?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Objective
The purpose of this research was to investigate sub-chronic toxicity in animal model.
Methods
A detailed study was done on the physical, hematological, biochemical and hormonal parameters of both male and female Sprague-Dawley rats after 28 days administration of naproxen and its metal complexes.
Results
There were no significant changes found in physical parameters on observation for both male and female rats without some minor differences. However, Naproxen metal complexes showed comparatively lower side effects than naproxen. Hematological report suggested that naproxen was in process of initiating inflammation which was justified by decreasing the mean value hemoglobin and hematocrit level and increasing the white blood cells level. There were no significant changes in biochemical parameters, however, the mean value of blood glucose level and cholesterol seemed to be higher and triglyceride was lower. Thyroid hormone levels were found lower, that was another indication inflammatory process. However, this might have the ability to lower the insulin secretion resulting in increasing blood glucose level.
Conclusion
In the present investigation, there were no significant alterations in histopathological studies and physical parameters though some signs of abnormalities had been found but hematological and hormonal data did not suggest any inflammatory or toxicological activity. However, we observed that naproxen showed more side effects than metal complexes which indicated that carboxylic group (–COOH) of naproxen may be responsible for showing those most of the side effects.
1 Introduction
Non steroidal anti-inflammatory drugs (NSAIDs) are one of the most widely utilized classes of drugs due to their potent anti-inflammatory, analgesic, and anti-pyretic properties in the world.Citation1 The treatment of inflammation and pain is an important area of therapeutics. In the last decade, nonsteroidal anti-inflammatory drugs (NSAIDs) like naproxen have played a central role in these indications and they are currently considered as the first choice, being one of the most widely prescribed drugs.Citation2,Citation3 Naproxen is a non steroidal anti-inflammatory, analgesic drug which is extensively used in the clinical treatment of acute and chronic pain and arthritis.Citation4 They inhibit both cyclooxygenase-1 (COX-1) and cyclooxygenase-2 (COX-2) isoenzymes involved in the synthesis of prostaglandins.Citation5 Prostaglandins are chemicals produced by the cells of the body promoting inflammation, pain and fever; supporting the blood clotting function of platelets and protecting stomach from the damaging effects of acid.Citation6,Citation7 Cyclooxygenase-1 is constitutively expressed and generates prostanoids involved in the maintenance of the integrity of gastrointestinal mucosa and platelet aggregationCitation8 whereas at sites of inflammation, cyclooxygenase-2 is induced to generate prostaglandins that mediate inflammation and pain.Citation9 They are highly effective for their anti-inflammatory and analgesic properties in treating different level of pains, such as osteoarthritis and rheumatoid arthritisCitation10 and may reduce the risk of colon cancer and probably various types of gastrointestinal (GI)-related cancers.Citation11 Despite all of those successes, many studies reveal that gastric or duodenal ulcers develop in 15–30 percent of patients who regularly take NSAIDsCitation12 and more than 100,000 patients are hospitalized and 16,500 die every year in America as a result of NSAID induced gastrointestinal events.Citation13,Citation14
As there is no information available on the sub-chronic toxicological effects of metal complexes of naproxen in the animal model compared to naproxen, we have motivated to study metal binding properties of naproxen with different transition metal ions with the aims of investigating their physical, hematological, biochemical and hormonal effects on the body by evaluating acute toxicity in animal model. In this paper, we report the synthesis of some organ metallic compounds of naproxen and their ability to reduce gastrointestinal toxicity.
2 Materials and methods
2.1 General procedure for synthesis of transition metal complexes of naproxen
All synthetic procedures were described in details by Hasan et al.Citation15
2.2 Method consideration, dose selection and route of administration
The sub-chronic model was used to evaluate NSAID induced pathological state in young, healthy rat model to see overall effect on the health of the animals over the 28 days dosing period. The sub-chronic model allows one to assess the test NSAID’s toxicity with regard to GI bleeding (hemoglobin, hematocrit reduction), the development of intestinal perforation and adhesions.Citation16 The dose of naproxen employed in the rat studies was equivalent to 10 mg/kg body weight. This dose was selected because it produced significant and comparable activity in reducing paw swelling in rats with adjuvant arthritis and also producing several side effects including ulceration.Citation17 Using this dose, other naproxen metal complexes are also administered at a dose of 10 mg/kg and test samples were orally administered twice daily for 18 days.Citation18
2.3 Experimental design
All experimental animals (Supplementary Sections 1.1 and 1.2) were randomly selected and divided into seven groups in .
Table 1 Experimental design of naproxen metal complexes.
2.4 Preparation of test samples
The calculated amount of the test samples were measured and normal saline was added with 1–2 drops of a suspending agent. To stabilize the suspension, it was stirred well by vortex mixture. Finally the volume was adjusted up to such so as to have final volume with concentration vol-dose/group/2 administrations per day.Citation18
2.5 Sacrificing of the animals and methods of observation and examination
At the end of treatment period (28 days), the animals were sacrificed, physical observation was performed and blood samples and organs were collected for further experiments (Supplementary Sections 1.3, 1.4 and 1.5).
2.6 Histopathologic studies
2.6.1 Microscopic evaluation of histopathological study
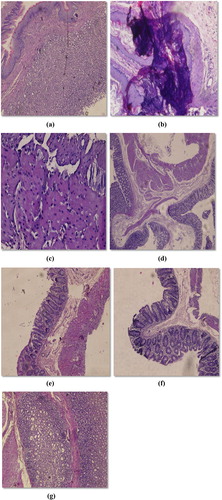
The stomach was opened along with greater curvature, rinsed with saline to remove gastric contents and blood clots using washout techniqueCitation19,Citation20 and examined by a 5× magnifying lens to assess the formation of ulcers. The stomach was removed and the number of ulcers was counted.
2.6.2 Measurement of Ulcer score
Ulcer index was measured by using following formula.Citation19 Ulcer scoring was described in .
Table 2 The number of ulcers was counted. Ulcer scoring was undertakenCitation21 as following manner.
UI = Ulcer Index.
UN = Average number of ulcers per animal.
US = Average number of severity score.
UP = Ulcer probability (incidence%) for each group.
Percentage inhibition of ulceration was calculated as below:
2.7 Statistical analysis
All the grouped data were statistically evaluated with SPSS version 17 software. All the results were expressed as Mean ± SEM (Standard Error of Mean) values for seven animals in each group. Means were compared by two tailed independent sample t-test. Probability (p) value of 0.05 or less (p < 0.05) was considered as significant.
3 Results
3.1 Characterization of metal complexes of naproxen
Physical, analytical and thermal properties, NMR spectra, FTIR spectra, scanning electron microscopy and HPLC study of naproxen metal complexes were described by Hasan et al.Citation15
3.2 Toxicological evaluation
3.2.1 Physical parameters
No significant changes were observed in both male and female rats without some minor alterations within any sample groups throughout the dosing period. No mortality was observed in all groups of both male and female rats during the experiment (see ).
3.2.2 Weight variation of rats during the study period
In an attempt to observe any change in the body weight of the tested animals induced by naproxen and its metal complexes, the weights were taken at initial period (0 day), 9th day, 18th day and 28th day of study. The alteration of the body weight of the experimental animals was not so prominent. No significant changes were observed.
3.2.3 Hematology assessment
Animal treated with the test samples showed no significant changes in hematological parameters. However, there were some considerations about naproxen treated groups. Naproxen showed lowering the RBC count, hemoglobin and hematocrit (HCT) level and increasing the number of white blood cells count, neutrophils, lymphocytes and monocytes ( and ). As the values are statistically significant, this was a clear indication of inflammation and might create pathological condition in near future. Other parameters were found in normal compared to control. However, in case of metal complexes there were no alterations in the value of those parameters in both male and female rats. This suggested that those complexes were comparatively safer drugs than parent naproxen.
Table 3 Hematological parameter changes of male rats during treatment of test compounds.
Table 4 Hematological parameter changes of female rats during treatment of test compounds.
3.2.4 Biochemical assessment
All the biochemical parameters studied were found to be comparable with control group. In both male and female rats groups, no significant changes were seen in any parameters ( and ). However, the blood glucose and cholesterol level seemed to be higher and triglyceride was found lower for naproxen treated groups.
Table 5 Biochemical parameter changes of male rats during treatment of test compounds.
Table 6 Biochemical parameter changes of female rats during treatment of test compounds.
3.2.5 GI side effects assessment
GI side effects (ulceration/bleeding) can be assessed by measuring hematocrit (index of anemia caused by GI bleeding), and inspecting the GI tract for lesions, perforations and adhesions (). The stomach and small intestine were then blindly evaluated for hemorrhagic damage. This involved measuring the lengths, in mm, of all hemorrhagic lesions. Separate gastric and intestinal damage scores were then calculated by summing the lengths of all lesions for each rat.Citation21
Table 7 GI effects of different groups of rats under the experimental period.
4 Discussion
In our previous paper,Citation22 we showed that naproxen metal complexes had better pharmacological properties than naproxen in mice. That’s why we thought there might be possibilities that they could have any toxicity profiles. In the present investigation, there were no significant alterations in histopathological studies and physical parameters though some signs of abnormalities had been found but hematological and hormonal data did not suggest any inflammatory or toxicological activity. However, we observed that naproxen showed more side effects than metal complexes that indicated that carboxylic group (–COOH) of naproxen may be responsible for showing those most of the side effects. Based on this result we strongly believe that –COOH group of naproxen predominantly responsible for showing side effects in various compartments of the body. Hence metal complexes lack the –COOH group because of complication with metals they did not show any considerable side effects than naproxen. No any major changes in behavior or tremors/convulsions or body weight were seen during the study period. Hematological reports indicated that there was increase in the number of white blood cell count, neutrophils, lymphocytes and monocytes and simultaneously decrease in hemoglobin, hematocrit that clearly stated that the inflammation was on progress for naproxen. However, all of these parameters were found normal in metal complexes of naproxen and also a clear indication of safety compared to parent naproxen. Biochemical parameters related to kidney function were found normal for all the samples. However, glucose level of the naproxen treated animals showed higher value than control and other samples. In overall, we tried to figure out a detailed scenario of the toxicity profiling of naproxen metal complexes. In general it can be said that they are comparatively safer than naproxen.
Conflict of interest statement
We declare that we have no conflict of interest.
Supplementary data 1
Download MS Word (12.1 KB)Acknowledgments
Authors are grateful to the Head of Department of Clinical Pharmacy and Pharmacology, Faculty of Pharmacy, University of Dhaka, Dhaka, Bangladesh for providing necessary chemical, instrument and other laboratory facility to conduct research.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 18 January 2017
References
- Misoprostol for co-prescription with NSAIDsDrug There Bull2819902526
- P.M.PelosoStrategies and practice for use of nonsteroidal anti-inflammatory drugsScand J Rheumat119962943
- L.S.SimonActions and toxicity of nonsteroidal anti-inflammatory drugsCurr Opin Rheumatol71995159166
- J.G.LombardinoL.G.OtternessE.H.WisemanArzneim Forsch2519751629
- B.CryerNonsteroidal anti-inflammatory drugs and gastrointestinal disease6th ed.M.FeldmanB.F.ScharschmidtM.H.SleisengerSleisenger and Fordtran’s Gastrointestinal and Liver Disease: Pathophysiology/Diagnosis/ManagementVol 11998W.B. SaundersPhiladelphia343357
- J.O.ShawK.M.MoserThe current status of prostaglandins and the lungsChest6819757580
- C.B.HigginsE.BrasunwaldThe prostaglandins: biochemical, physiologic and clinical considerationsAm J Med53197292112
- E.A.MeadeW.L.SmithD.L.DeWittDifferential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isoenzymes by aspirin and other non-steroidal anti inflammatory drugsJ Biol Chem268199366106614
- E.FossleinAdverse effects of nonsteroidal anti-inflammatory drugs on the gastrointestinal systemAnn Clin Lab Sci2819986781
- J.L.WallaceBuilding a better aspirin: gaseous solutions to a century-old problemBrit J Pharmacol1522007421428
- E.J.JacobsM.J.ThunE.B.BainA large cohort study of long-term daily use of adult-strength aspirin and cancer incidenceJ Natl Cancer Inst992007608615
- L.LaineNonsteroidal anti-inflammatory drug gastropathyGastroint Endosc Clin North Am61996489504
- M.M.WolfeD.R.LichtensteinG.SinghGastrointestinal toxicity of nonsteroidal antiinflammatory drugsN Engl J Med340199918881899 [Erratum, N Engl J Med. 1999;341:548
- G.SinghD.Rosen RameyNSAID induced gastrointestinal complications: the ARAMIS perspective — 1997J Rheumatol Suppl511998816
- Md. SharifHasanRuhulKayeshFaridaBegumS.M.Abdur RahmanTransition metal complexes of naproxen: synthesis, characterization, forced degradation studies, and analytical method verificationJ Anal Meth Chem20162016356069510.1155/2016/3560695 10 pages
- L.M.LichtenbergerJ.J.RomeroW.M.De RuijterPhosphotidylcholine association increases the anti-inflammatory and analgesic activity of ibuprofen in acute and chronic rodent models of joint inflammation: relationship to alterations in bioavailability and cyclooxygenase-inhibitory potencyJ Pharmacol Exp Ther2982001279287
- R.BlacklerS.SyerM.BollaE.OnginiJ.L.WallaceGastrointestinal-sparing effects of novel NSAIDs in rats with compromised mucosal defencePLoS ONE72012e35196
- C.CicalaA.IanaroS.FiorucciNO-naproxen modulates inflammation, nociception and downregulates T cell response in rat Freund’s adjuvant arthritisBrit J Pharmacol130200013991405
- W.H.VogelB.A.ScholkensJ.SandowG.MullerW.F.VogelDrug Discovery And Evaluation2nd ed.2002SpringerNew Yorkp.670–725. ISBN-13: 978-3540423966
- A.S.SalimGastric diversion: a method for H+ output estimation in the ratDigestion3919884751
- Y.CuryJ.Garcia-LemeThe inflammatory response of hyperthyroid and hypothyroid rats. Role of adrenocortical steroidsAgents Act151984377385
- Md. SharifHasanNarhariDasZobaerAl MahmudS.M.Abdur RahmanPharmacological evaluation of naproxen metal complexes on antinociceptive, anxiolytic, cns depressant, and hypoglycemic propertiesAdv Pharmacol Sci20162016304072410.1155/2016/3040724 7 pages
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://doi:10.1016/j.ajme.2016.12.005.