?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present study was designed to investigate the free radical defence and antihyperglycemic property of S. mundagam. Petroleum ether, ethyl acetate, methanol and hot water extracts of bark were determined for the total phenolic, tannin, flavonoid content and antioxidant property using DPPH, ABTS+, phosphomolybdenum, FRAP, superoxide, nitric oxide and metal chelating assays. The antioxidant response was best observed in ABTS+ (109686.87 μM TE/g extract), phosphomolybdenum (268.54 g AAE/100 g extract) and superoxide radical scavenging assays (84.30%). Bark methanol extract was found highly efficient in scavenging the free radicals than other extracts. The higher phenolic content (54.44 g GAE/100 extract) could be attributed to this effect. The glucose homeostasis was observed till 180th min in glucose loaded rats treated with the bark methanol extract. The extract could also induce potent hypoglycaemia in STZ induced diabetic rats. The antioxidant defence system could be one of the prime mechanisms of S. mundagam leaf and bark extracts that needs to be studied further for the exact molecular action leading to antidiabetic effect.
1 Introduction
Diabetes mellitus (DM) is grabbing an alarming interest among the scientists and researchers globally. It is one of the most prevalent diseases with the highest number of patients in India. Type-2 diabetes occurs when there is a primary defect in insulin resistance, coupled with a relative insulin deficiency. The major complications of DM include aggravated atherosclerotic disease of the heart, myocardial infarction, heart failure, predisposition to infection, limited joint mobility, hardening of the skin and cataract formation.Citation1 The pathogenesis of type-2 diabetes is not fully understood. Animal models of type-2 diabetes have been proved to be useful to study the pathogenesis of and to find a new therapy for, the disease.Citation2 Oxidative stress and free radicals makes the diabetic condition more severe. Various mechanisms have been suggested to contribute the formation of these reactive oxygen-free radicals in diabetic condition. Glucose oxidation is believed to be the main source of free radicals. Glucose in enediol form get oxidized in a transition-metal dependent reaction to an enediol radical anion that is converted into reactive ketoaldehydes and to superoxide anion radicals.Citation3,Citation4 The superoxide anion radicals can lead to production of extremely reactive hydroxyl radicals, superoxide anion radicals which can also react with nitric oxide to form reactive peroxynitrite radicals.Citation5,Citation6 Hyperglycemia is also found to promote lipid peroxidation of low density lipoprotein (LDL) by a superoxide-dependent pathway resulting in the generation of free radicals.Citation7,Citation8 Living organism has its own enzymatic and non-enzymatic antioxidant defence system against free radicals. When the balance between these antioxidants and pro-oxidants collapses, there becomes a need of supplementing antioxidants in diet or as medicine which could also block the progression of diabetes. Plants could be the best source to fulfil the need as medicine and food against oxidative stress induced diabetes. The adverse effect by continuous use of synthetic drugs has encouraged the use of plant based medicine which could provide maximum healing with minimum or no side effect. Traditional medical practitioners have identified several such plants which are needed to be validated scientifically. The most common drug metformin recommended as a hypoglycaemic drug was developed from Galega officinalis. Thus, it is obvious that the plants with such wonder drugs are in urgent search to treat diabetes but this fact is yet to gain importance among people throughout the world.
Syzygium sp. are well known for their medicinal properties especially for its antidiabetic potential.Citation9–Citation12 Hence, the study concentrated on the search for the plants from the genus Syzygium which could be another demanding source of antidiabetic drug. Syzygium mundagam of the family Myrtaceae, is endemic to Western Ghats. The plant is one of the antidiabetic ingredients in formulations with tulsi.Citation13 The fruits of the tree are eaten by the Paniya and Kuruma tribes of Wayanad.Citation14 Hence the attempt has been made to investigate the antioxidant property of S. mundagam that would also help in controlling diabetic conditions.
2 Material and methods
2.1 Collection of plant materials
The leaves and bark of S. mundagam were collected during October 2011 from Chanthanathodu, Wayanad district of Kerala, Western Ghats, India. the authenticity of S. mundagam was confirmed (CMPR 7932) by Dr. M. Prabhukumar, Scientist and Head-in-charge, Plant Systematic and Genetic Resources Division, Centre for Medicinal Plant Research, Arya Vaidya Sala, Kottakkal, India. Freshly collected leaves and bark were cleaned to remove adhering dust and then dried under shade. The dried sample was powdered and used for further studies.
2.2 Chemicals
Ferric chloride, 2,2-diphenyl-1-picryl-hydrazyl (DPPH), potassium persulfate, 2,2′aninobis(3-ethylbenzothiozoline-6-sulfonic acid) disodium salt (ABTS), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), ferrous chloride, 2,4,6-tripyridyl-s-triazine (TPTZ), polyvinyl polypyrrolidone (PVPP), ethylenediamine tetracetic acid (EDTA) disodium salt streptozotocin, nicotinamide were obtained from Himedia (Mumbai, India), Merck (Hyderabad, India) and Sigma (Thane, India). All other reagents used were of analytical grade.
2.3 Successive solvent extraction
The powdered plant material (100 g) was extracted in Soxhlet extractor successively with petroleum ether, ethyl acetate and methanol (300 mL). Finally, the material was macerated using hot water with occasional stirring for 24 h and the aqueous extract was filtered. Each time before extracting with the next solvent, the material was dried in hot air oven below 40 °C. The different solvent extracts were concentrated by rotary vacuum evaporator and air dried. The dried extract obtained with each solvent was weighed. The percentage yield was expressed in terms of air dried weight of plant material.
2.4 Quantification of total phenolics, tannins and flavanoids
The total phenol content of leaf and bark extracts was determined according to the method described by Siddhuraju and Becker.Citation15 The results are expressed as gallic acid equivalents. The extracts incubated with polyvinyl polypyrrolidone (PVPP) were used for tannin estimation.Citation16 The tannin content of the sample was calculated as follows:
The flavonoid contentsCitation17 were quantified as it acts as major antioxidants in plants reducing oxidative stress. The amount of flavonoid was calculated in rutin equivalents.
2.5 Total antioxidant activity assay by radical cation 2,2′-azinobis (3-ethylebenzothiozoline-6-sulphonic acid) (ABTS+)
ABTS radical cation decolorization assay was done to determine the total antioxidant activity of the samples according to the method of Re et al.Citation18 The total antioxidant activity (TAA) was expressed in μM trolox equivalence/g extract.
2.6 Ferric reducing antioxidant power (FRAP) assay
The antioxidant capacities of phenolic extracts of samples were estimated according to the procedure described by Pulido et al.Citation19 The FRAP value is expressed as mmol Fe (II) equivalent/mg extract.
2.7 Phosphomolybdenum assay
The antioxidant activity of samples was evaluated by the phosphomolybdenum method.Citation20 The results were reported in ascorbic acid equivalents per gram extract (AEAC).
2.8 Radical scavenging activity using DPPH⋅ method
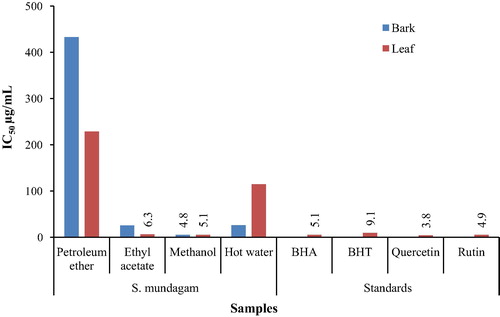
The DPPH radical scavenging activity of extracts was determined by the method of Blois.Citation21 More significantly the IC50 of the extracts were also calculated.
2.9 Metal chelating activity
The chelating of ferrous ions by leaf and bark extracts was estimated by the method of Dinis et al.Citation22 The metal chelating activity was determined in percentage of inhibition.
2.10 Assay of superoxide radical (O2−) scavenging activity
The ability to inhibit formazan formation by scavenging superoxide radicals by the extracts was studied by the method of Beauchamp and Fridovich.Citation23 The percentage inhibition of superoxide anion generation was calculated using the following formula:
2.11 Nitric oxide radical scavenging activity
The procedure is based on the method, where sodium nitroprusside in aqueous solution at physiological pH, spontaneously generates nitric oxide, which interacts with oxygen to produce nitrite ions that can be estimated using Greiss reagent. Scavengers of nitric oxide (extract and standard at 100 μg) compete with oxygen leading to reduced production of nitrite ions. The absorbance of the chromophore formed was read at 546 nm.Citation24 The percentage inhibition of nitric oxide was calculated using the formula:
2.12 Animals
Healthy Wistar albino rats (100–150 g) and Swiss albino mice (20–25 g) of either sex and of approximately the same age were used in this study. They were fed with standard chow diet and water ad libitum and were housed in polypropylene cages under controlled temperature.
2.13 Acute toxicity
Acute oral toxicity studies were performedCitation25 according to the OECD (Organization for Economic Co-operation and Development) guidelines. Male mice (n = 6/each dose) were selected for acute toxicity study. The animals were fasted overnight with free access to water. Extract (suspended in 0.6% carboxy methyl cellulose) was administered orally at a dose of 5 mg/kg. The general behaviours such as motor activity, tremors, convulsions, straub reaction, aggressiveness, piloerection, loss of lighting reflex, sedation, muscle relaxation, hypnosis, analgesia, ptosis, lacrimation, diarrhoea and skin colour were observed for 3 days. If mortality observed in 4/6 or 6/6 animals, the dose administered was considered as toxic dose. However, if the mortality was observed in only one mouse, then the dose was repeated with higher doses such as 100, 200, 500, 1000 and 2000 mg/kg.
2.14 Oral glucose tolerance test (OGTT)
In this method, the rats were fasted for 12 h and blood was taken from the tail end 30 min before administration of Syzygium mundagam bark (SMBM) extract (100 and 200 mg/kg). Glibenclamide was administered as standard drug (10 mg/kg). The control group kept untreated. Thirty minutes later, the rats from all groups were given glucose (2 g/kg) orally. The groups were divided as: group I: control, group II and III: 100 and 200 mg/kg SMBM extract and group IV: glibenclamide. Blood were collected from the tail vein just prior to glucose administration (0 min), 30, 60, 120 and 180 min after glucose loading.Citation26 The blood glucose level was determined at each time intervals and compared with control.
2.15 Anti-hyperglycemic activity
Hyperglycemia was induced in overnight fasted animals by a single intraperitoneal injection of 60 mg/kg streptozotocin (STZ), 15 min after the i.p. administration of 120 mg/kg nicotinamide.Citation27 After 1 h 20% glucose was orally administered to avoid the hypoglycaemic condition in diabetic rats. Before the injection initial blood glucose was noted. On 10th day, blood glucose levels were checked and the rats with glucose levels >250 mg/dL were grouped for the study. Diabetic rats were treated with oral doses of SMBM extracts (100 and 200 mg/kg) and standard glibenclamide (10 mg/kg) and after 1 h of drug administration blood glucose levels were observed for 5 h at an interval of 1 h. The diabetic rats kept untreated were grouped as controlled. The groups were divided as: group I: STZ control, group II and III: 100 and 200 mg/kg SMBM extract and group IV: glibenclamide.
2.16 Statistical analysis
The results were expressed as Mean ± SD/SEM. The data were statistically analyzed using SPSS version 17.0 (SPSS, ANNOVA statistical software, TULSA, USA) by means of one way ANOVA followed by Duncan’s test for antioxidant studies (determined in Mean ± SD) and Dunnett’s test for in vivo studies (determined in Mean ± SEM). Mean values were considered statistically significant when p < 0.05, p < 0.01 and p < 0.001.
3 Results
3.1 Quantification of total phenolics, tannins and flavanoids
The total phenolics, tannins and flavonoids contents of different extracts of leaf, and bark of S. mundagam is shown . The extractable phenolics were found to be higher in methanol extract of bark (54.44 g GAE/100 g extract) while it was low in bark petroleum ether (1.61 g GAE/100 g extract). The total tannins estimated from the total phenolics were high in ethyl acetate extract of bark (47.96 g GAE/100 g). Leaf methanol extract also exhibited a good level of total phenolics and tannin. Flavonoid content of bark was higher both in ethyl acetate and methanol (12.78 and 12.48 g RE/100 g extract respectively).
Table 1 Total phenolics, tannins and flavonoids content of S. mundagam leaf and bark.
3.2 Total antioxidant activity assay by radical cation 2,2′-azinobis (3-ethylebenzothiozoline-6-sulphonic acid) (ABTS+)
The ability of extracts to neutralize the ABTS+ cation radicals is presented in . Among the extracts, bark methanol extract showed the best potential to scavenge ABTS radicals (109686.87 μM TE/g extract). Other extracts depicted far lower activity where ethyl acetate extract of bark and methanol extract of leaf were comparatively better in their scavenging property. Petroleum ether extract of leaf (1592.99 μM TE/g extract) was least active.
Table 2 Activity of S. mundagam leaf and bark in ABTS+, FRAP and phosphomolybdenum assays.
3.3 Ferric reducing antioxidant power (FRAP) assay
Ferric reducing potential of S. mundagam was estimated from their ability to reduce TPTZ - Fe (III) complex to TPTZ- Fe (II) and shown in . The methanol (6612.54 mM Fe (II)/mg extract) extract of bark revealed higher ferric reducing activity. The activity of other extracts ranged between 7.60 (leaf petroleum ether) to 3514.72 mM Fe (II)/mg extract (leaf methanol).
3.4 Phosphomolybdenum assay
The molybdenum reducing ability of bark methanol extract was found to be higher (268.54 g AAE/100 g extract) where the petroleum ether extract showed lower activity (1.85 g AAE/100 g extract) (). Except ethyl acetate extract of bark, other extracts showed moderate to low potential of reducing the radical.
3.5 Radical scavenging activity using DPPH method
The methanol extract of bark (4.8 μg/mL) and leaf (5.1 μg/mL) were highly active in counteracting the DPPH radicals generated in the methanol solution (). The ethyl acetate extract of both parts were also equally capable of scavenging the radical. The IC50 of plant extracts were comparable to standards quercetin, rutin, BHA and BHT.
3.6 Metal chelating activity
The metal ion chelating ability of S. mundagam extracts were estimated and are shown in . In contrast to other assays, petroleum ether extract of leaf (73.9%) and bark (58.0%) exhibited best activity. The observed activity was well higher than the standard BHT which showed a chelation of 48% ().
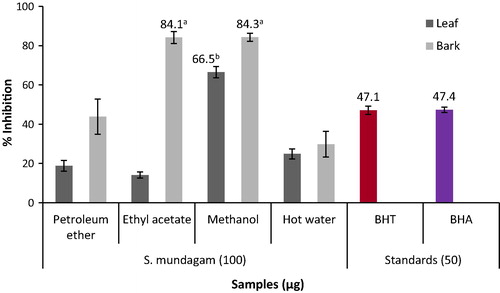
3.7 Assay of superoxide radical (O2−) scavenging activity
The ability of S. mundagam to scavenge the superoxide radicals generated in the reagent solution to inhibit formazan is directly proportional to the inhibition percentage of plant extract. The ability of ethyl acetate (84.1%) and methanol extract (84.3%) of bark to scavenge the radicals was much superior to other extracts ().
3.8 Nitric oxide radical scavenging activity
S. mundagam extracts could also display 25.6% nitric oxide radical scavenging ability being higher in methanol extract of bark (). The extracts were found to be less active compared to standard BHT (92.4%).
3.9 Acute toxicity
The acute oral toxicity study showed the safety of the extract up to a maximum dose of 2000 mg/kg body weight. The extract administration neither caused any significant change in the behaviours nor the death of animals. 1/10th and 1/20th of the maximum dose was fixed for further analyses. The animals were divided into 4 groups of six animals each, Group I treated with standard glibenclamide, group II and III with 100 and 200 mg extract/kg respectively and group IV kept untreated.
3.10 Oral glucose tolerance test (OGTT)
The rats treated with SMBM showed significant reduction of blood glucose levels after an initial rise in the 30th min (). The higher dose (200 mg/kg) could lower down the glucose level by 59.70% which indicates the ability of the extract to provide tolerance to the glucose loaded rats. The extract could reduce 177 mg/dL of blood glucose at 180th min. The 100 mg/kg dose was also able to bring down the glucose level in blood to near normal after 180 min.
3.11 Anti-hyperglycemic activity
SMBM showed a good ability in reducing the glucose level by 38.16%. When compared to control rats, hypoglycemic effect of the extract was much higher. There was no significant reduction (p < 0.05) observed in control rats as the blood glucose was brought down by only 0.35% ().
4 Discussion
The present study highlights the antioxidant and hypoglycemic activity of Syzygium mundagam. Excessive production of free radicals may lead to oxidative stress which may lead to subsequent mechanisms in pancreatic beta-cell death and the development of insulin-dependent diabetes mellitus-induced by STZ.Citation28 Hence the antioxidant property shown by the plant extracts would support hypoglycemic and antihyperglycemic property. The attempt has been made to determine the ability of the plant to assess the action over the acute diabetic condition. The results from the investigation could also encourage carrying out a detailed study using chronic diabetic models in rat.
Flavonoids and phenolics acids are the most important groups of secondary metabolites and bioactive compounds in plants and good sources of natural antioxidants in human diets,Citation29 therefore the quantification of these metabolites was done to confirm the presence of antioxidant activity in this plant. Methanol was found highly efficient in extracting phenolic compounds from bark than leaves. This indicates the physiological process of the plant where the secondary metabolite like phenolics might have produced higher in bark than leaves. The tannin and flavonoid content was also higher in bark than leaf, indicating that bark could be the best antioxidant agent. Similar results were observed in a closely related species of S. mundagam.Citation11
ABTS is one of the simplest radicals that can be used to estimate even a small amount of antioxidant molecule. The activity can be confirmed by the reduction in the colour of reaction mixture from dark green to a colourless solution. The bark methanol extract has shown the best ability to scavenge ABTS radical produced when reacted with potassium persulphate and depicts the presence of phytocompounds that are very strong scavengers equivalent to trolox. The DPPH radical scavenging activity was determined by the decrease in absorbance caused by the extracts. The lower IC50 depicts the higher activity. It was noted that the activity of methanol extract of leaf and bark was close to the activity shown by standard antioxidants. It has been found that antioxidant molecules such as ascorbic acid, tocopherol, flavonoids and tannins reduce and decolourize DPPH due to their hydrogen donating ability.Citation30 This could be predicted that the phenolic molecule in the extract could have high hydrogen donating ability. Several studies reported that higher phenolic compounds in plant extracts are capable of forming metal complexes and stabilizing transition metal ions, making them unable to participate in metal-catalysed initiation and hydroperoxide decomposition reactions.Citation31 As observed in previous assays the methanol extract of bark was found to be efficient reducing agent indicating the presence of higher phenolics that are capable of forming stable complexes. However, petroleum ether extract was found to be the active extract which could chelate the metal ion.
It has been observed that superoxide radicals may give rise to other reactive radicals like hydrogen peroxides, hydroxyl radicals and singlet oxygen that may lead to lipid peroxidation, DNA damage, damage to proteins and lipids.Citation32 Flavanoids could reduce the levels of these radicals and prevent the damage caused to biomolecules.Citation33 The ability of antioxidants can be detected by the decrease in the absorbance of reaction mixture where the phytochemicals in the extract are able to inhibit the blue NBT formation.Citation34 The inhibiting property of methanol extract (84.30%) is an indication of its ability to protect damage of cellular components. The phosphomolybdenum activity shown by the methanol extract (268.54 g AAE/100 g extract) was in agreement with the study by Chandran et al.Citation11 in S. calophyllifolium. Nitric oxide radical may cause various disease conditions carcinomas and inflammation, juvenile diabetes, multiple sclerosis, arthritis and ulcerative colitis.Citation35 The scavenging property shown by the extract could reduce such risk factors. The antioxidant factor in the extract could compete with the oxygen molecules thereby blocking the nitric oxide radical formation. The radical scavenging activity of methanol extract clearly shows that it contains higher concentration of phytochemicals that can act as antioxidants in a synergetic manner.
Oxidative stress is believed to increase the severity of diabetes and its complications. Several studies have also indicated the role of free radicals in the pathogenesis of diabetes.Citation36 Hence drug with antioxidant activity can have an effective role in treatment of diabetes. Our study has shown antioxidant property of S. mundagam with the best being the methanol extract (SMBM) was checked for antihyperglycemic activity. The acute toxicity study shows safety level of the extract even at the dose of 2000 mg/kg and thus can be administered as drug without any adverse effects. The extract could reduce the blood glucose level in both glucose loaded rats and STZ induced diabetic rats. A similar study was done with bark methanol extract of S. calophyllifolium in rats and proved to be a good hypoglycemic agent.Citation11 The extract could have enhanced the ability of tissues to uptake the glucose by decreasing the insulin resistance. As discussed earlier, another mechanism could be that the extract might have reduced the free radical generation under diabetic condition and resulting hypoglycaemia. This study also signifies the antidiabetic compounds present in methanol extract which needs to be characterized.
5 Conclusion
In summary, this study represents the first report on the antioxidant and antihyperglycemic activity of S. mundagam. The results clearly depict the role of antioxidants in lowering blood glucose level in both glucose loaded and STZ induced diabetic rats. The extractability of methanol and higher concentration of bioactive molecules might have generated the higher antioxidant activity that in-turn induced the hypoglycaemia in rats. Hence the study signifies the need of much detailed study on antioxidant compounds responsible for the antihyperglycemic activity to validate the extract as a competent therapeutic agent for the treatment of diabetes.
Acknowledgement
The authors are thankful to Department of Science and Technology, Govt. of India and INSPIRE programme (DST/INSPIRE Fellowship 2010/IF10618) for providing financial support for carrying out the work.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 3 January 2017
References
- A.J.KnentzM.NattrasDiabetic ketoacidosis, non ketotic hyperosmolar coma and lactic acidosisJ.C.PickupG.WilliamsHandbook of Diabetes2nd ed.1991Blackwell Science479494
- Y.LinZ.SunCurrent views on type 2 diabetesJ Endocrinol2042010111
- S.P.WolffR.T.DeanGlucose autoxidation and protein modification. The potential role of ‘autoxidative glycosylation’ in diabetesBiochem J2451987243250
- Z.Y.JiangA.C.WoollardS.P.WolffHydrogen peroxide production during experimental protein glycationFEBS Lett26819906971
- B.HalliwellJ.M.C.GutteridgeRole of free radicals and catalytic metal ions in human disease: an overviewMeth Enzymol1861990185
- N.HoggB.KalyanaramanJ.JosephA.StruckS.ParthasarathyInhibition of low-density lipoprotein oxidation by nitric oxide. Potential role in atherogenesisFEBS Lett3341993170174
- M.KawamuraJ.W.HeineckeA.ChaitPathophysiological concentrations of glucose promote oxidative modification of low density lipoprotein by a superoxide dependent pathwayJ Clin Invest941994771778
- E.C.TsaiI.B.HirschJ.D.BrunzellA.ChaitReduced plasma peroxyl radical trapping capacity and increased susceptibility of LDL to oxidation in poorly controlled IDDMDiabetes43199410101014
- P.S.M.PrinceV.P.MenonL.PariHypoglycemic activity of Syzygium cumini seeds: effects on lipid peroxidation in alloxan diabetic ratsJ Ethnopharmacol61199817
- K.GurusamyR.KokilavaniK.S.Ananta TeepaEffect of Syzygium calophyllifolium Walp. seed extract on transaminases and phosphatases in alloxan induced diabetic ratsAncient Sci Life2620072833
- R.ChandranS.SathyanarayananM.RajanM.KasipandiT.ParimelazhaganAnti-oxidant, hypoglycemic and anti-hyperglycemic properties of Syzygium calophyllifoliumBangladesh J Pharmacol102015672680
- R.ChandranT.ParimelazhaganS.ShanmugamS.ThankarajanAntidiabetic activity of Syzygium calophyllifolium in Streptozotocin-Nicotinamide induced Type-2 diabetic ratsBiomed Pharmacother822016547554
- Oudhia P. Drawbacks of indigenous medicinal orchids and ferns and Syzygium mundagam (Bourd.) Chithra based herbal formulations. Pankaj Oudhia’s expert comments on world patents on herbal medicines. Audio bank on biodiversity and traditional healing. <http://www.pankajoudhia.com>; 2012.
- M.K.R.NarayananN.AnilkumarV.BalakrishnanM.SivadasanA.H.AhmedA.A.AlatarWild edible plants used by the Kattunaikka, Paniya and Kuruma tribes of Wayanad District, Kerala, IndiaJ Med Plants Res5201135203529
- P.SiddhurajuK.BeckerStudies on antioxidant activities of Mucuna seed (Mucuna pruriens var. utilis) extracts and certain non-protein amino/imino acids through in vitro modelsJ Sci Food Agri83200315171524
- R.SiddhurajuS.ManianThe antioxidant activity and free radical scavenging capacity of dietary phenolic extracts from horse gram (Macrotyloma uniflorum (Lam.) Verdc.) seedFood Chem1052007950958
- J.ZhishenT.MengechengW.JianmingThe determination of flavonoid contents on mulberry and their scavenging effects on superoxide radicalFood Chem641999555559
- R.ReN.PellegriniA.ProteggenteA.PannalaM.YangC.Rice-EvansAntioxidant activity applying an improved ABTS radical cation decolorization assayJ Free Rad Biol Med26199912311237
- R.PulidoL.BravoF.Sauro-CalixtoAntioxidant activity of dietary polyphenols as determined by a modified ferric reducing/antioxidant power assayJ Agri Food Chem48200033963402
- P.PrietoM.PinedaM.AguilarSpectophotometric quantitative of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin EAnal Biochem2691999337341
- M.S.BloisAntioxidants determination by the use of a stable free radicalNature4617195811991200
- T.C.P.DinisV.M.C.MadeiraL.M.AlmeidaAction of phenolic derivatives (acetoaminophen, salycilate and 5-aminosalycilate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengersArch Biochem Biophy3151994161169
- C.BeauchampI.FridovichSuperoxide dismutase: improved assays and an assay applicable to acrylamide gelsAnal Biochem441971276277
- N.SreejayanM.N.A.RaoNitric oxide scavenging by curcuminoidsJ Pharm Pharmacol491997105107
- D.J.EcobichonThe Basis of Toxicology Testing1997CRC PressNew York4386
- A.M.M.JalilA.IsmailP.P.ChongM.HamidS.H.S.KamaruddinEffects of cocoa extract on glucometabolism, oxidative stress, and antioxidant enzymes in obese-diabetic (Ob-db) ratsJ Agri Food Chem56200878777884
- P.MasielloC.BrocaR.GrossDevelopment of a new model of type 2 diabetes in adult rats administered with streptozotocin and nicotinamideDiabetes471998224229
- K.B.KooH.J.SuhK.S.RaJ.W.ChoiProtective effect of cyclo (his-pro) on streptozotocin-induced cytotoxicity and apoptosis in vitroJ Microbiol Biotechnol212011218227
- D.KimS.JeondC.LeeAntioxidant capacity of phenolic phytochemicals from various cultivars of plumsFood Chem812003321326
- A.KumaranR.J.KarunakaranIn vitro antioxidant activities of methanol extracts of five Phyllanthus species from IndiaLebensm-Wiss Technol402007344352
- S.BourgouR.KsouriA.BellilaI.SkandraniH.FallehB.MarzoukPhenolic composition and biological activities of Tunisian Nigella sativa L. shoots and rootsCompte Rendu de Biologies33120084855
- A.P.WickensAging and the free radical theoryRespi Physiol1282001379391
- P.G.PiettaFlavonoids as antioxidantsJ Nat Prod6320001035
- I.ParejoF.ViladomatJ.BastidaComparison between the radical scavenging activity and antioxidant activity of six distilled and non-distilled Mediterranean herbs and aromatic plantsJ Agri Food Chem5020026882
- R.E.HuieS.PadmajaThe reaction of no with superoxideFree Radic Res Commun181993195199
- R.RahimiN.ShekoufehL.BagherA.A.MohammadA review on the role of antioxidants in the management of diabetes and its complicationsBiomed Pharmacother592005365373