Abstract
Glutamate delta-1 (GluD1) and glutamate delta-2 (GluD2) form the delta family of ionotropic glutamate receptors (iGluRs) and are distinct from other (iGluRs) in that they do not exhibit typical agonist-induced ion channel currents. Recent studies have demonstrated a crucial role of the delta receptors in synapse formation by interacting with presynaptic proteins such as Neurexin1. This review presents current knowledge regarding the expression, structure and function of Glu delta receptors (GluD1, GluD2) in brain, focusing on synapse formation, function and dysfunction.
1 Introduction
Glutamate is the main excitatory neurotransmitter in the vertebrate central nervous system. During the quest for ionotropic glutamate receptors (iGluRs) two peculiar candidates, GluRdelta1 (GluD1) and GluRdelta2 (GluD2), were cloned by sequence homology with iGluRs subunits of the AMPA, Kainate, and NMDA subtypes.Citation5–Citation7 However, delta subunits are unresponsive to glutamate and progress in identifying their functions has been slower than for other iGluRs.Citation8
In the central nervous system (CNS), GluD1 is expressed diffusely throughout the forebrain during early developmentCitation6,Citation11; however, its functional significance remains elusive. Recombinant GluD1 is endowed with a functional channel pore domain and promotes synapse formation in vitro.Citation25–Citation28 GluD1 knockout mice (GluD1 KO) have normal learning in the Morris water maze test and intact hippocampal long-term potentiation.Citation10 GluD1 is highly expressed in the inner ear hair cells.Citation9,Citation10 Deletion of GluD1 leads to a deficit in high frequency hearing in mice.Citation10 Genetic association studies have established the GRID1 gene, which codes for GluD1, as a strong candidate gene for schizophrenia, bipolar disorder, and major depressive disorder.Citation12–Citation19 GRID1 knockout (KO) mice exhibit behavioral correlates of schizophrenia symptoms, such as hyperaggressiveness and deficits in social interaction.Citation10,Citation32,Citation48 Copy number variation studies have also implicated GRID1 in autism spectrum disorder (ASD).Citation20–Citation22 In addition, GRID1 gene is localized to the 10q22–q23 genomic region which is a site for recurrent deletions associated with cognitive and behavioral abnormalities.Citation23,Citation24
GluD2 is required for proper development and function of the cerebellum.Citation1,Citation2 GluD2 acts as a synapse organizer via interactions with postsynaptic scaffold and signaling proteins, and with presynaptic parallel fiber terminals.Citation1,Citation44,Citation43 Moreover, the metabotropic glutamate receptor mGlu1 associates with GluD2Citation3 and triggers the opening of the GluD2 channel, which is critically involved in the slow glutamatergic current at the parallel fiber-to-Purkinje cell synapse.Citation4,Citation3
2 Expression of GluD1 and GluD2 in mammalian brain
GluD1 is highly expressed in the forebrain including the cortex and hippocampusCitation6,Citation10,Citation32,Citation37 and recent studies also indicate expression in cerebellar interneurons.Citation33 In the cortex and hippocampus high level of GluD1 mRNA and protein appears in pyramidal neurons.Citation10,Citation34,Citation33 Original studies of delta subunits mRNA distribution in the rodent brain report selective GluD2 expression in Purkinje cells and rapid postnatal decrease of GluD1 expression down to low levels in the adult.Citation5,Citation6 However, subsequent reports indicate that adult expression of both subunits is more widespread than originally described.Citation10,Citation11,Citation32,Citation36 Recently Hepp et al. used a combination of in situ hybridization, RTPCR, Western blot and immunohistochemistry to characterize the expression patterns of GluD1 and GluD2 in the rodent brain. GluD1 was expressed in neurons throughout the brain, with higher levels in the forebrain and lower levels in the cerebellum. GluD1 was localized at the postsynaptic density of excitatory synapses on hippocampal pyramidal cells. GluD2 expression was also widespread but was markedly enriched in the cerebellum. Likewise, the GluD1/GluD2 mRNA ratio was high in the cortex and low in the cerebellum.Citation29 Their results support a role for the delta family of glutamate receptors in neuronal networks throughout the adult brain.
3 Molecular structure of GluD1 and GluD2
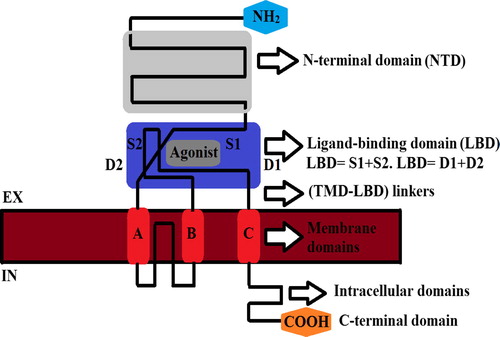
GluD1 and GluD2 consist of a N-terminal domain and a bipartite ligand-binding domain on the extracellular side of the plasma membrane, three transmembrane domains and an ion-channel-forming re-entrant loop segment, and a cytoplasmic C-terminal domain. Four subunits assemble to form a functional receptor. NTD, N-terminal domain; S1 and S2, sequence segments that form the ligand binding domain (LBD); D1 and D2, globular domains of the LBD, corresponding to the two lobes of the clamshell-like structure; A, B, C, transmembrane domains; P, pore helix and pore loop; CTD, C-terminal domain. The main difference between delta receptors and other ionotropic glutamate receptors lies within their LBDs. However, there are also some subtle differences in electrophysiological and gating properties, demonstrating that in delta receptors the ion channel and the linkers are connecting it to the LBD function slightly differently than in other glutamate receptorsCitation26 .
Figure 1 Modular domain structure of delta receptors (GluD1, GluD2). Four subunits assemble to form a functional receptor. NTD, N-terminal domain; S1 and S2, sequence segments that form the ligand binding domain (LBD); D1 and D2, globular domains of the LBD, corresponding to the two lobes of the clamshell-like structure; A, B, C, transmembrane domains.
4 Role of GluD1 receptor in synaptogenesis
Synaptogenesis is the formation of synapses between neurons in the nervous system. Although it occurs throughout a healthy person’s life span, an explosion of synapse formation occurs during early brain development, known as exuberant synaptogenesis.Citation39 During development, early spherical neural progenitor cells give rise to many processes, the neurites; one of these early neurites subsequently transforms into an axon while others develop into dendrites. The growing axons that come in contact with other neurons form terminal presynaptic swellings. These presynaptic swellings possess specific neurotransmitter as well as cognate receptors; they also influence the post-synaptic neurons to express desired receptors. The number of presynaptic swellings, their morphometric characteristics, receptor decoration and other properties may be increased, modified or lost. These individual or collective changes influence somatic and autonomic behaviors including cognition as well as consciousness, sensitivity, new and/or existing learning and memory, or recovery processes following an injury or a disease.Citation40–Citation42
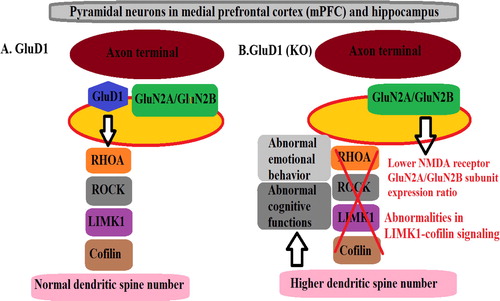
Recent studies have demonstrated a crucial role of the delta receptors in synapse formation by interacting with presynaptic proteins such as Neurexin1.Citation43,Citation44,Citation27,Citation46 Although the synaptic function of GluD2 expressed in Purkinje cells has been extensively studied, the function of GluD1 in native system remains poorly understood. Yadav et al. showed that deletion of GluD1 leads to abnormal emotional and social behaviors. They found that GluD1 knockout mice (GluD1 KO) were hyperactive, manifested lower anxiety-like behavior, depression-like behavior in a forced swim test and robust aggression in the resident-intruder test. Chronic lithium rescued the depression-like behavior in GluD1 KO. GluD1 KO mice also manifested deficits in social interaction. They proposed that deletion of GluD1 leads to aberrant circuitry in prefrontal cortex and amygdala owing to its potential role in presynaptic differentiation and synapse formation.Citation32 In another study Yadav et al., evaluated GluD1 KO in learning and memory tests. They proposed that GluD1 receptor is essential for normal synapse formation and maintenance and deletion of GluD1 leads to synaptic abnormalities in the amygdala, prefrontal cortex and hippocampus that lead to social and emotional deficits as well as deficits in learning and memory.Citation48 The results of Gupta et al., demonstrated a critical role of GluD1 in maintaining spine dynamics. They found that pyramidal neurons in adult GluD1 KO medial prefrontal cortex (mPFC) and hippocampus have higher dendritic spine number that may occur due to impaired spine pruning or excessive spine generation. They also observed abnormalities in LIMK1-cofilin signaling which is involved in regulating spine dynamics and a lower NMDA receptor GluN2A/GluN2B subunit expression ratio suggesting a potential impairment in the GluN2B to GluN2A developmental switch. Moreover, inhibition of GluN2B-containing receptors was found to reverse signaling abnormalities and spine density as well as stereotyped behavior and depression-like behavior in GluD1 KO miceCitation37 . These results may have implications for disorders such as autism spectrum disorder (ASD).
Figure 2 Signaling pathway of GluD1 receptor in the pyramidal neurons in medial prefrontal cortex (mPFC) and hippocampus. A normal LIMK1-cofilin signaling and expression of NMDA receptor GluN2A/GluN2B subunit expression ratio and normal dendritic spine number in the pyramidal neurons in the presence of GluD1 receptor in medial prefrontal cortex (mPFC) and hippocampus. B show abnormalities in LIMK1-cofilin signaling and lower expression of NMDA receptor GluN2A/GluN2B subunit expression ratio, higher dendritic spine number in the GluD1 KO in the pyramidal neurons in medial prefrontal cortex (mPFC) and hippocampus and abnormal emotional behavior and abnormal cognitive functions in mice.
5 Role of GluD2 receptor in cerebellar long-term depression (LTD) and synaptogenesis
GluD2 receptors are predominantly localized in the postsynaptic density of excitatory synapses in the central nervous system (CNS). GluD2 was previously designated as an “orphan” iGluR, as no endogenous ligands had been identified that could bind and activate the receptor.Citation5,Citation6 D-Ser and glycine have now been identified as ligands for GluD2.Citation51
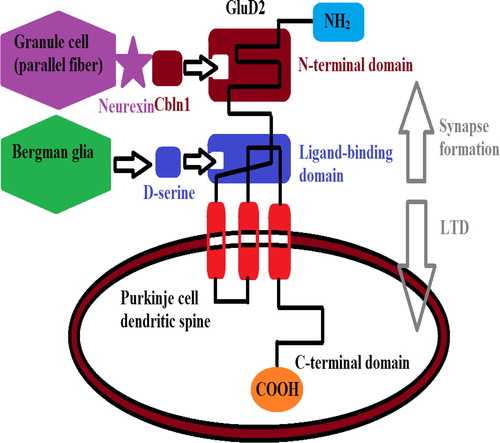
The role of GluD2 in the CNS is most studied in the cerebellum, where GluD2 receptors are expressed in glutamatergic synapses of the Purkinje-type neurons.Citation52–Citation54 A key role for GluD2 in postsynaptic functions in cerebellar Purkinje neurons, including induction of cerebellar long-term depression (LTD), a form of synaptic plasticity that underlies motor learning, has been demonstrated.Citation52,Citation75 Endogenous D-Ser binding to GluD2 has been shown to regulate LTD in Purkinje neurons.Citation55 This modulation required the intact intracellular C-terminal domain (CTD) of GluD2, which interacts with a range of scaffolding and signaling proteins.Citation73 C-terminal portion of GluD2 consists of a PDZ binding motif, to which PDZ proteins, such as PSD-93, protein tyrosine phosphatase (PTPMEG), synaptic scaffolding molecule SSCAM, n-PIST, and delphilin can bind.Citation54 Furthermore, D-serine released from Bergmann glia can bind to the ligand binding domain of GluD2 and induce AMPA receptor endocytosis and LTD.Citation55 Similarly, application of an antibody against the ligand-binding domain of GluD2 induces AMPA receptor endocytosis and LTD in the wild-type adult cerebellum.Citation52 Importantly, D-serine binding to GluD2 fails to induce LTD when the C-terminal domain is deleted or when PKC inhibitory peptide is included in the patch pipette.Citation55 It can be speculated that D-Ser binding to the extracellular LBD may induce conformational changes at the CTD that potentially control GluD2 interactions with intracellular effector proteins required for LTD induction.
In addition to a direct signaling role, the extracellular part of GluD2 binds the protein Cbln1, which is secreted from cerebellar granule cells, and this interaction is essential for synapse integrity between Purkinje cells and cerebellar granule cells in adult mice.Citation58,Citation44,Citation60–Citation62,Citation75 Together, these results indicate that GluD2 contributes to two major functions at PF–Purkinje cell synapses—synapse formation/maintenance and LTD induction.
Precise neuronal circuitry is established by the coordinated formation of excitatory and inhibitory synapses. A loss of balance between excitation and inhibition leads to aberrant information processing, which is associated with various forms of neurodevelopmental and neuropsychiatric disorder.Citation63–Citation67 Purkinje cells (PCs), which send the only output from the cerebellar cortex, receive two excitatory inputs, from parallel fibers (PFs; axons of the granule cells) and climbing fibers; they receive inhibitory input from two groups of molecular-layer interneurons (MLIs), basket cells and stellate cells. A key molecule that induces excitatory synaptogenesis between PCs and PFs is Cbln1, a C1q-family glycoprotein that is secreted from PFs. Among synapse organizers, Cbln1 is unique because it is indispensable for the formation and maintenance of synapses in vivo.Citation68,Citation69,Citation61 The role of Cbln1 in excitatory synaptogenesis is well defined, whether and how Cbln1 regulates inhibitory synapses on PCs has remained unclear. A recent study by Ito-Ishida et al., showed that Cbln1–GluD2 signaling shifts the excitatory–inhibitory balance toward excitation in PCs, by downregulating MLI–PC synapses while increasing the number of excitatory synapses from PFsCitation72 . Because activation of MLI–PC synapses is essential to fine-tune the onset of PC action potentials, which regulate motor coordination and learning,Citation70,Citation71 it can be speculated that the Cbln1-mediated suppression of MLI–PC synapse functions has a significant physiological impact on such behaviors.
Figure 3 GluD2 signaling pathway. Cbln1 released from parallel fibers (the axons of the granule cells) binds to neurexin containing splice-site 4 (S4+) at the presynaptic site and the N-terminus of GluD2 at the postsynaptic site. The neurexin/Cbln1/GluD2 tripartite complex traverses the synaptic cleft and may function as a bidirectional synaptic organizer. D-serine released from Bergmann glia binds to the ligand-binding domain of GluD2 and regulates AMPA receptor endocytosis and long-term depression (LTD) via the C-terminus of GluD2.
6 Concluding remarks
Defects in synapses, including their formation, function, and maintenance, however, are of particular interest not only because they are basic functional units of the brain and continue to be modified by experience throughout life, but also because molecular and structural changes occurring in synapses may be the most immediately targetable for therapeutic interventions after birth.Citation76–Citation79 Recent studies have demonstrated a crucial role of the delta receptors (GluD1, GluD2) in synapse formation.
A major hypothesis for the underlying etiology of autism and SCZ is that of synaptic dysfunction. Recent studies have implicated GRID1 in autism spectrum disorder (ASD) and SCZ. GRID1 knockout (KO) mice exhibit behavioral correlates of schizophrenia symptoms, such as hyperaggressiveness and deficits in social interaction.Citation10,Citation32,Citation48 Studies have shown that delta receptors (GluD1, GluD2) play significant role in synapse formation and might play a role in the underlying pathophysiology of the autism and SCZ. However, there is a lack of clear data supporting its role in autism and SCZ; therefore, complementary studies are needed to fully clarify delta receptors functions. This emphasizes the need to evaluate its role in the brain by using different animal models of the autism and SCZ.
Conflict of interest
The author declare that there is no conflict of interest.
Acknowledgments
We gratefully acknowledge funding from National 12th Five year Plan “Major Scientific and Technological Special Project for Significant New Drugs Creation” project of “Novel G protein coupled receptor targeted drug screening system and key technology research” (No. 2012ZX09504001-001) and Program for New Century Excellent Talents in University (No. NCET-10-0817), which have supported aspects of our research covered in this review. The author would like to thank Professor Atlas khan for his expert comments and contribution to English editing of this manuscript.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 20 October 2016
References
- W.KakegawaT.MiyazakiK.EmiK.MatsudaK.KohdaJ.MotohashiM.MishinaS.KawaharaM.WatanabeM.YuzakiDifferential regulation of synaptic plasticity and cerebellar motor learning by the C-terminal PDZ-binding motif of GluRdelta2J Neurosci286200814601468
- N.KashiwabuchiK.IkedaK.ArakiT.HiranoK.ShibukiC.TakayamaY.InoueT.KutsuwadaT.YagiY.KangImpairment of motor coordination, Purkinje cell synapse formation, and cerebellar long-term depression in GluR delta 2 mutant miceCell8121995245252
- A.S.KatoM.D.KniermanE.R.SiudaJ.T.IsaacE.S.NisenbaumD.S.BredtGlutamate receptor delta2 associates with metabotropic glutamate receptor 1 (mGluR1), protein kinase Cgamma, and canonical transient receptor potential 3 and regulates mGluR1-mediated synaptic transmission in cerebellar Purkinje neuronsJ Neurosci324420121529615308
- V.AdyJ.PerroyL.TricoireC.PiochonS.DadakX.ChenI.DusartL.FagniB.LambolezC.LevenesType 1 metabotropic glutamate receptors (mGlu1) trigger the gating of GluD2 delta glutamate receptorsEMBO Rep1512014103109
- K.ArakiH.MeguroE.KushiyaC.TakayamaY.InoueM.MishinaSelective expression of the glutamate receptor channel delta 2 subunit in cerebellar Purkinje cellsBiochem Biophys Res Commun1973199312671276
- H.LomeliR.SprengelD.J.LaurieG.KohrA.HerbP.H.SeeburgW.WisdenThe rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor familyFEBS Lett31531993318322 pii: 0014–5793(93)81186–4
- M.YamazakiK.ArakiA.ShibataM.MishinaMolecular cloning of a cDNA encoding a novel member of the mouse glutamate receptor channel familyBiochem Biophys Res Commun18321992886892
- S.M.SchmidM.HollmannTo gate or not to gate: are the delta subunits in the glutamate receptor family functional ion channels?Mol Neurobiol372–32008126141
- S.SafieddineR.J.WentholdThe glutamate receptor subunit delta1 is highly expressed in hair cells of the auditory and vestibular systemsJ Neurosci17199775237531
- J.GaoS.F.MaisonX.WuK.HiroseS.M.JonesOrphan glutamate receptor delta1 subunit required for high-frequency hearingMol Cell Biol27200745004512
- E.MayatR.S.PetraliaY.X.WangR.J.WentholdImmunoprecipitation, immunoblotting, and immunocytochemistry studies suggest that glutamate receptor delta subunits form novel postsynaptic receptor complexesJ Neurosci15199525332546
- M.D.FallinV.K.LasseterD.AvramopoulosK.K.NicodemusP.S.WolyniecBipolar I disorder and schizophrenia: a 440-single-nucleotide polymorphism screen of 64 candidate genes among Ashkenazi Jewish caseparent triosAm J Hum Genet772005918936
- S.Z.GuoK.HuangY.Y.ShiW.TangJ.ZhouA case-control association study between the GRID1 gene and schizophrenia in the Chinese Northern Han populationSchizophr Res932007385390
- T.VenkenM.AlaertsD.SoueryD.GoossensS.SluijsChromosome 10q harbors a susceptibility locus for bipolar disorder in Ashkenazi Jewish familiesMol Psychiatry132008442450
- J.TreutleinT.W.MuhleisenJ.FrankM.MattheisenS.HermsDissection of phenotype reveals possible association between schizophrenia and Glutamate Receptor Delta 1 (GRID1) gene promoterSchizophr Res1112009123130
- Y.ZhuT.KalbfleischM.D.BrennanY.LiA MicroRNA gene is hosted in an intron of a schizophrenia-susceptibility geneSchizophr Res10920098689
- T.A.GreenwoodL.C.LazzeroniS.S.MurrayK.S.CadenheadM.E.CalkinsAnalysis of 94 candidate genes and 12 endophenotypes for schizophrenia from the consortium on the genetics of schizophreniaAm J Psychiatry1682011930946
- M.OrsettiF.Di BriscoP.L.CanonicoA.A.GenazzaniP.GhiGene regulation in the frontal cortex of rats exposed to the chronic mild stress paradigm, an animal model of human depressionEur J Neurosci27200821562164
- M.OrsettiF.Di BriscoM.RinaldiD.DallortoP.GhiSome molecular effectors of antidepressant action of quetiapine revealed by DNA microarray in the frontal cortex of anhedonic ratsPharmacogenet Genomics192009600612
- J.T.GlessnerK.WangG.CaiO.KorvatskaC.E.KimAutism genome-wide copy number variation reveals ubiquitin and neuronal genesNature4592009569573
- M.SmithM.A.SpenceP.FlodmanNuclear and mitochondrial genome defects in autismsAnn N Y Acad Sci11512009102132
- G.M.CooperB.P.CoeS.GirirajanJ.A.RosenfeldT.H.VuA copy number variation morbidity map of developmental delayNat Genet432011838846
- J.BalciunieneN.FengK.IyaduraiB.HirschL.CharnasRecurrent 10q22–q23 deletions: a genomic disorder on 10q associated with cognitive and behavioral abnormalitiesAm J Hum Genet802007938947
- B.W.van BonJ.BalciunieneG.FruhmanS.C.NagamaniD.L.BroomeThe phenotype of recurrent 10q22q23 deletions and duplicationsEur J Hum Genet192011400408
- T.KuroyanagiM.YokoyamaT.HiranoPostsynaptic glutamate receptor delta family contributes to presynaptic terminal differentiation and establishment of synaptic transmissionProc Natl Acad Sci USA10612200949124916
- A.OrthD.TapkenM.HollmannThe delta subfamily of glutamate receptors: characterization of receptor chimeras and mutantsEur J Neurosci3710201316201630
- K.RyuM.YokoyamaM.YamashitaT.HiranoInduction of excitatory and inhibitory presynaptic differentiation by GluD1Biochem Biophys Res Commun41712012157161
- R.YadavR.RimermanM.A.ScofieldS.M.DravidMutations in the transmembrane domain M3 generate spontaneously open orphan glutamate delta1 receptorBrain Res1382201118
- R.HeppY.A.HayC.AguadoR.LujanL.DauphinotM.C.PotierS.NomuraO.PoirelS.E.MestikawyB.LambolezL.TricoireGlutamate receptors of the delta family are widely expressed in the adult brainBrain Struct Funct201410.1007/s00429-014-0827-4 July 8
- R.YadavS.C.GuptaB.G.HillmanJ.M.BhattD.J.StairsS.M.DravidDeletion of glutamate delta-1 receptor in mouse leads to aberrant emotional and social behaviorsPLoS One72012e32969
- K.KonnoK.MatsudaC.NakamotoM.UchigashimaT.MiyazakiM.YamasakiK.SakimuraM.YuzakiM.WatanabeEnriched expression of GluD1 in higher brain regions and its involvement in parallel fiber-interneuron synapse formation in the cerebellumJ Neurosci342014e7412e7424
- E.S.LeinM.J.HawrylyczN.AoGenome-wide atlas of gene expression in the adult mouse brainNature4452007e168e176
- M.YamasakiT.MiyazakiH.AzechiM.AbeR.NatsumeT.HagiwaraA.AibaM.MishinaK.SakimuraM.WatanabeGlutamate receptor delta2 is essential for input pathwaydependent regulation of synaptic AMPAR contents in cerebellar Purkinje cellsJ Neurosci319201133623374
- S.C.GuptaR.YadavR.PavuluriB.J.MorleyD.J.StairsS.M.DravidEssential role of GluD1 in dendritic spine development and GluN2B to GluN2A NMDAR subunit switch in the cortex and hippocampus reveals ability of GluN2B inhibition in correcting hyperconnectivityNeuropharmacology932015274284 June
- P.R.HuttenlocherA.S.DabholkarRegional differences in synaptogenesis in human cerebral cortexJ Comp Neurol38721997167178
- S.R.Y.CajalThe croonian lecture: la fine structure des centres nerveuxProc R Soc London551894444468
- E.R.KandelW.A.SpencerCellular neurophysiological approaches in the study of learningPhysiol Rev48196865134
- M.MayfordS.A.SiegelbaumER.KandelSynapses and memory storageCold Spring Harb Perspect Biol20124
- T.UemuraS.J.LeeM.YasumuraT.TakeuchiT.YoshidaM.RaR.TaguchiK.SakimuraM.MishinaTrans-synaptic interaction of GluRdelta2 and neurexin through Cbln1 mediates synapse formation in the cerebellumCell1412010e1068e1079
- K.MatsudaE.MiuraT.MiyazakiCbln1 is a ligand for an orphan glutamate receptor delta2, a bidirectional synapse organizerScience (New York, N.Y.)3282010e363e368
- M.YasumuraT.YoshidaS.J.LeeT.UemuraJ.Y.JooM.MishinaGlutamate receptor delta1 induces preferentially inhibitory presynaptic differentiation of cortical neurons by interacting with neurexins through cerebellin precursor protein subtypesJ Neurochem1212012e705e716
- R.YadavB.G.HillmanS.C.GuptaP.SuryavanshiJ.M.BhattR.PavuluriD.J.StairsS.M.DravidDeletion of glutamate delta-1 receptor in mouse leads to enhanced working memory and deficit in fear conditioningPLoS One82013e60785
- P.NaurK.B.HansenA.S.KristensenS.M.DravidD.S.PickeringL.OlsenB.VestergaardJ.EgebjergM.GajhedeS.F.TraynelisIonotropic glutamate-like receptor delta2 binds D-serine and glycineProc Natl Acad Sci USA10420071411614121
- H.HiraiT.LauneyS.MikawaT.TorashimaD.YanagiharaT.KasauraA.MiyamotoM.YuzakiNew role of delta2-glutamate receptors in AMPA receptor trafficking and cerebellar functionNat Neurosci62003869876
- M.YuzakiThe delta2 glutamate receptor: a key molecule controlling synaptic plasticity and structure in Purkinje cellsCerebellum320048993
- M.YuzakiNew (but old) molecules regulating synapse integrity and plasticity: Cbln1 and the delta2 glutamate receptorNeuroscience1622009633643
- W.KakegawaY.MiyoshiK.HamaseS.MatsudaK.MatsudaK.KohdaK.EmiJ.MotohashiR.KonnoK.ZaitsuD-serine regulates cerebellar LTD and motor coordination through the d2 glutamate receptorNat Neurosci142011603611
- E.MiuraK.MatsudaJ.I.MorganM.YuzakiM.WatanabeCbln1 accumulates and colocalizes with Cbln3 and GluRdelta2 at parallel fiber-Purkinje cell synapses in the mouse cerebellumEur J Neurosci292009693706
- M.YuzakiSynapse formation and maintenance by C1q family proteins: a new class of secreted synapse organizersEur J Neurosci322010191197
- M.YuzakiCbln1 and its family proteins in synapse formation and maintenanceCurr Opin Neurobiol212011215220
- K.MatsudaM.YuzakiCbln family proteins promote synapse formation by regulating distinct neurexin signaling pathways in various brain regionsEur J Neurosci33201114471461
- K.TabuchiJ.BlundellM.R.EthertonR.E.HammerX.LiuC.M.PowellT.C.SudhofA neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in miceScience31820077176
- H.T.ChaoH.ChenR.C.SamacoM.XueM.ChahrourJ.YooJ.L.NeulS.GongH.C.LuN.HeintzM.EkkerJ.L.RubensteinJ.L.NoebelsC.RosenmundH.Y.ZoghbiDysfunction in GABA signalling mediates autism-like stereotypies and Rett syndrome phenotypesNature4682010263269
- O.YizharL.E.FennoM.PriggeF.SchneiderT.J.DavidsonD.J.O’SheaV.S.SohalI.GoshenJ.FinkelsteinJ.T.PazK.StehfestR.FudimC.RamakrishnanJ.R.HuguenardP.HegemannK.DeisserothNeocortical excitation/inhibition balance in information processing and social dysfunctionNature4772011171178
- O.MarinInterneuron dysfunction in psychiatric disordersNat Rev Neurosci132012107120
- M.L.WallaceA.C.BuretteR.J.WeinbergB.D.PhilpotMaternal loss of Ube3a produces an excitatory/inhibitory imbalance through neuron type-specific synaptic defectsNeuron742012793800
- A.Ito-IshidaE.MiuraK.EmiK.MatsudaT.IijimaT.KondoK.KohdaM.WatanabeM.YuzakiCbln1 regulates rapid formation and maintenance of excitatory synapses in mature cerebellar Purkinje cells in vitro and in vivoJ Neurosci28200859205930
- W.KakegawaT.MiyazakiK.KohdaK.MatsudaK.EmiJ.MotohashiM.WatanabeM.YuzakiThe N-terminal domain of GluD2 (GluRdelta2) recruits presynaptic terminals and regulates synaptogenesis in the cerebellum in vivoJ Neurosci29200957385748
- P.WulffM.SchonewilleM.RenziL.ViltonoM.Sassoe-PognettoA.BaduraZ.GaoF.E.HoebeekS.van DorpW.WisdenM.FarrantC.I.De ZeeuwSynaptic inhibition of Purkinje cells mediates consolidation of vestibulo-cerebellar motor learningNat Neurosci12200910421049
- C.I.De ZeeuwF.E.HoebeekL.W.BosmanM.SchonewilleL.WitterS.K.KoekkoekSpatiotemporal firing patterns in the cerebellumNat Rev Neurosci122011327344
- A.Ito-IshidaW.KakegawaK.KohdaE.MiuraS.OkabeM.YuzakiCbln1 downregulates the formation and function of inhibitory synapses in mouse cerebellar Purkinje cellsEur J Neurosci398201412681280 April
- A.S.KristensenK.B.HansenP.NaurL.OlsenN.L.KurtkayaS.M.DravidT.KvistF.YiJ.PøhlsgaardR.P.ClausenM.GajhedeJ.S.KastrupS.F.TraynelisPharmacology and structural analysis of ligand binding to the orthosteric site of glutamate-likeGluD2 receptorsMol Pharmacol8922016253262 February
- M.YuzakiCerebellar LTD vs. motor learning-lessons learned from studying GluD2Neural Netw472013364110.1016/j.neunet.2012.07.001 November
- M.Z.KhanL.HeThe role of polyunsaturated fatty acids and GPR40 receptor in brainNeuropharmacology201510.1016/j.neuropharm.2015.05.013 May 22. pii: S0028–3908(15)00194-X
- M.Z.KhanL.HeX.ZhaungThe emerging role of GPR50 receptor in brainBiomed Pharmacotherapy782016121128
- M.Z.KhanX.ZhuangL.HeGPR40 receptor activation leads to CREB Phosphorylation and improves cognitive performance in an Alzheimer’s disease mouse modelNeurobiol Learning Memory201610.1016/j.nlm.2016.03.006
- M.Z.KhanA possible significant role of zinc and GPR39 zinc sensing receptor in Alzheimer disease and epilepsyBiomed Pharmacotherapy792016263272 April
