Abstract
Cerium oxide nanoparticles (CeO2NPs) is an efficient neuroprotective agent and showed promising effects in some neurodegenerative diseases such as Alzheimer’s disease and multiple sclerosis. However, the implication of CeO2NPs in Parkinsonism remains to be investigated.
The aim of this study was to assess the possible role of CeO2NPs as a neuroprotective agent against the development of behavioral and biochemical changes in rat model of Parkinson’s disease. Thirty rats were included and received left intrastriatal injection of either saline (controls, n = 10) or 6-hydroxy dopamine (6-OHDA) in untreated group (n = 10) and 10 rats were received intraperitoneal injection of low dose CeO2NPs two hours before surgery, and continued once daily for 6 weeks (preventive group). At the end of experimental period, rats were subjected to behavioral assessment and then killed for biochemical analysis of striatal dopamine levels, oxidative stress markers and caspase-3 activity. Results showed that CeO2NPs resulted in partial neuroprotection against disturbances in motor performance. It also partially decreased apoptosis and oxidative stress in preventive group, while it failed to increase striatal dopamine level as compared to untreated rats. The present study verified some neuroprotective effects of CeO2NPs in 6-OHDA-induced Parkinsonian rats through their antioxidant and anti apoptotic effects. Some of these effects persisted till the end of six weeks whereas others declined after three weeks. A larger dose may be needed to produce more valuable effects and to maintain protection for a longer period.
1 Introduction
Parkinson’s disease (PD) is currently regarded as the most common degenerative disorder of the aging brain after the Alzheimer’s disease with extremely high psychosocial impacts and noticeable declines in patients’ quality of life.Citation1 The cardinal biochemical abnormality in PD is the profound deficit in brain dopamine level attributed to the loss of neurons of the nigrostriatal dopaminergic pathway. The exact pathogenic mechanism for neurodegeneration observed in PD is not fully understood. However, The concept that free radical–mediated neuronal injury has been suggested as the main hypothesis for PD pathogenesis.Citation2,Citation3 Multiple genetic and environmental risk factors interfere with mitochondrial function, increase free radicals production with eventual release of apoptosis-initiating factors.Citation4–Citation8
Fig. 7 Correlations between dopamine levels, oxidative stress markers (TAC, MDA & Nitrite) and caspase 3 measured in striatal tissue of all studied groups.
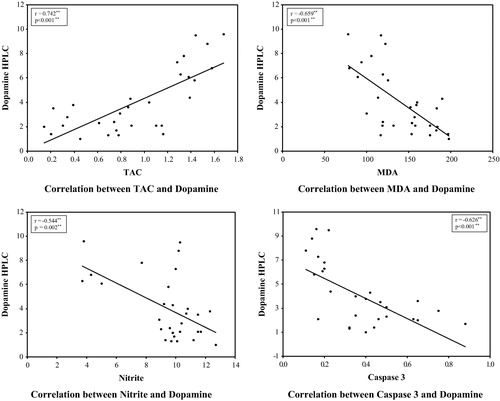
Table 1 Neuro-behavior finding in control, 6-OHDA untreated and preventive groups 3 weeks after intrasrtiatal injection of 6-OHDA.
Table 2 Neuro-behavior findings in control, 6-OHDA untreated group and preventive group 6 weeks after intrastriatal injection of 6-OHDA.
Table 3 Neuro-behavior findings in the preventive group 3 and 6 weeks after intrastriatal injection of 6-OHDA.
6-Hydroxydopamine (6-OHDA), a hydroxylated analog of dopamine, was found to induce degeneration of dopaminergic neurons and results in rat model of Parkinsonism.Citation9 Intracellular uptake of 6-OHDA is mediated by dopamine or noradrenaline membrane transporters (DAT and NAT respectively) due to its structural similarity with endogenous catecholamines.Citation10 Once taken up into neurons, 6-OHDA accumulates in the cytosol where it is readily oxidized leading to the generation of reactive oxygen species and ultimately, oxidative stress-related cytotoxicity.Citation11
Recently, nanotechnology has become a main focus of biomedical research.Citation12 In particular, cerium oxide nanoparticles (CeO2NPs) have distinctive properties that can be effective in nanotherapeutics. The small size of these nanoparticles gives them a high surface area to volume ratio, in addition to the ability of cerium to exist in either a +3 (reduced) or +4 (oxidized) state at the particle surface: Ce3+are associated with oxygen vacancies. Therefore, CeO2NPs can be used as a scavenger of superoxide anions.Citation13,Citation14 Moreover, CeO2NPs were shown to exhibit superoxide dismutase (SOD) and catalase enzymes mimetic activities in a redox-state dependent manner.Citation14
CeO2NPs were effective to prevent macular degenerationCitation15 and the formation of neovascular lesions in the retina,Citation16 to decrease hepatic oxidative stress linked to the progression of diabetesCitation17 and to promote wound-healing activity.Citation18 Additionally, CeO2NPs were proven to be extremely protective for cardiacCitation19 and neuronal cells.Citation20 The use of CeO2NPs in the prevention of neurodegenerative diseases in which oxidative stress plays a major role in its pathology may hold promising prophylactic potential. However, the application of CeO2NPs in PD remains to be investigated.
The aim of the present work was to assess the possible effects of cerium oxide nanoparticles (CeO2NPs) in prevention of motor, behavioral and neurochemical changes in rat model of Parkinson’s disease.
2 Materials and methods
2.1 Experimental animals
30 Adult male Wistar rats weighing 200–220 g were used (pro-cured from Experimental Animal house in Physiology Department, Alexandria University). Animals were maintained at room temperature under standard conditions of a 12-h light-dark cycle with food and water ad libitum. They were allowed to acclimatize for 1 week prior to experimentation. All experiments were carried out during the light phase between 9:00 and 15:00. Procedures involving animals and their care were conducted in conformity with ethical guide-lines of Alexandria University on laboratory animals and the protocol was approved by the Faculty of Medicine, Alexandria University Ethics Committee.
The animals were divided into the following groups:
2.1.1 Control (sham-operated) group
This group included 10 rats received a single left intrastriatal (IS) stereotaxic injection of 2 μl of vehicle (0.9% saline with 0.1% ascorbic acid, pH 5.5) followed after 3 weeks by daily intraperitoneal (i.p) injection of 0.5 ml phosphate buffered saline (PBS) for another 3 weeks.
2.1.2 6-OHDA- untreated group
It included 10 rats received a single dose of left intrastriatal injection of 10 μg of 6-hydroxydopamine hydrobromide (6-OHDA- HBr) (Sigma-Aldrich) dissolved in 2 μl of the same vehicle to induce PD model. Ascorbic acid was used to stabilize 6-OHDA-HBr, as it prevents oxidation of 6-OHDA-HBr to an inactive form. After 3 weeks, these rats were injected with 0.5 ml PBS i.p daily for 3 weeks.Citation21
2.1.3 Cerium oxide nanoparticles (CeO2NPs)- preventive group
10 rats were injected stereotaxically with 10 μg of 6-OHDA- HBr dissolved in 2 μl of vehicle into the left striatum for 3 weeks. Rats were received i.p injection of low dose CeO2NPs (0.1 mg/kg) in 0.5 ml PBS two hours before surgery, and continued once daily for 6 weeks.Citation22
2.2 Experimental procedure
The rats were anaesthetized (by ketamine 100 mg/kg and xylazine 5 mg/kg, i.p.) and fixed in a stereotaxic frame (David kopf instrument) with the incisor bar set at −3.3 mm, to adjust head level.
The scalp was incised and the skull was exposed. A small burr hole was made in the skull by dental drill above the left striatum 1 mm anterior to bregma and 2.6 mm left of midline. The dura was cut and a 26 gauge blunt-tipped needle attached with 5 μL Hamilton syringe was slowly lowered to a depth of 5.0 mm below the surface of the skull (bregma) according to the Atlas of Paxinos and WatsonCitation21,Citation23.
Rats were subjected to unilateral (left) intrastriatal injection with either of 2 μl vehicle (in the control group) or 10 μg of 6-OHDA- HBr dissolved in vehicle (in 6–OHDA- injected group) was slowly injected at a rate of 0.4 μl/min. The needle was left in place for an additional 5 min following the injection and then slowly withdrawn to prevent the reflux of the drug. The burr holes were filled with Gelfoam and the incision was closed with sutures.Citation21
The animals were placed individually in a heated recovery chamber until they recovered from anesthesia. Then rats were housed together in a group of four animals per cage. Food and water was kept inside the cages for the first week so that animals could easily access it without any physical trauma. After surgery, all rats received gentamicin (5 mg/kg, i.p) for 3 days to prevent sepsis, and meloxicam (1 mg/kg, i.p.) for analgesia. Rats were also given daily injection of saline (500 μl, s.c.), to prevent dehydration until they regained their pre-surgery weight.Citation21
2.2.1 Behavioral tests
All rats included in this study were subjected to behavioral tests 2–3 weeks after surgery to confirm the manifestations of Parkinsonism and development of PD in rat modes and to compare between untreated and preventive group. The tests were once more performed at the end of experimental period (at the end of 6 weeks) to assess the preventive role of cerium oxide nanoparticles against behavioral and motor changes that accompany PD. Behavioral tests include the followings:
| 1. | Open field test.Citation24 | ||||
| 2. | Rotarod test.Citation25 | ||||
| 3. | Stepping test and initiation time.Citation26 | ||||
2.2.2 Striatal tissue neurobiochemical assays
On the last experimental day, the rats were sacrificed by decapitation immediately after behavioral assessments. The whole brain was removed and washed with ice cold saline and the left striatum was dissected as previously described.Citation27 For dopamine estimation, the striatum (20% w/v) was homogenized and deproteinized in 0.2 M perchloric acid containing 100 μM EDTA2Na. The homogenate was left for 30 min. to deproteinize. Then, the homogenate was centrifuged at 10,000g for 15 min at 0 °C (Hettich Universal 320R refrigerated centrifuge). After centrifugation, the supernatant was adjusted to pH = 3.35 by adding 1 M acetic acid, and then filtered through 0.45-μm membrane filter (Millipore, Bedford, MA, USA). For oxidative stress parameters assays, part of striatum was homogenized in 10 times (w/v) ice cold 0.1 M phosphate buffer (pH 7.4) and centrifuged at 10,000g for 15 min at 4 °C.For caspase 3 activity assay, another part of the specimen was homogenized with caspase 3 reaction buffer. Aliquots of brain homogenate supernatant were analyzed in duplicate for total protein concentration by the Lowry’s method using Folin phenol reagent with bovine serum albumin as a standard.Citation28
2.2.2.1 Dopamine high performance liquid chromatography (HPLC) assay
For dopamine HPLC assay, Arsene et al. method was adopted with slight modifications. Briefly, 20 μl of the filtered striatal supernatant was injected into an HPLC reversed–phase system (Agilent Technologies) with a Zorbax SB-C18 250 × 4.6 mm column (Agilent Technologies). The mobile phase consisted of 0.8 mM EDTA-Na2, 0.12 M NaH2PO4·H2O, 0.646 g sodium heptane sulphonate and 60% methanol. Dopamine was detected using an UV detector (210 nm). HPLC results were read off dopamine calibration curve and were normalized to total tissue proteins to be expressed as ng/mg protein.Citation29,Citation30
2.2.2.2 Oxidative stress assay
Total antioxidant capacity (TAC), the lipid peroxidation marker, malondialdehyde (MDA) and nitrite were determined in striatal tissue supernatant by colorimetric technique using commercial kits (Biodiagnostic, Egypt), according to the manufacturer’s protocol. The results of TAC were normalized to total tissue proteins to be expressed as mmol/mg protein,Citation31 whereas the results of MDA were normalized to tissue weight to be expressed as ng/gm tissue.Citation32 The results of nitrite were normalized to total tissue proteins to be expressed as μmol/mg protein.Citation33
2.2.2.3 Caspase-3 activity assay
As a marker for apoptosis in striatal tissue, caspase-3 enzymatic activity was measured by colorimetric reaction provided by R&D Systems. Caspase-3 activity was expressed as the ratio to the control levels and its results were normalized to total tissue proteins.Citation34
2.3 Statistical analysis
Statistical analysis was performed using Statistical Package for Social Sciences 20.0 for Windows (SPSS, Chicago, IL). The distributions of quantitative variables were tested for normality using Shapiro-Wilk’s test. If revealed normal distribution, data were described as mean ± S.E.M. and analyzed by one way ANOVA followed, when significant, with Post Hoc Tukey test. For abnormally distributed data, Kruskal Wallis test was used to compare between different groups and pair wise comparison was assessed using Mann-Whitney test. Wilcoxon signed ranks test was used to compare performance of the diseased limb versus the opposite one in the same group in the stepping test. Significance of the obtained results was judged at the 5% level.
3 Results
3.1 Behavioral findings: (–)
3.1.1 Open field test
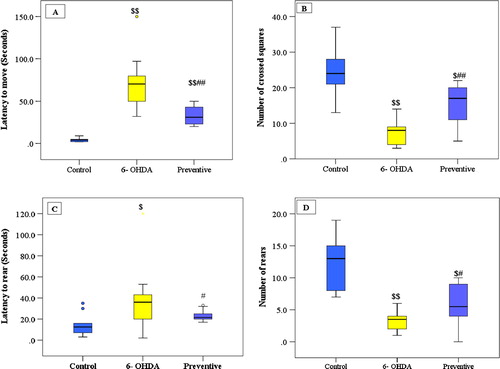
Intrastriatal injection of 6-OHDA in the untreated group resulted in a significant impairment in locomotor and exploratory behavior as seen after three weeks by decreased number of crossed squares and number of rears and increased latency to start moving and rearing versus the control group. This impairment was persistent after six weeks giving the same significant difference from control group and no significant difference was detected in comparing the results of open field test in untreated group after three and six weeks.
Treatment of rats with low dose of (CeO2NPs) in the preventive group succeeded in preventing the development of impairment in locomotor behavior after three weeks as determined by significant decrease in latency to move and rear with a significant increase in number of crossed squares and number of rears as compared to untreated group, although, this dose failed to return these tests back to normal, where a significant change was still detected in comparison to control group except for latency to rear where no significant difference was noticed.
After six weeks, the low dose of (CeO2NPs) was not effective in improving open field test as noticed after three weeks. As regard latency to rear, a significant increase was detected in preventive group after six weeks when compared to results after three weeks. In addition, number of rears decreased as compared to number of rears after three weeks and no significant difference was detected between preventive and untreated group. On the other hand, the low dose of (CeO2NPs) in the preventive group was able to maintain the improvement in latency to move and number of crossed squares as observed after three weeks and no significant difference was detected after six weeks when compared to results after three weeks.
3.1.2 Rotarod test
A significant decrease in the mean of latency to fall time was reported in 6-OHDA group after three weeks as compared to control group which persisted also after six weeks. This reduction was in part prevented by low dose administration of CeO2NPs in the preventive group as a significant increase was detected in this group after three and six weeks when compared to untreated group but still significantly lower than control group. However, the results of treatment of rats with (CeO2NPs) were less effective after six weeks than after three weeks as significant difference was detected in comparing rotarod test results after three and six weeks in the preventive group.
3.1.3 Stepping test and initiation time
The motor performance of right forepaw (contralateral to injection site) of 6-OHDA untreated group was significantly impaired after three and six weeks when compared to either the left paw of the same group or right paw of the control group. The number of steps taken by right forelimb of 6-OHDA group was significantly less as well as the mean time to initiate steps by this limb was substantially delayed compared to left forelimb of the 6 OHDA group and to the right forelimb of control group. In control group, there was no significantly difference in motor performance of both forelimbs. Early injection of low dose of CeO2NPs in preventive group resulted in partial protection against this motor performance impairment of right forelimb after three and six weeks as the number of steps taken by right forelimb were significantly increased and the mean time to initiate steps by this limb was significantly decreased in comparison to untreated group but they did not return back to control values. There was no significant difference in stepping test and initiation time in both untreated and preventive group in comparing results after three and six weeks, except for the time taken to initiate steps by right forelimb in preventive group which was significantly increased after six weeks as compared to that after three weeks but it was still significantly lower than untreated group (see ).
Fig. 1 Open field parameters: (A) latency to move (seconds), (B) number of crossed squares (C) latency to rear and (D) number of rears, three weeks following intrastriatal injection 6-OHDA injection. Data are expressed as medians (intra-quartile rang), $ P ⩽ 0.05, $$ P ⩽ 0.001versus control group, # P ⩽ 0.05, ## P ⩽ 0.001 versus 6-OHDAgroup.
3.2 Neurobiochemical markers
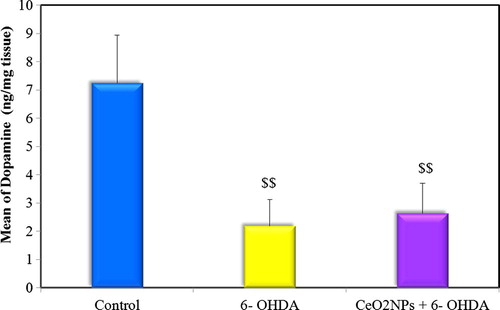
3.2.1 Striatal dopamine level ()
There was a significant decrease in striatal dopamine level in 6-OHDA untreated group versus control rats. Meanwhile, low preventive dose of CeO2NPs were not able to keep high dopamine levels as controls and dopamine levels were not significantly different from those of untreated 6 OHDA rats.
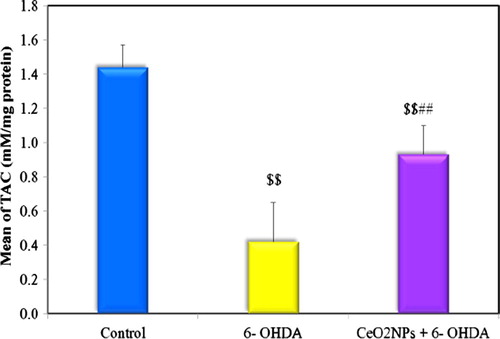
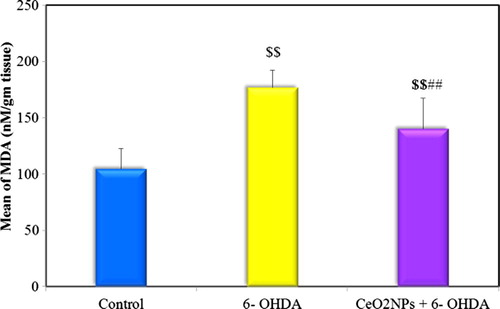
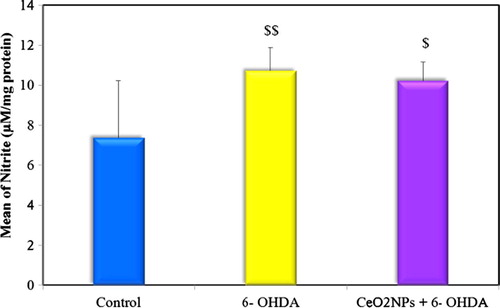
3.2.2 Oxidative stress markers (TAC, MDA and nitrite) (–)
The present study revealed a significant increase in striatal MDA and nitrite levels and decrease in striatal TAC level in 6-OHDA untreated group compared to the control indicating increased oxidative stress. Low dose administration of CeO2NPs in the preventive group succeeded in ameliorating this oxidative stress where a significant difference was observed between preventive group and untreated group in comparing MDA and TAC levels but not for nitrite level. Although this low dose of CeO2NPs failed to return the oxidative stress markers back to normal (see ).
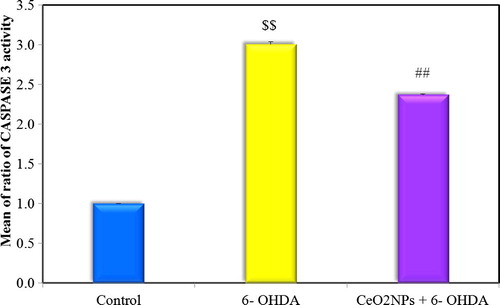
3.2.3 Striatal caspase-3 activity
The present study showed that there was a significant increase in striatal caspase-3 activity in 6-OHDA untreated group versus control rats. The low dose of CeO2NPs decreased striatal caspase-3 activity as compared to 6-OHDA untreated group and returned back to levels comparable with the control group.
3.3 Correlation analysis
3.3.1 Correlation between neurobehavioral findings and biochemical markers in all studied groups
The levels of dopamine and TAC showed significant negative correlations with the time of latency to move and significant positive correlations with the number of crossed squares and number of rears. On the other hand, MDA, nitrite and caspase 3 activity were positively correlated with the time of latency to start moving and rearing and negatively correlated with number of rears and crossed squares. The Mean time of latency to fall was positively correlated with dopamine and TAC but negatively correlated with MDA, nitrite and caspase 3 activity.
The total number of steps taken by RT forelimb showed significant positive correlations with dopamine and TAC levels and significant negative correlations with MDA, nitrite and caspase 3 activity while the time to initiate stepping was positively correlated with MDA, nitrite and caspase 3 activity and negatively correlated with dopamine and TAC. However, correlation between biochemical markers and left forelimb results were insignificant ().
Table 4 Correlation between neurobehavioral findings and biochemical markers in all studied rats.
3.3.2 Correlations between neurobiochemical findings in all studied groups: ()
A significant positive correlations was observed between dopamine and TAC (r = 0.742∗∗ & p = <0.001) whereas a negative correlation was noticed between dopamine and MDA(r = −0.659∗∗ & p = <0.001), nitrite (r = −544∗∗ & p = 0.002∗∗) as well as caspase 3(r = −0.626∗∗ & p = <0.001).
In addition, TAC was negatively correlated with MDA, nitrie and caspase 3 activity where r = −0.779, −0.578∗∗, −0.822 respectively and P = <0.001 for all. As regard MDA, it showed a significant positive correlations with nitrite (r = 00.639∗∗ & p = <0.001) and caspase 3 activity (r = 0.553∗∗ & p = 0.002). Caspase 3 activity also showed a significant negative correlation with TAC (r = −0.822 & P = <0.001) and a significant positive correlation with MDA (r = 0.553 & P = 0.002) and nitrite (r = 0.442 & P = 0.014)
4 Discussion
In the current study, compared to control rats, 6-OHDA injected group showed manifestations of PD as demonstrated by a significant impairment of motor skills. There was reduction in the number of rears and squares crossed, and increase in latency to start moving and rearing suggesting bradykinesia. This was in agreement with Rizelio et al.Citation35 who found impaired motor activity in 6-OHDA rat model. It has been reported that 6-OHDA lesion induced bradykinesia with the contralateral forelimb.Citation36 In our study, right side affection was detected in rats injected with 6-OHDA in the left striatum compared with control rats or the left forelimb of the same rat which is consistent with previous studies.Citation37,Citation38 This may be explained by striato-cortical connections directly or indirectly through the thalamus (indirect and direct pathway).Citation39 Moreover, 6-OHDA injected rats also exhibited incoordination and loss of stability as shown by reduction in the mean latency to fall as compared with control rats. This finding is supported by the study of Iancu et al.Citation37 who found that the time spent in rotating rod was inversely correlated with cell loss.
The exact pathogenic mechanism for neurodegeneration in PD is not fully understood. Oxidative stress in brain is an important factor in the neuropathology of PD.Citation40 In the present study, the central administration of 6-OHDA leads to cellular oxidative stress as demonstrated by the significant reduction of TAC level when compared to controls. This is in agreement with the study by Lefter et al.Citation41 who found that 6-OHDA lesion resulted in significant depression in total antioxidant activity and increased oxidative stress levels in rats’ brains. The present study also showed a significant elevation in striatal MDA level in 6-OHDA injected rats compared to controls which is in parallel with previous studies.Citation42,Citation43 The nitrite level was also significantly higher in 6-OHDA injected rats compared to controls. This is consistent with the study of Guo et al.Citation44 who found that ROS-NO pathway was strongly involved in the toxicity of 6-OHDA-induced nigrostriatal lesions.
The dopamine depletion noted in our study in 6-OHDA untreated rats can be explained by the associated oxidative stress state observed in these rats. Previous studies have shown that 6-OHDA is transported into dopaminergic neurons where it is oxidized to produce hydrogen peroxide, superoxide, and hydroxyl radicals and resulted in potent inhibition of the mitochondrial respiratory chain complexes I and IV with subsequent depletion of dopamine level.Citation45,Citation46 It is known that oxidative stress plays an important role in the degeneration of dopaminergic neurons and dopamine depletion in Parkinson’s disease.Citation47 This explanation found more support in our study where a significant positive correlation was detected between striatal dopamine and TAC levels as well as the significant negative correlation between dopamine, MDA and nitrites levels. These findings were in agreement with Nagatsu et al.Citation48 who reported a role of oxidative stress in dopamine depletion.
Cell death is assumed to be one of the possible pathogenic mechanisms leading to neurodegeneration in PDCitation49 The present study showed a significant elevation of brain caspase 3 activity ratio in 6-OHDA rats compared to control rats. Several studies have pinpointed a role for the mitochondrial-caspase cascade in 6-OHDA-induced apoptosis, which initiates the activation of the main effector caspase- 3.Citation50–Citation52 It is reported that 6-OHDA initiates cellular oxidative stress, enters neurons via DAT and initiates the activation of cell death pathways by generation of intracellular free radicals and mitochondrial inhibition.Citation11 In addition. The current study revealed a significant negative correlation between striatal levels of caspase 3 and dopamine as well as TAC, whereas a positive relationship between caspase 3, MDA and nitrite was noticed. These findings supported the fact that caspase 3 plays an important role as a mediator of cell death in PD.
The correlation between oxidative stress markers and motor performance in this study was also verified from the positive correlation found between striatal MDA, nitrite and caspase 3 with latency to start movement (latency to move, latency to rear, time taken to initiate steps by RT and LT forelimb). Moreover, a negative correlation was detected with motor activities (number of crossed squares, number of rears and total steps taken by RT and LT forelimb). Therefore, it could be suggested that 6-OHDA induced oxidative stress and neuronal cell apoptosis with subsequent neurological motor deficits.
Cerium oxide nanoparticles, one of the most interesting nanomaterials for their catalytic properties, show a promise for application in medicine. Due to the presence of oxygen on its surface and autoregenerative cycle of its two oxidation states, Ce3+ and Ce4+, CeO2NPs can be used as an antioxidant agent. Thus, cerium oxide nanoparticles may be used as a tool for the treatment of many disorders which are associated with oxidative stress and apoptosis.Citation53
The use of low dose of CeO2NPs in the current study as a neuroprotective agent in 6-OHDA- injected group was variably effective in improving the motor functions and ameliorating the changes that accompanied PD. CeO2NPs succeeded in providing some protection against the impairment of locomotor behavior after three weeks although theses dose failed to preserve the motor functions as in normal rats. After six weeks CeO2-NPs failed to produce the same protective effect and showed only partial improvement in some open field parameters (numbers of squares crossed, and latency to move only). Regarding the motor performance in rotarod test; there was also a significant improvement of latency scores after three and six weeks from the use of low doses of CeO2NPs treatments compared to 6-OHDA untreated rats, although it was less effective after six weeks. In addition, this study showed slight improvement in initiation time and number of steps taken by right forelimb in the preventive group after three and six weeks but it also produced less improvement after six weeks.
This partial improvement in motor performance and coordination in neurobehavioral results was also reported by Heckman et al.Citation54 who found that CeO2NPs improved motor functions in autoimmune neurodegenerative disease. In addition, several studies approved neuroprotective effects of CeO2NPs on motor dysfunction.Citation55,Citation56 Thus, CeO2NPs preserved motor performance in 6-OHDA- injected group but they failed to preserve almost motor performance scores as control level. They also failed to produce the same protective effect with persistent neurodegeration for long period as it was observed in our study after six weeks. This may be attributed to the low dose used in this study which was insufficient to prevent the marked neurodegeneration and produce valuable neuroprotection especially for long period.
This neuroprotective effect of CeO2NPs has been attributed to different mechanisms. Enhancement of antioxidant capacity, prevention of reactive oxygen species (ROS) generation and attenuation of apoptosis are important mechanisms carried by CeO2 NPs with subsequent restoration of striatal dopamine levels.Citation57,Citation14 Our results provide some support to these mechanisms as treatment of 6-OHDA injected rats with low dose of CeO2 NPs caused elevation in striatal TAC levels compared 6-OHDA untreated group. This was in agreement with the study of Yu et al.Citation58 and Hochella et al.Citation59 who found that the antioxidative effect of CeO2 nanoparticles is attributed to its direct exchange between ROS and the high ratio of electrons on the larger surface area of the nanoparticles.
CeO2 NPs treatment also caused significant reduction in striatal MDA level compared to 6-OHDA untreated group. As previously observed, CeO2NPs, can scavenge almost all types of reactive species, has excellent regeneration ability and can cross the blood brain barrier (BBB) because of its nano size.Citation60
Dowding and co-workersCitation61 explored and demonstrated the NO scavenging ability of CeO2NP. The possible mechanism by which CeO2NPs scavenges NO is through formation of an electropositive nitrosyl ligand caused by internal electron transfer from NO to a Ce4.Citation62 This was contrary to our results where the low dose of CeO2NPs failed to produce such effect.
Several studies reported the potential therapeutic benefits of CeO2NPs in many diseases where they have been shown to scavenge ROS and provide neuroprotection.Citation15–Citation17 Because of its multiple antioxidant-enzyme-like activities, including SOD, catalase, peroxidase-like activities, and hydroxyl radical and nitric oxide radical scavenging properties, CeO2NPs was expected to scavenge almost all types of reactive species. This makes it superior to any antioxidant enzyme or molecule because they often scavenge only a single type of free radical before being inactivated.Citation16
In the present study, CeO2NPs caused a significant reduction in caspase-3 when compared to 6-OHDA untreated group. It is thought that CeO2 NPs protect cells and tissues from damage by its regenerative free radical scavenging property. This was in accordance with study of Colon et al.Citation63 who investigated the protective effect of CeO2NPs against radiation induced damage. Estevez and coworkersCitation64 reported that CeO2 could reduce ischemic cell death in mice hippocampal brain slices by 50%.
Although the low dose of CeO2NPs succeeded in improving the oxidative stress and cell death partially but they were unable to regain it back to normal condition. Moreover, this low dose of CeO2NPs failed to reduce nitrite level. Thus, this low dose of CeO2NPs was not able to cause complete amelioration of the profound oxidative stress state which was produced by 6-OHDA. This could explain the inability of such dose of CeO2NPs to provide complete protection of dopaminergic neurons and to preserve dopamine within normal levels. The Negative correlation observed between dopamine and nitrite as well as MDA provided more support to this explanation. The low dose of CeO2NPs showed improvements in only some biochemical aspects which led to improvement in some motor performances.
The use of such low dose in the current work was based on the results of many previous studies which revealed that extensive application of CeO2 nanoparticles may induce oxidative stress, apoptosis and cytotoxicity.Citation65–Citation67 Dillon et al.Citation68 reported that once the dose achieved a certain maximum level of benefit, neuroprotective effect declined. Kim et al.Citation55 also found that the indiscriminate use of nanoparticles without considering the optimal dose may not be protective and may even be harmful.
In conclusion, the use of low dose of CeO2NPs showed partial protection against the neurochemical disturbances and motor disturbances in 6-OHDA induced parkinsonian rat model. CeO2NPs represent a novel neuroprotective agent in PD and they can improve motor performance through their antioxidant and anti apoptotic effects. Further studies are needed to examine the neuroprotective effect of higher doses of CeO2NPs and protection against the development of behavioral and biochemical changes in Parkinson’s disease.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 20 January 2017
References
- D.W.DicksonParkinson’s disease and parkinsonism: neuropathologyCold Spring Harb Perspect Med282012a00925
- S.FahnG.CohenThe oxidant stress hypothesis in Parkinson’sdisease: evidence supporting itAnn Neurol321992804812
- OnyouHwangRole of oxidative stress in parkinson's diseaseExp Neurobiol2220131117
- M.T.LinM.F.BealMitochondrial dysfunction and oxidative stress in neurodegenerative diseasesNature4432006787795
- A.H.SchapiraMitochondria in the aetiology and pathogenesis of Parkinson’s diseaseLancet Neurol7200897109
- S.J.ChintaJ.K.AndersenRedox imbalance in Parkinson’s diseaseBiochim Biophys Acta1780200813621367
- E.C.HirschS.HunotB.FaucheuxDopaminergic neuronsdegenerate by apoptosis in Parkinson’s diseaseMov Disord141999383385
- W.G.TattonC.W.OlanowApoptosis in neurodegenerative disease: the role of mitochondriaBiochim Biophys Acta14101999195213
- U.Ungerstedt6-Hydroxydopamine induced degeneration of central monoamine neuronsEur J Pharmacol51968107110
- Nicola simola, Micaela morreli and annar .cartaThe 6-Hydroxydopamine Model of Parkinson's DiseaseNeurotox Res112007151167
- D.BlumS.TorchN.LambengMolecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: Contribution to the apoptotic theory in Parkinson’s diseaseProg Neurobiol652001135172
- O.SalataApplications of nanoparticles in biology and medicineNanoBiotechnology220043
- T.PirmohamedJ.M.DowdingS.SinghNanoceria exhibit redox state-dependent catalase mimetic activityChem Commun (Camb)46201027362738
- E.G.HeckertA.S.KarakotiS.SealW.T.SelfThe role of ceriumredox state in the SOD mimetic activity of nanoceriaBiomaterials29200827052709
- L.KongX.CaiX.ZhouNanoceria extend photoreceptor cell lifespan in tubby mice by modulation of apoptosis/survival signaling pathwaysNeurobiol Dis422011514523
- X.ZhouL.L.WongA.S.KarakotiS.SealJ.F.Mc-GinnisNanoceria inhibit the development and promote the regression of pathologic retinal neovascularization in the Vldlr knockoutmousePLoS One62011e16733
- N.PourkhaliliA.HosseiniA.Nili-AhmadabadiBiochemical and cellular evidence of the benefit of a combination of cerium oxide nanoparticles and selenium to diabetic rats. WorldJ Diabetes22011204210
- R.DavanR.G.S.V.PrasadV.S.JakkaCerium oxide nanoparticles promotes woundhealing activity in in-vivo animal modelJ Bionanosci620127883
- J.NiuK.WangP.KolattukudyCerium oxide nanoparticles inhibitsoxidative stress and nuclear factor-κb activation in H9c2cardiomyocytes exposed to cigarette smoke extractJ Pharmacol Exp Ther33820115361
- D.SchubertR.DarguschJ.RaitanoS.W.ChanCerium andyttrium oxide nanoparticles are neuroprotectiveBiochem Biophys Res Commun34220068691
- Michael P.SmithWayne A.CassOxidative stress and dopamine depletion in an intrastriatal 6-hydroxydopamine model of Parkinson’s diseaseNeuroscience144200710571066
- C.KimT.KimI.ChoiCeria Nanoparticles that can protect against Ischemic StrokeAngew Chem Int Ed5120121103911043
- G.PaxinosC.WatsonThe Rat Brain in Stereotaxic Coordinates4th ed.1998Academic PressSan Diego
- K.GuentherR.M.DeaconV.H.PerryJ.N.RawlinsEarly behavioural changes in scrapie-affected mice and the influence of dapsoneEur J Neurosci142001401409
- Y.S.AbadaH.P.NguyenR.SchreiberB.EllenbroekAssessment of motor function, sensory motor gating and recognition memory in a novel BACHD transgenic rat model for Huntington diseasePLoS One82013e68584
- M.OlssonG.NikkhahC.BentlageA.BjorklundForelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping testJ Neurosci155 Pt 2199538633875
- S.SpijkerDissection of rodent brain regionsW.K.LiNeuroproteomics2011Humana PressTotowa, NJ1326
- O.H.LowryN.J.RosebroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem1931951265275
- P.TripathiA.SinghS.AgrawalO.PrakashM.P.SinghCypermethrin alters the status of oxidative stress in the peripheral blood: relevance to ParkinsonismJ Physiol Biochem702014915924
- A.L.ArseneC.AramăN.MitreaA.N.CristeaExperimental assessment of cerebral monoaminergic status in a murine model of behaviorFarmacia572009492499
- D.KoracevicG.KoracevicV.DjordjevicS.AndrejevicV.CosicMethod for the measurement of antioxidant activity in human fluidsJ Clin Pathol542001356361
- E.D.WillsMechanisms of lipid peroxide formation in animal tissuesBiochem J991966667676
- H.A.MontgomeryJ.F.DymockThe determination of nitrite in waterAnalyst861961414416
- L.Casciola-RosenD.W.NicholsonT.ChongApopain/CPP32 cleaves proteins that are essential for cellular repair: a fundamental principle of apoptotic deathJ Exp Med183199619571964
- V.RizelioR.E.SzawkaL.L.XavierLesion of the subthalamic nucleus reverses motor deficits but not death of nigrostriatal dopaminergic neurons in a rat 6-hydroxydopamine-lesion model of Parkinson's diseaseBraz J Med Biol Res4320108595
- E.DowdC.MonvilleE.M.TorresS.B.DunnettThe corridor task: a simple test of lateralised response selection sensitive to unilateral dopamine deafferentation and graft-derived dopamine replacement in the striatumBrain Res Bull6820052430
- R.IancuP.MohapelP.BrundinG.PaulBehavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in miceBehav Brain Res1622005110
- T.SchallertD.NortonT.JonesA clinically relevant unilateral rat model of Parkinsonian akinesiaNeural Plast31992332333
- M.R.DeLongT.WichmannCircuits and circuit disorders of the basal gangliaArch Neurol6420072024
- U.AdigaJ.D’souzaR.KousalyaG.M.RaoM.NandiniV.D’souzaTotal antioxidant activity in Parkinson’s diseaseBiomed Res1722006145147
- R.LefterA.CiobicaL.HritcuB.StoicaD.CojocaruZ.OlteanuThe effects of a 6-OHDA induced lesion in murine nuccleus accumbens on memory and oxidative stress statusEur J Med82013443449
- M.T.MansouriY.FarboodM.J.SameriA.SarkakiB.NaghizadehM.RafeiradNeuroprotective effects of oral gallic acid against oxidative stress induced by 6-hydroxydopamine in ratsFood Chem138201310281033
- C.M.ChenJ.L.LiuY.R.WuIncreased oxidative damage in peripheral blood correlates with severity of Parkinson's diseaseNeurobiol Dis332009429435
- S.GuoJ.YanT.YangX.YangE.BezardB.ZhaoProtective effects of green tea polyphenols in the 6-OHDA rat model of Parkinson's disease through inhibition of ROS-NO pathwayBiol Psychiatry62200713531362
- Y.GlinkaM.GassenM.B.YoudimMechanism of 6-hydroxydopamine neurotoxicityJ Neural Transm Suppl19975566
- J.BovéC.PerierNeurotoxin-based models of Parkinson's diseaseNeuroscience20125176
- S.FahnD.SulzerNeurodegeneration and neuroprotection in Parkinson diseaseNeuroRx12004139154
- T.NagatsuM.SawadaMolecular mechanism of the relation of monoamine oxidase B and its inhibitors to Parkinson's disease: possible implications of glial cellsJ Neural Transm Suppl7120065365
- E.C.HirschS.HunotB.FaucheuxDopaminergic neurons degenerate by apoptosis in Parkinson’s diseaseMov Disord141999383385
- G.ChenK.A.BowerC.MaS.FangC.J.ThieleJ.LuoGlycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal deathFaseb J18200411621164
- E.E.OchuN.J.RothwellC.M.WatersCaspases mediate 6-hydroxydopamine-induced apoptosis but not necrosis in PC12 cellsJ Neurochem70199826372640
- N.TakaiH.NakanishiK.TanabeInvolvement of caspase-like proteinases in apoptosis of neuronal PC12 cells and primary cultured microglia induced by 6-hydroxydopamineJ Neurosci Res5421998214222
- R.W.TarnuzzerJ.ColonS.PatilS.SealVacancy engineered ceria nanostructures for protection from radiation-induced cellular damageNano Lett5200525732577
- K.L.HeckmanW.DeCoteauA.EstevezCustom cerium oxide nanoparticles protect against a free radical mediated autoimmune degenerative disease in the brainACS Nano720131058210596 (23)
- C.KimT.KimI.ChoiCeria Nanoparticles that can protect against ischemic strokeAngew Chem Int Ed5120121103911043
- N.SinghC.A.CohenB.A.RzigalinskiTreatment of neurodegenerative disorders with radical nanomedicineAnn N Y Acad Sci11222007219230
- T.PirmohamedJ.M.DowdingS.SinghNanoceria exhibit redox state-dependent catalase mimetic activityChem Commun (Camb)46201027362738
- J.C.YuL.ZhangJ.LinDirect sonochemical preparation of high-surface-area nanoporous ceria and ceria-zirconia solid solutionsJ Colloid Interface Sci2602003240243
- M.F.HochellaJrS.K.LowerP.A.MauriceNanominerals, mineral nanoparticles, and Earth systemsScience319200816311635
- A.KarakotiS.SinghJ.M.DowdingS.SealW.T.SelfRedox-active radical scavenging nanomaterialsChem Soc Rev39201044224432
- J.M.DowdingT.DosaniA.KumarS.SealW.T.SelfCerium oxide nanoparticles scavenge nitric oxide radical (NO)Chem Commun (Camb)48201248964898
- C.XuX.QuCerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applicationsNPG Asia Mater62014e90
- J.ColonN.HsiehA.FergusonCerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2Nanomedicine62010698705
- A.Y.EstevezS.PritchardK.HarperNeuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemiaFree Radic Biol Med51201111551163
- G.ChengW.GuoL.HanCerium oxide nanoparticles induce cytotoxicity in human hepatoma SMMC-7721 cells via oxidative stress and the activation of MAPK signaling pathwaysToxicol In Vitro27201310821088
- D.AliS.AlarifiS.AlkahtaniA.A.AlKahtaneA.AlmalikCerium oxide nanoparticles induce oxidative stress and genotoxicity in human skin melanoma cellsCell Biochem Biophys2014 (in Press)
- E.J.ParkJ.ChoiY.K.ParkK.ParkOxidative stress induced by cerium oxide nanoparticles in cultured BEAS-2B cellsToxicology245200890100
- C.DillonM.BillingsK.HockeyL.DelagrazB.RzigalinskiCerium oxide nanoparticles protect against MPTP-induced dopaminergic neurodegeneration in a mouse model for parkinson's diseaseNanotechnology32011451454