procedure described by Prieto et al.Citation27 The assay is based on the reduction of Mo (VI) to Mo (V) by the extract and subsequent formation of a green phosphate/Mo (V) complex at an acidic pH. CA or SM (0.3 ml) was mixed with 3 ml of reagent solution (0.6 M sulphuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate). Test tubes containing the reaction solution were incubated at 95 °C for 90 min. The absorbance was read at 695 nm. The total antioxidant capacities were expressed as mg gallic acid equivalent per g dry extract.
2.6 In vitro evaluation of antioxidant activities
2.6.1 1,1-Diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity
The free radical scavenging potential of the extracts was determined according to the procedure of Awah et al.Citation28 with some minor modifications. Briefly, an aliquot of 50 μL of CA or SM (10–100 μg/ml) was mixed with 950 μL of ethanolic solution of DPPH (3.4 mg/100 ml). The mixture was vortexed and incubated in the dark at 37 °C for 1 h. The free radical scavenging potential of CA or SM was expressed as the disappearance of the initial purple colour. The absorbance of the reaction mixture was recorded at 517 nm using a UV–Visible spectrophotometer (Agilent 8453, Germany). Gallic acid was used as the positive control. The percentage inhibition of DPPH free radical was calculated according to the formula:
2.6.2 Assay for nitric oxide scavenging activity
Sodium nitroprusside is known to decompose in an aqueous solution at physiological pH (7.2) producing NO∗. Under aerobic condition, NO reacts with oxygen to produce stable products (nitrite and nitrate). The effect of CA or SM on nitrite generation was evaluated using the Griess reagent as modified by Mirkov et al.Citation29 Griess reagent consists of sulphanilamide (1%), naphthylethylene diamine dihydrochloride (2%) and H3PO4 (0.1%). Briefly, 4 ml of CA or SM (10–100 μg/mL) was mixed with 1 ml of 5 mM sodium nitroprusside in phosphate buffer saline (PBS) at pH 7.4. The reaction mixture was incubated for 2 h at room temperature (31 °C). Two millilitres of the CA or SM reaction mixture or standard nitrite was mixed with 1.2 ml of Griess reagent and the absorbance of the reaction mixture was measured at 550 nm. The phosphate buffered saline was used as a blank. The concentration of nitrite was calculated with reference to the absorbance of the standard nitrite. The percentage inhibition was calculated according to the following:where A0 = absorbance of standard, A1 = absorbance of the extracts.
2.6.3 Ferric ion reducing power assay
The reducing capacity of CA or SM may serve as a good indicator of its potential antioxidant property. The reducing power of CA or SM was investigated using the method of Oyaizu.Citation30 Various concentrations of CA, SM or gallic acid solution (1.0 ml) were mixed with 2.5 ml of potassium buffer (0.2 M, pH 6.6) and 2.5 ml of potassium ferricyanide [K3Fe(CN)6] (1% w/v). After 30 min of incubation at 50 °C, 2.5 ml of 10% trichloroacetic acid solution was added to each test tube and the mixture was centrifuged at 10,000g for 10 min. Then, 2.5 ml of the supernatant solution was withdrawn from the tube and mixed with 2.5 ml of distilled water and 0.5 ml of ferric chloride solution (0.1%, w/v). The absorbance was measured at 700 nm.
2.7 Acute toxicity test
The acute toxicity of CA or SM was determined using the fixed dose protocol of the Organization of Economic Co-operation and Development (OECD) guidelines for testing of chemicals, TG420 (OECD, 2001) for oral administration. 22 female mice were given CA or SM (250 mg/kg, p.o., n = 1; 2500 mg/kg, p.o., n = 5; and 5000 mg/kg, p.o., n = 5). Behavioural signs of toxicity and mortality were observed; during the first 30 min, then the second, fourth, sixth hour and once daily for 14 days for delayed toxicity or mortality. Also, the effect of the extracts on spontaneous locomotor activity was observed using the open field test.Citation31
2.8 Behavioural cognition tests
2.8.1 Y-maze test
The test relies on the innate tendency of rats to explore a novel environment to assess spatial recognition. Rats (150–170 g) were randomly divided into 10 groups (n = 6) and treated as follows for 3 days: Group 1-vehicle (10 ml/kg; p.o., normal control), Group 2-vehicle (10 ml/kg, p.o., amnesic model), Group 3-tacrine (5 mg/kg; i.p.), Groups 4–6: CA (50, 100 and 200 mg/kg; p.o., respectively), Groups 7–9: SM (50, 100 and 200 mg/kg; p.o., respectively) or Group 10: SM + CA (50 mg/kg; 1:1 dilution). One hour after vehicle or drug administration on day 3, scopolamine (3 mg/kg, i.p.) was administered to rats in Groups, 2–10. Five minutes post-scopolamine injection, cognitive function of the rat was evaluated by placing it in the centre of the Y-maze facing the south arm ‘B’ and allowing it to explore the maze freely for a period of 5 min. The number and the sequence of arm entries were observed. An arm entry was scored when all four paws were in the arm. Alternation behaviour was defined as consecutive entries into all three arms (i.e., ABC, CAB, or BCA but not BAB).Citation32 The percentage of spontaneous alternation was measured as an index of working memory by calculating the ratio of the actual number of alternations to the possible number (defined as the total number of arm entries minus two) multiplied by 100, i.e., % alternation [(number of alternations)/(total number of arm entries – 2)] × 100. The total number of arm entries was recorded as an index of locomotor activity.
2.8.2 Morris water maze (MWM) test
Male albino rats were randomly divided into 10 groups (n = 6) and treated as follows for 3 consecutive days: Group 1-vehicle (10 ml/kg; p.o., normal control), Group 2-vehicle (10 ml/kg, p.o., amnesic model), Group 3-tacrine (5 mg/kg; i.p.), Groups 4–6: CA (50, 100 and 200 mg/kg; p.o., respectively), Groups 7–9: SM (50, 100 and 200 mg/kg; p.o., respectively) or Group 10: SM + CA (50 mg/kg; 1:1 dilution). One hour after vehicle or drug administration on day 3 of treatment, scopolamine (3 mg/kg, i.p.) was administered to rats in Groups, 2–10. Five minutes post-scopolamine injection, spatial learning was evaluated using the Morris water maze task for 5 consecutive days (days 3–7). The MWM apparatus consisted of a circular water tank (110 cm diameter and 60 cm height) filled with water (26 ± 2 °C) to a depth of 30 cm. Four equally spaced points around the edge of the pool were designated as N (North), E (East), W (West), and S (South). A black round platform of diameter 10 cm was placed 2 cm below the surface of the water in a constant position in the middle of the southwest quadrant in all trials. The rats were given a maximum of 60 s (cut-off time) to locate the hidden platform and were allowed to stay on it for 10 s. The time taken for the rat to locate the escape platform was recorded using a stopwatch. In the event that the animal was unable to locate the hidden platform within 60 s, it was gently guided to it and was allowed to stay on it for 10 s. Each rat was subjected to a daily session of 3 trials per day for 5 days consecutively. Escape latency time (ELT) (time to locate the hidden platform in the water maze) was used as an index of learning.Citation8,Citation33
2.9 Brain tissue preparation
After the MWM test on day 7, all the rats were anaesthetized with chloral hydrate (300 mg/kg, i.p.) and rapidly perfused with cold chilled normal saline. The skull was cut open and the brain was exposed from its dorsal side. The whole brain was quickly removed and the prefrontal cortex (PFC), striatum (STR) and hippocampus (HIP) were isolated on an ice-cold plate. The isolated brain areas were weighed and homogenized in 0.03 M sodium phosphate buffer, pH-7.4 with an Ultra-Turrax T25 (LA, USA) homogenizer at a speed of 9500 rpm. The homogenate was used to assay for malondialdehyde (MDA), reduced glutathione (GSH), superoxide dismutase (SOD), and nitrite.
2.10 Biochemical estimations
2.10.1 Estimation of MDA level
MDA, which is an indicator of lipid peroxidation, was spectrophotometrically measured using the thiobarbituric acid assay procedure.Citation8 Two hundred microlitres of supernatant was added and briefly mixed with 1 mL of 50% trichloroacetic acid in 0.1 M HCl and 1 mL of 26 mM thiobarbituric acid. After mixing on a vortex, samples were maintained at 95 °C for 20 min, after which samples were centrifuged at 960g for 10 min and supernatants were read at 532 nm. The results were expressed as U/mg protein.
2.10.2 Measurement of GSH
GSH was determined by its reaction with 5,5′-dithiobis (2-nitrobenzoic acid) (Ellman’s reagent) to yield a yellow chromophore which was measured spectrophotometrically.Citation7 The brain homogenate was mixed with an equal amount of 10% trichloroacetic acid (TCA) and centrifuged (Remi cold centrifuge) at 2000g for 10 min at 4 °C. The supernatant was used for GSH estimation. To 100 μl of processed tissue sample, 2 ml of phosphate buffer (pH 8.4), 0.5 ml of 5,5′-dithiobis (2-Nitrobenzoic acid) (DTNB) and 0.4 ml of double-distilled water were added and the mixture was shaken vigorously on a vortex mixer. The absorbance was read at 412 nm within 15 min. The results were expressed as U/mg protein.
2.10.3 Determination of catalase activity
Catalase (CAT) activity was assayed according to the method of Sinha.Citation34 Twenty-five microlitre aliquots of the supernatant of tissue homogenate was mixed with 975 μL of 0.1 M phosphate buffer at pH 8.0 and 500 μL of 10 mM H2O2. The reaction was stopped by the addition of 2.0 ml of dichromate-acetic acid reagent (5% potassium dichromate and glacial acetic acid were mixed in 1:3 ratio). The decomposition of H2O2 was directly estimated by the net decrease in absorbance at 240 nm. The reaction mixture (1.5 ml) contained 1.0 ml of 0.01 M phosphate buffer (pH 7.0), 0.4 ml of 2 M H2O2. The results were expressed as U/mg protein.
2.10.4 Determination of superoxide dismutase (SOD) activity
The activity of superoxide dismutase (SOD, EC 1.15.1.1) was assayed according to the method of Winterbourn et al.Citation35 by monitoring its ability to inhibit the photochemical reduction in nitroblue-tetrazolium (NBT). Each 1.5 mL reaction mixture contained 100 mM TRIS/HCl (pH 7.8), 75 mM NBT, 2 μM riboflavin, 6 mM EDTA, and 200 μL of supernatant. Monitoring the increase in absorbance at 560 nm followed the production of blue formazan. One unit of SOD is defined as the quantity required to inhibit the rate of NBT reduction by 50%. The enzyme activity is expressed as U/mg protein.
2.10.5 Nitrite estimation
Nitrite was estimated in the rat’s brain using the Griess reagent (an indicator of nitric oxide (NO) production). NO has a short half-life and is rapidly converted to the stable end products such as nitrate (NO3−) and nitrite (NO2−). One hundred microlitre of Griess reagent (1:1 solution of 1% sulphanilamide in 5% phosphoric acid and 0.1% napthylamine diamine dihydrochloric acid in water) was mixed with 100 μl of supernatant and vortexed. The absorbance was measured at 542 nm.Citation8 Nitrite concentration was calculated using a standard curve for sodium nitrite.
2.10.6 Protein estimation
Protein was measured in all brain samples by the method of Lowry et al.Citation36 Bovine serum albumin (BSA) (1 mg/ml) was used as standard and measured in the range of 0.01–0.10 mg/ml.
2.11 Statistical analysis
Results obtained are expressed as mean ± SEM (n = 6). The statistical level of significance was determined by one- or two-way ANOVA followed by Tukey post hoc multiple comparison test (whichever is applicable) using Graphpad prism version 6 (Graphpad prism Inc, CA, USA).
3 Results
3.1 TPC, TFC and TAC
lists gallic acid equivalents of total phenolic content of SM and CA. The phenolic compounds in the extract varied from 29.28 mg GAE/g DE in SM to 35.48 mg GAE/g DE in CA. In addition, the total flavonoid contents (major components of the phenolic compounds) of SM and CA were estimated, and the total flavonoids ranged from 12.84 to 16.44 mg RE/g DE, in SM and CA, respectively. Moreover, the extracts showed potent antioxidant capacity ranging from 41.06 mg ascorbic acid equivalent per gram of SM extract to 60.13 mg ascorbic acid equivalent per gram of CA extract ().
Table 1 Table of total antioxidant capacity, total phenolic and flavonoid content.
3.2 DPPH, NO and FRAP estimation
The antioxidant activity of SM and CA was assessed by DPPH, which is based on the ability of DPPH to react with proton donors such as phenols. SM and CA exhibit DPPH free radical scavenging potential comparatively similar to the effect of ascorbic acid (). In another experiment, the ability of the extracts to scavenge NO production was evaluated. SM, CA and ascorbic acid attenuated NO production as shown in . In the reducing power assay, SM, CM and ascorbic acid showed concentration dependent increase in absorbance indicating high reducing power ().
Table 2 Free radical scavenging, nitric oxide inhibition and ferric ion reducing power activity of SM and CA.
3.3 Acute toxicity test
The extract of SM and CA up to 5000 mg/kg did not induce mortality but the observed toxicity behaviours include restlessness and tachypnoea. However, CA caused no significant change in exploratory behaviour (rearing and grooming) and locomotor activity (number of lines crosses) as shown in . SM on the other hand produced significant change in rearing (P < 0.01) and grooming (P < 0.05) behaviour but caused no significant change in locomotor activity ().
Table 3 Effect of SM and CA on spontaneous locomotor activity.
3.4 Y-maze test
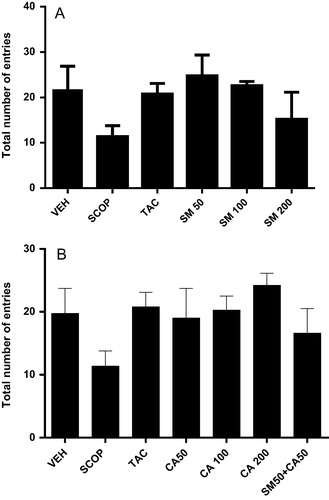
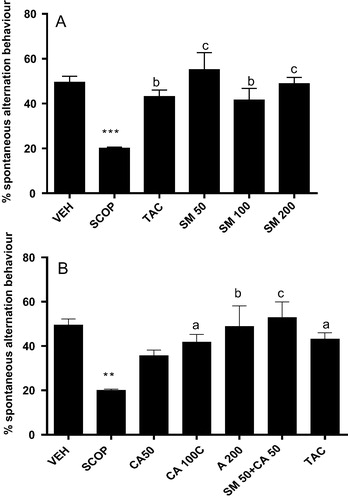
The effects of SM and CA on short-term or working memory were investigated in the spontaneous alternation behaviour in Y-maze. One way ANOVA revealed significant effect of treatment on alternation behaviour [F(5, 24) = 8.409, P < 0.01]. Post hoc test showed that intraperitoneal administration of scopolamine induced significant deficit in spontaneous alternation behaviour when compared with vehicle-treated control (A). However, pretreatment of rats with SM reversed the decrease in alternation behaviour induced by scopolamine which was similar to the effect of tacrine. Similarly, CA (100 and 200 mg/kg) produced significant increase in percentage alternation behaviour [F(6, 28) = 5.103, P < 0.01] when compared with vehicle-scopolamine treated rats (B). Interestingly, the combination of SM and CA (50 mg/kg) increased [F(6, 28) = 5.103, p < 0.01] the spontaneous alternation behaviour compared with vehicle-scopolamine treated. However, in all the treated groups no significant change in the number of entries was observed (A and B).
Figure 1 Effect of (A) SM or (B) CA on spontaneous alternation behaviour in scopolamine-treated mice in the Y-maze test. Values are expressed as mean ± SEM (n = 6), ∗∗P < 0.01; ∗∗∗P < 0.001 versus vehicle-treated; aP < 0.05; bP < 0.01; cP < 0.05 versus scopolamine-treated group (one-way ANOVA followed by Tukey post hoc multiple comparison test).
3.5 MWM task
Vehicle treated rats quickly acquired the spatial task as depicted by a gradual session-dependent decrease in escape latency [F(4, 20) = 32.07, P < 0.001]. However, scopolamine (3 mg/kg, i.p.) administration caused spatial memory impairment as indicated by no significant change [F(4, 20) = 5.473, P < 0.01] in ELT from 2nd to the 5th sessions when compared with session 1. In contrast, tacrine pretreatment before scopolamine injection, produced a significant decrease [F(4, 20) = 167.5, P < 0.001] in ELT from sessions 2–5 when compared to session 1. Similarly, oral administration of SM prevented scopolamine-induced memory impairment, and SM (50, 100 and 200 mg/kg) significantly decreased [F(4, 20) = 37.79, P < 0.001], [F(4, 20) = 33.95, P < 0.001], [F(4, 15) = 4.31, P < 0.01] ELT from session 2–5 when compared with session 1 (A). Similarly, CA administration prevented scopolamine-induced memory impairment in rats. As shown in B, CA (50, 100 and 200 mg/kg) significantly decreased [F(4, 20) = 34.56, P < 0.001], [F(4, 20) = 43.47, P < 0.001], [F(4, 20) = 281.9, P < 0.001] ELT from sessions 2–5 in comparison with session 1. The combination of SM 50 mg/kg and CA 50 mg/kg significantly decreased [F(4, 20) = 70.55, P < 0.001] ELT from sessions 2–5 when compared with session 1.
Figure 3 Effect of (A) SM and (B) CA on spatial learning following scopolamine-induced memory impairment in the Morris water maze test in rats. Values are expressed as mean ELT (s) ± S.E.M (n = 6). aP < 0.05, bP < 0.01, cP < 0.001 versus session 1 (two-way ANOVA followed by Tukey post hoc multiple comparison test).

3.6 MDA levels
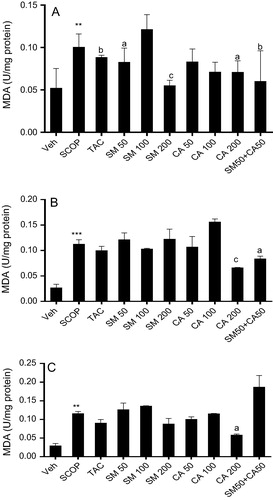
Scopolamine administration significantly increased [F(2, 15) = 35.10, P < 0.001] MDA level in the prefrontal cortex of rats in comparison with vehicle-treated group. However, treatment with tacrine, significantly decreased [F(4, 25) = 3.080, P < 0.05] MDA levels by 5.6 folds. Similarly, pretreatment of rats with SM (50 and 200 mg/kg) significantly attenuated [F(4, 25) = 3.080, P < 0.05] MDA generation in comparison with scopolamine treated group. In the same vein, pretreatment of rats with CA (200 mg/kg) significantly ameliorated [F(5, 30) = 3.179, P < 0.05] MDA levels when compared with scopolamine-vehicle treated. The combination of SM and CA (50 mg/kg) significantly decreased [F(5, 30) = 3.080, P < 0.05] MDA level by 7.7 folds in comparison with scopolamine-vehicle treated group (A). In the hippocampus, scopolamine administration significantly increased [F(2, 15) = 35.10, P < 0.001] MDA level by 3.12 folds in comparison with vehicle-control treated. Interestingly, tacrine and SM treatment showed no significant change in MDA level in comparison with scopolamine treated group. However, CA treatment at 200 mg/kg as well as the combination of SM 50 mg/kg and CA 50 mg/kg significantly reduced [F(4, 25) = 10.59, P < 0.001] and [F(2, 5) = 5.619, P < 0.05] MDA level by 2.53 and 1.60 folds, respectively, versus scopolamine-vehicle treated group (B). One way ANOVA revealed significant effect of scopolamine administration [F(2, 15) = 31.10, P < 0.001]. Post hoc analysis showed that intraperitoneal administration of scopolamine produced significant (P < 0.001) increase in MDA levels in the striatum of rats brain in comparison with vehicle treated group. However, pretreatment of rats with tacrine, SM and CA (50 and 100 mg/kg) failed to reverse the increase in MDA level induced by scopolamine. On the other hand, pretreatment of rats with CA (200 mg/kg) significantly reduced [F(3, 20) = 9.058, P < 0.01] MDA levels. The combination of SM 50 mg/kg and CA 50 mg/kg could not reverse the increase in MDA levels induced by scopolamine injection (C).
Figure 4 Effect of SM and CA on MDA levels in the (A) prefrontal cortex, (B) hippocampus and (C) striatum of scopolamine-treated rats after the MWM task. Values are expressed as Mean ± SEM (n = 6). ∗∗P < 0.01, ∗∗∗P < 0.001 versus vehicle-treated control, aP < 0.05, bP < 0.01, cP < 0.001 versus vehicle + scopolamine treated (one-way ANOVA followed by Tukey post hoc multiple comparison test).
3.7 GSH levels
Scopolamine injection produced significant decrease in the prefrontal cortex GSH level when compared with vehicle-treated control group. Conversely, the pretreatment of rats with SM (100 mg/kg) or CA (50 mg/kg) increased GSH level [F(4, 25) = 3.424, P < 0.05] by 0.56 and 0.46 folds, respectively. However, there was no significant change in the level of GSH in the prefrontal cortex in combined treatment groups (A). Scopolamine injection also induced deficit in GSH level in the hippocampus which was reversed by pretreatment of rats with tacrine, SM (50, 100 and 200 mg/kg) or CA (100 mg/kg) [F(4, 25) = 6.182, P < 0.05]. Furthermore, the combination of SM 50 mg/kg and CA 50 mg/kg synergistically enhanced GSH level in the hippocampus (B).
Figure 5 Effect of SM and CA on GSH level in the (A) prefrontal cortex, (B) hippocampus and (C) striatum of scopolamine-treated rats after the MWM task. Values are expressed as mean ± SEM (n = 6). ∗P < 0.05, ∗∗P < 0.01 versus vehicle-treated control, aP < 0.05, bP < 0.01, cP < 0.001 versus vehicle + scopolamine treated (one-way ANOVA followed by Tukey post hoc multiple comparison test).
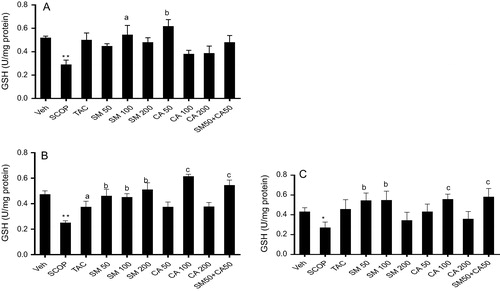
One way ANOVA revealed significant effect of treatment on GSH level in the striatum [F(9, 50) = 11.67, P < 0.001]. Post hoc analysis showed that scopolamine injection caused significant decrease (P < 0.05) in the level of GSH when compared with vehicle control treated. Similarly, tacrine produced no significant change in the level of GSH in the striatum in comparison with scopolamine treated group. However, pretreatment of rats with SM (50 and 100 mg/kg) (p < 0.001) or CA (100 mg/kg) (p < 0.001) increased GSH level by 0.59 and 0.57 folds, respectively. Moreover, treatment of rats with combined doses of SM 50 mg/kg and CA 50 mg/kg increased (P < 0.001) the level of GSH by 0.55 folds [F(3, 20) = 8.858, P < 0.001] (C).
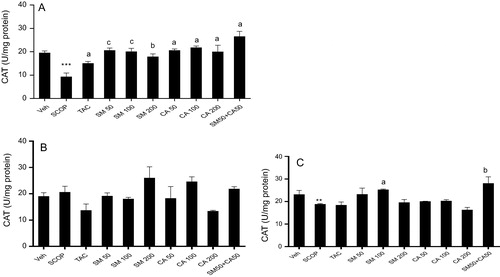
3.8 Catalase activity
One way ANOVA revealed significant effect of treatment on catalase level in the prefrontal cortex [F(9, 50) = 8.76, P < 0.01], hippocampus [F(9, 50) = 2.85, P < 0.01] and striatum [F(9, 50) = 4.85, P < 0.01]. Post hoc analysis showed that scopolamine injection caused significant decrease (P < 0.001, P < 0.05) in catalase level in the prefrontal cortex and striatum, respectively, when compared with vehicle control treated (A–C). Meanwhile, tacrine significantly increased (P < 0.05) levels of catalase in the prefrontal cortex but not in the hippocampus and striatum. Moreover, the pretreatment of rats with SM (50, 100 and 200 mg/kg) significantly increased [F(3, 20) = 14.35, p < 0.001] catalase levels by 0.45, 0.46. 0.52 folds, respectively, in the prefrontal cortex when compared with vehicle-scopolamine treated group. Similarly, treatment with CA showed significant increase [F(4, 25) = 11.88, p < 0.001] in catalase levels at 50 mg/kg, 100 mg/kg and 200 mg/kg by 0.45, 0.43, 0.46 folds, respectively. Moreover, the combination of SM 50 mg/kg and CA 50 mg/kg significantly increased [F(4, 25) = 11.88, p < 0.0001] catalase levels by 0.35 folds (A). Pretreatment with SM showed a significant increase [F(3, 20) = 3.651, p <= 0.05] in catalase levels by 0.74 folds at 100 mg/kg in comparison with scopolamine treated group. However, CA pretreatment produced no significant increase in catalase level. Interestingly, a significant increase [F(4, 25) = 9.088, p < 0.001] in catalase levels by 0.67 folds was produced following combination of SM 50 mg/kg and CA 50 mg/kg in comparison with scopolamine treated group (C).
Figure 6 Effect of SM and CA on catalase level in the (A) prefrontal cortex, (B) hippocampus and (C) striatum of scopolamine-treated rats after the MWM task. Values are expressed as mean ± SEM (n = 6). ∗∗P < 0.01, ∗∗∗P < 0.001 versus vehicle-treated control, aP < 0.05, bP < 0.01, cP < 0.001 versus vehicle + scopolamine treated (one-way ANOVA followed by Tukey post hoc multiple comparison test).
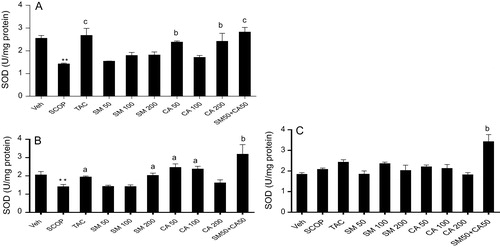
3.9 SOD activity
One way ANOVA revealed significant effect of treatment on SOD activity in the prefrontal cortex [F(9, 50) = 8.12, P < 0.01], hippocampus [F(9, 50) = 7.51, P < 0.01] and striatum [F(9, 50) = 7.72, P < 0.01]. Post hoc analysis showed that scopolamine injection caused significant decrease (P < 0.01) in SOD activity by 1.79 and 0.85 folds in the prefrontal cortex and hippocampus, respectively, when compared with vehicle-treated control (A–C). The decrease in SOD activity within the prefrontal cortex and hippocampus was reversed by the pretreatment of rats with tacrine. In contrast, pretreatment of rats with SM produced no significant change in SOD activity in the prefrontal cortex but SM (200 mg/kg) treatment significantly increased the activity of SOD within the hippocampus when compared with vehicle-scopolamine treated group. Moreover, CA treatment (50 and 200 mg/kg) significantly increased [F(3, 20) = 7.130, P < 0.001] SOD activity in the prefrontal cortex compared with vehicle-scopolamine treated group. In addition, pretreatment of rats with CA (50 and 100 mg/kg) significantly increased [F(4, 25) = 3.895, p < 0.0136] SOD activity in the hippocampus by 1.48 folds when compared with vehicle-scopolamine treated group. Interestingly, the combination of SM 50 mg/kg and CA 50 mg/kg increased [F(3, 20) = 11.14, p = 0.0002] SOD activity by 0.5 folds versus vehicle-scopolamine group (A and B). In the striatum, no significant change in SOD activity was observed in SM and CA pretreated rats but the combination of SM 50 mg/kg and CA 50 mg/kg increased [F(2, 15) = 10.60, p = 0.0013] SOD activity by 0.61 folds when compared with vehicle-scopolamine treated group (C).
Figure 7 Effect of SM and CA on SOD activity in the (A) prefrontal cortex, (B) hippocampus and (C) striatum of scopolamine-treated rats after the MWM task. Values are expressed as mean ± SEM (n = 6). ∗∗P < 0.01 versus vehicle-treated control, aP < 0.05, bP < 0.01, cP < 0.001 versus vehicle + scopolamine treated (one-way ANOVA followed by Tukey post hoc multiple comparison test).
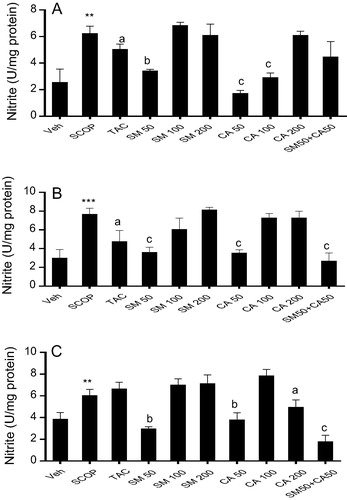
3.10 Nitrite level
One way ANOVA revealed significant effect of treatment on nitrite generation in the prefrontal cortex [F(9, 50) = 8.34, P < 0.01], hippocampus [F(9, 50) = 7.47, P < 0.01] and striatum [F(9, 50) = 11.22, P < 0.01]. Post hoc analysis showed that the intraperitoneal injection of scopolamine significantly (P < 0.01) increased nitrite levels by 2.44, 2.55 and 1.56 folds in the prefrontal cortex, hippocampus and striatum, respectively, when compared with vehicle-treated control. However, pretreatment of rats with tacrine attenuated nitrite generation by 1.23 and 1.61 folds, respectively, in the prefrontal cortex and hippocampus but no significant change in nitrite level was seen in the striatum when compared with vehicle-scopolamine treated group. Similarly, the pretreatment of rats with SM (50 mg/kg) significantly decreased nitrite level by 1.24 and 1.83 folds in the prefrontal cortex and hippocampus, respectively, when compared with scopolamine treatment (A and B). Moreover, pretreatment of rats with CA (50 and 100 mg/kg) significantly attenuated scopolamine-induced nitrite generation in the prefrontal cortex, hippocampus and striatum. In addition, the combination of SM 50 mg/kg and CA 50 mg/kg significantly ameliorated scopolamine-induced increase in nitrite level in the hippocampus and striatum (A–C).
Figure 8 Effect of SM and CA on nitrite level in the (A) prefrontal cortex, (B) hippocampus and (C) striatum of scopolamine-treated rats after the MWM task. Values are expressed as mean ± SEM (n = 6). ∗∗P < 0.01; ∗∗∗P < 0.01 versus vehicle-treated control, aP < 0.05; bP < 0.01; cP < 0.001 versus vehicle + scopolamine treated (one-way ANOVA followed by Tukey post hoc multiple comparison test).
4 Discussion
In this study, we demonstrated that the pretreatment of rats with Spondias mombin or Cola acuminata prevented scopolamine-induced memory impairment in rats. Moreover, the combination of Spondias mombin and Cola acuminata produced synergistic improvement in learning and working memory in rats without affecting the spontaneous motor activity. It is of interest to state that the extracts or their combination improved antioxidant defence system in the prefrontal cortex and hippocampus of scopolamine treated rats.
Scopolamine, a non-selective muscarinic antagonist induces dysregulation of the cholinergic neuronal pathway and memory circuits in the central nervous system, resulting in serious impairments in learning, acquisition, and short-term retention of spatial memory tasks.Citation37 In the present study, we used two well-characterized memory tasks: Y-maze test and MWM test in rats to investigate the protective effect of Spondia mombin and Cola acuminata against scopolamine-induced memory impairment. Scopolamine is well known for its amnesic effect in animals and humanCitation38,Citation39 and its pretrial administration caused cognitive impairment in both the Y-maze and MWM tests.Citation8 Scopolamine-induced cognitive dysfunction has been reported to be associated with impairment in central cholinergic system which plays an important role in learning and memory.Citation7,Citation40
The Y maze task is a specific and sensitive test of spatial recognition or working memory in rodents which relies on an innate tendency of rats to explore a novel environment.Citation32,Citation41 In agreement with previous studies, scopolamine administration impaired spatial working memory evidenced in the significant reduction in spontaneous alternation behaviour.Citation32 However, the pretreatment of rats with Spondias mombin or Cola acuminata as well as their combination significantly improved spatial working memory evidenced in the increase in relative proportion of spontaneous alternation percentage as compared with scopolamine treated rats. Moreover, neither scopolamine administration nor pretreatment of rats with the extracts of Spondias mombin or Cola acuminata affected the total number of arm entries thus ruling out possible psychostimulant effect at the tested doses. These results suggested that the extracts when used alone or in combination could enhance short term or working memory.Citation32
The Morris water maze learning task is used to assess hippocampal-dependent spatial learning ability.Citation42 In the present study, scopolamine produced impairment of acquisition and retrieval of memory evidenced in significant in escape latency. However, pre-trial administration of S. mombin, C. acuminata or their combination for three consecutive days before scopolamine injection on day 3 significantly decreased escape latency time in a non-dose dependent manner from day 2 to 5. The reduction in escape latency from day to day reflects learning with respect to reference or long-term memory. This suggests that S. mombin and C. acuminata significantly ameliorated the deficit in long-term memory induced by scopolamine.
Previous studies showed that scopolamine induced amnesia is associated with increased oxidative stress in brain.Citation7,Citation39 In the present study, we found increased oxidative and nitrosative stresses evidenced in elevated MDA and nitrite levels in the prefrontal cortex, hippocampus and striatum of scopolamine-treated rats brain. Scopolamine was also found to disturb metabolism, especially for low molecular weight antioxidants such as glutathione, and therefore intensify the level of lipids peroxidation within the brain,Citation42 which is particularly vulnerable to reactive oxygen species (ROS) action due to high abundance of highly oxidizable polyunsaturated fatty acids. However, the pretreatment of rats with C. acuminata, S. mombin or their combinations ameliorated scopolamine-induced increase in MDA and nitrite levels in the prefrontal cortex. GSH on the other hand provides major protection in oxidative injury by participating in the cellular defence system against oxidative damage.Citation43 In this study, scopolamine injection caused significant decrease in the GSH, catalase and superoxide dismutase which were reversed by pretreatment of rats with S. mombin, C. acuminata or their combinations, which possibly renders them as promising antioxidants. The nitrite scavenging ability of S. mombin and C. acuminata extracts further expands their role as potent antioxidants. Furthermore, the results of the DPPH and FRAP assays indicate potent free radical scavenging effect of S. mombin and C. acuminata. DPPH is a stable free radical, when antioxidant reacts with DPPH, the electron is paired off and the DPPH solution is decolorized. The scavenging activity of the antioxidant or the bleaching of the colour stoichiometrically depends on the number of electrons taken up. This may be as a result of the hydrogen donating ability of the phenols in the extracts. The total phenolic and flavonoid content assays showed that the extracts are rich in polyphenolic compounds most importantly, flavonoids. It is presumed that the hydroethanolic extracts owe their antioxidant activity due to high phenolic and flavonoid contents.Citation44 Flavonoids have also been reported to act as acetylcholinesterase inhibitor.Citation45 In addition, the ferric ion reducing power capacity of the extracts further supports the potential antioxidant capacity of S. mombin and C. acuminata extracts. The reducing properties are generally connected with the presence of reductones.Citation45 The ferrous ion increases lipid oxidation through the breakdown of hydrogen and lipid peroxides to reactive free radicals via the Fenton reaction. It can also accelerate peroxidation by decomposing lipid hydroperoxides into peroxyl and alkoxyl radicals.Citation46 These radicals can themselves attract hydrogen and perpetuate the chain reaction of lipid peroxidation. Thus, inhibition of lipid peroxidation by extracts of S. mombin and C. acuminata can be a fundamental property through which they can mitigate the initiation and/or propagation of oxidative stress related diseases. Further study is ongoing in our laboratory to elucidate the active principles responsible for the observed effect and their action on cholinergic neurotransmission.
Findings from this study showed that the extracts of Spondias mombin and Cola acuminata possess memory enhancing effects possibly through enhancement of antioxidant defence systems. Thus, they could be potential phytotherapeutic agents in the treatment of dementia of Alzheimer’s type.
Conflicts of interest
The authors declare that the are no conflict of interest.
Acknowledgement
The authors are grateful to Mr. C. Micah of the Department of Pharmacology, Therapeutics and Toxicology, and Mr. S.A. Adenekan of the Department of Biochemistry, College of Medicine, University of Lagos, Lagos, Nigeria, for their technical assistance.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 6 April 2017
References
- X.ZhuB.SuX.WangM.A.SmithG.PerryCauses of oxidative stress in Alzheimer diseaseCell Mol Life Sci17200722022210
- F.MangialascheM.C.PolidoriR.MonasteroS.ErcolaniC.CamardaR.CecchettiBiomarkers of oxidative and nitrosative damage in Alzheimer’s disease and mild cognitive impairmentAgeing Res Rev842009285305
- M.W.DeckerJ.L.McGaughThe role of interactions between the cholinergic system and other neuromodulatory systems in learning and memorySynapse721991151168
- R.SchliebsT.ArendtThe cholinergic system in aging and neuronal degenerationBehav Brain Res22122011555563
- G.M.BoresF.P.HugerW.PetkoA.E.MutlibF.CamachoD.K.RushPharmacological evaluation of novel Alzheimer’s disease therapeutics: acetylcholinesterase inhibitors related to galantamineJ Pharmacol Exp Ther27721996728738
- H.AllainD.Bentué-FerrerO.TributS.GauthierB.F.MichelC. Drieu-LaRochelleAlzheimer’s disease: the pharmacological pathwayFundam Clin Pharmacol1742003419428
- S.TotaK.HanifP.K.KamatA.K.NajmiC.NathRole of central angiotensin receptors in scopolamine-induced impairment in memory, cerebral blood flow, and cholinergic functionPsychopharmacol (Berl)22222012185202
- I.O.IsholaS.TotaO.O.AdeyemiE.O.AgbajeT.NarenderR.ShuklaProtective effect of Cnestis ferruginea and its active constituent on scopolamine-induced memory impairment in mice: a behavioral and biochemical studyPharmaceut Biol5172013825835
- M.Y.AksenovM.V.AksenovaD.A.ButterfieldJ.W.GeddesW.R.MarkesberyProtein oxidation in the brain in Alzheimer’s diseaseNeuroscience10322001373383
- J.N.KellerF.A.SchmittS.W.ScheffQ.DingQ.ChenD.A.ButterfieldEvidence of increased oxidative damage in subjects with mild cognitive impairmentNeurology647200511521156
- A.M.SwomleyD.A.ButterfieldOxidative stress in Alzheimer disease and mild cognitive impairment: evidence from human data provided by redox proteomicsArch Toxicol8910201516691680
- T.IlićM.JovanovićA.JovicićM.TomovićOxidative stress and Parkinson’s diseaseVojnosanit Pregl5551998463468
- R.DringenJ.HirrlingerGlutathione pathways in the brainBiol Chem38442003505516
- C.M.MaierP.H.ChanRole of superoxide dismutases in oxidative damage and neurodegenerative disordersNeuroscientist842002323334
- G.E.CrichtonJ.BryanK.J.MurphyDietary antioxidants, cognitive function and dementia – a systematic reviewPlant Foods Hum Nutr6832013279292
- J.CorthoutL.PietersM.ClaeysD.V.BergheA.J.VlietinckAntivirally Active Gallotannins from Spondias mombinPlanta Med5461988573
- K.F.RodriguesM.HasseAntimicrobial activities of secondary metabolites produced by endophytic fungi from Spondias mombinJ Basic Microbiol402000261267
- K.F.RodriguesG.J.SamuelsFungal endophytes of Spondias mombin leaves in BrazilJ Basic Microbiol3919991518
- K.A.AboV.O.OgunleyeJ.S.AshidiAntimicrobial potential of Spondias mombin, Croton zambesicus and Zygotritonia croceaPhytother Res1361999494497
- A.O.AyokaR.O.AkomolafeE.O.IwalewaM.A.AkanmuO.E.UkponmwanSedative, antiepileptic and antipsychotic effects of Spondias mombin L. (Anacardiaceae) in mice and ratsJ Ethnopharmacol10322006166175
- T.O.ElufioyeA.T.OladeleC.M.Cyril-OlutayoJ.M.AgbedahunsiS.A.AdesanyaEthnomedicinal study and screening of plants used for memory enhancement and antiaging in Sagamu, NigeriaEur J Med Plants232012262275
- C.M.Cyril-OlutayoA.T.OladeleT.O.ElufioyeEthnobotanical survey of medicinal plants used in the management of memory loss and antiaging in Ondo State, NigeriaInt J Pharm2120122632
- N.NiemenakP.OnomoR.LiebereiD.NdoumouPurine alkaloids and phenolic compounds in three cola species and Garcinia kola grown in CameroonSouth Afr J Botany7442008629635
- G.ObohO.AgunloyeA.AkinyemiA.AdemiluyiS.AdefeghaComparative study on the inhibitory effect of caffeine and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rat’s brain in vitroNeurochem Res3822013413419
- Y.S.VeliogluG.MazzaL.GaoB.D.OomahAntioxidant activity and total phenolics in selected fruits, vegetable products and grain productsJ Agric Food Chem46199841134117
- A.KumaranR.J.KarunakaranAntioxidant activity of Cassia auriculata flowersFitoterapia78120074647
- P.PrietoM.PinedaM.AguilarSpectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin EAnal Biochem2691999337341
- F.M.AwahP.N.UzoegwuJ.O.OyugiJ.RutherfordP.IfeonuX.YaoFree radical scavenging activity and immunomodulatory effect of Stachytarpheta angustifolia leaf extractFood Chem119201014091416
- S.M.MirkovA.N.DjordjevicN.L.AndricS.A.AndricT.S.KosticG.M.BogdanovicNitric oxide-scavenging activity of polyhydroxylated fullerenol, C60(OH)24Nitric Oxide1122004201207
- M.OyaizuStudies on products of browning reaction prepared from glucosamineJapan J Nutr441986307314
- I.O.IsholaA.A.AkinyedeA.M.SholarinAntidepressant and anxiolytic properties of the methanolic extract of Momordica charantia Linn (Cucurbitaceae) and its mechanism of actionDrug Res (Stuttg)6472014368376
- M.SarterG.BodewitzD.N.StephensAttenuation of scopolamine-induced impairment of spontaneous alternation behavior by antagonist but not inverse agonist and antagonist β-carbolinePsychopharmacology941988491495
- R.G.MorrisDevelopment of a water maze procedure for studying spatial learning in the ratJ Neurosci Methods1119844760
- A.K.SinhaColorimetric assay of catalaseAnal Biochem4721972389394
- C.C.WinterbournR.E.HawkinsM.BrianR.W.CarrellThe estimation of red cell superoxide dismutase activityJ Lab Clin Med8521975337341
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem1931951265275
- I.KlinkenbergA.BloklandThe validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studiesNeurosci Biobehav Rev348201013071350
- E.J.JeongK.Y.LeeS.H.KimS.H.SungY.C.KimCognitive- enhancing and antioxidant activities of iridoid glycosides from Scrophularia buergeriana in scopolamine-treated miceEur J Pharmacol588120087884
- V.V.GiridharanR.A.ThandavarayanT.KonishiAmelioration of scopolamine induced cognitive dysfunction and oxidative stress by Inonotus obliquus – a medicinal mushroomFood Funct262011320327
- R.T.BartusR.L.DeanB.BeerA.S.LippaThe cholinergic hypothesis of geriatric memory dysfunctionScience2171982408417
- O.O.AdeyemiI.O.IsholaH.A.AdedejiNovel action of metformin in the prevention of haloperidol-induced catalepsy in mice: potential in the treatment of Parkinson’s disease?Prog Neuro-Psychopharmacol Biol Psychiat4832014245251
- D.A.El-SherbinyA.E.KhalifaA.S.AttiaEel-D.EldensharyHypericum perforatum extract demonstrates antioxidant properties against elevated rat brain oxidative status induced by amnestic dose of scopolaminePharmacol Biochem Behav763–42003525533
- N.BraidyM.ZarkaJ.WelchW.BridgeTherapeutic approaches to modulating glutathione levels as a pharmacological strategy in Alzheimer’s diseaseCurr Alzheimer Res1242015298313
- S.KumarA.K.PandeyChemistry and biological activities of flavonoids: an overviewScient World J201310.1155/2013/162750
- D.VauzourEffect of flavonoids on learning, memory and neurocognitive performance: relevance and potential implications for Alzheimer’s disease pathophysiologyJ Sci Food Agric946201410421056
- T.AhmedA.H.GillaniInhibitory effect of curcuminoids on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia may explain medicinal use of turmeric in Alzheimer’s diseasePharmacol Biochem Behav912009554559