Abstract
Background
Colorectal cancer is one of the most frequently seen cancers worldwide. Currently, CEA is the most commonly used tumor marker in colorectal cancer. The changes in IGF/IGFBP equilibrium is also known to cause carcinogenesis. In this study, we aimed to monitor IGF-I/IGFBP-3 levels, the changes in IGF-I/IGFBP-3 ratio and correlations of these peptides with the common tumor marker CEA.
Materials and methods
55 colorectal cancer patients and 35 control group patients were included in this study. Serum CEA, IGF-I and IGFBP-3 levels of all specimens were measured with chemiluminescence method.
Results
In colorectal cancer patients, IGF-I levels was found to be increased, IGFBP-3 levels decreased and IGF-I/IGFBP-3 ratio was increased; when compared to control group (p < 0.05). A moderately significant correlation was found between the conventional tumor marker CEA and IGF-I and IGF-BP3 (p = 0.001, r = 0.533 and p = 0.001, r = −0.573 respectively).
Conclusions
IGF-I/IGFBP-3 ratio seems to be increased in the colorectal cancer patients. When considered with the moderate correlation levels of these peptides with CEA, this increase in IGF-I/IGF-BP3 ratio may be useful in monitoring carcinogenesis in colorectal cancer patients among with CEA but more detailed and extensive studies in larger study groups needed to be carried out.
1 Introduction
The frequency of colorectal cancer in developed countries increased dramatically in recent years. Colorectal cancer is the second most common cancer in females and the third most common cancer in males worldwide. It is more common in developed countries with North America, Europe, and Australia having the highest incidence rates.Citation1,Citation2 Different laboratory techniques are used in the diagnosis, treatment and follow up of colorectal cancer. Currently, carcinoembryonic antigen (CEA) is the most widespreadly used tumor marker. CEA is one of the most common oncofetal proteins to be used as a tumor marker as it functions as a homotypic intercellular adhesion molecule that promotes the aggregation of human colorectal carcinoma cells.Citation3 Clinical applications of CEA includes many different categories like diagnosis (distinction between benign and malignant tumors), treatment monitoring (therapeutic effect, tumor recurrence), special pathological techniques (immunohistochemistry, immunocytochemistry), localization (tumor monitoring with radiolabelled antibodies) and therapy (antibody-bound cytotoxic agents and vaccine vectors carrying the CEA gene).Citation4–Citation6
IGF-I is a 7.5 kDa, 70 aminoacid single chain polypeptide including three disulfide bonds. IGF-I mediates the growth stimulating effects of growth hormone (GH).Citation7,Citation8 Studies conducted in the last two decades revealed that IGF-I is included in tumorigenesis process of various cancer types. The levels of IGF-I, IGF-II and IGF-IR are found to be elevated in malignancies like glioblastoma, neuroblastoma, meningioma and GIS, colorectal, pancreas and ovarian cancers.Citation9–Citation12
IGFBP-3 is found as a 150 kDa triple complex in circulation.Citation13 It serves as a depot by binding more than 90% of serum IGF-I.Citation14 This binding protein not only controls the level of free IGF-I, but it also blocks the effects of IGF-I and increases the half-life of IGF-I. Besides it’s effects on IGF-I; IGFBP-3 plays important roles in cell survival, growth and differentiation in IGF-I independent manners.Citation15 Recent epidemiological studies have shown that low serum IGFBP-3 level is a negative risk factor for breast, prostate and colorectal cancers.Citation16
Recent studies have shown that IGF/IGFBP ratio is a better predictor of disease progression.Citation17 IGF/IGFBP ratio alterations have proven to cause carcinogenesis in different levels and models.Citation18 There are also correlation studies about using IGF-I/IGFBP-3 ratio in various cancer types among with commonly used tumor markers.Citation19
In this study, we aimed to detect IGF-I and IGFBP-3 levels and IGF-I/IGFBP-3 ratio changes in colorectal cancer patients. Another goal of the study is to determine the correlation of these peptides with CEA; the common tumor marker in colorectal cancer.
2 Materials and methods
Patient group was consisted of 55 newly diagnosed colorectal cancer patients who did not recieve any anti-cancer therapy or undergone cancer surgery, and who did not have any other malignancies, hypertension, diabetes mellitus, coronary artery disease, central or peripheric nervous system problems, liver disease, kidney failure or any other chronical illnesses and who had serum CEA levels above the reference limits and had no any other exclusion criteria. Control group was consisted of 35 healthy people with no signs of any acute or chronic diseases mentioned above and no signs of any malignancy; and who had CEA levels within the reference limits. Patient and control group subjects were all admitted to General Surgery Clinics at Ankara Numune Training and Research Hospital between August-October 2010. Blood samples were collected and allowed to coagulate for 30 min at room temperature and centrifuged at 1500g for 10 min. The extracted sera were aliquoted into eppendorf tubes and kept at −80 °C until the time of analysis.
Serum CEA level detection of all specimens were made with chemiluminescence method in Beckman Coulter UniCel® DxI 800 Immunoassay System analyzers. Serum IGF-I and IGFBP-3 levels were detected with chemiluminescence immunometrical method in Siemens Immulite 1000 analyzer. The reference values for the related tumor marker CEA was accepted as 0–3 ng/mL (inter and intra-assay CV values of 6.3% and 5.8 respectively). The reference values for IGF-I was 78–222 ng/L (inter and intra-assay CV values of 7.4% and 4.5% respectively) and for IGF-BP3 was 3.4–6.9 ng/mL (inter and intra-assay CV values of 6.8% and 3.1% respectively).
3 Statistical analysis
Data analysis was made with SPSS for Windows 11.5 programme. The distribution patterns of continuous variables were checked with Kolmogorov-Smirnov test. Descriptive statistics of normally distributed continuous variables are illustrated as mean ± standard deviation. Descriptive statistics of non-normally distributed continuous variables are illustrated as median (interquartile range) values. Among group differences of normally distributed variables were analyzed with Student’s t test while non-normally distributed variables were analyzed with Mann Whitney U test. Correlation analysis between CEA/IGF-I and CEA/IGF-BP3 pairs was made with Spearman’s rho test and p < 0.05 was accepted as statistically significant.
4 Results
Age, sex, serum CEA, IGF-I and IGFBP-3 levels of patient and control groups are listed in .
Table 1 The sex distribution, mean ages, CEA,IGF-I,IGFBP-3 levels and IGF-I/IGFBP-3 ratios of the study groups.
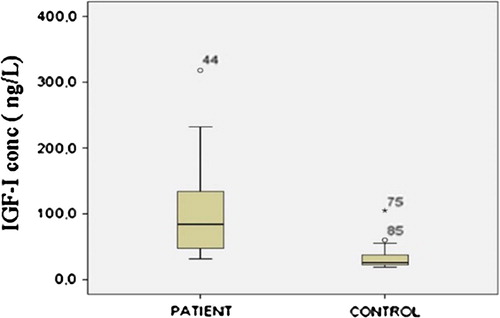
IGF-I levels of control and patient groups are shown in , in a Box-Whiskar plot. There is a statistically significant (p < 0.05) difference between patient and control groups.
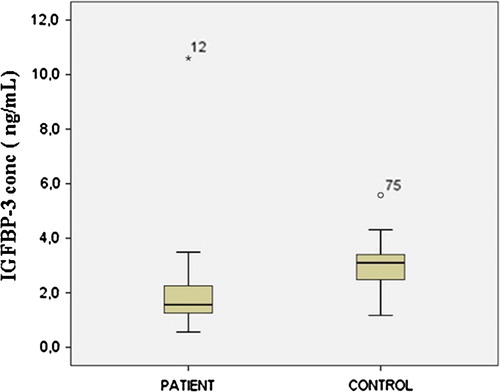
There is a statistically significant (p < 0.05) difference between the IGFBP-3 levels of the patient and control groups. IGFBP-3 levels of control and patient groups are shown in a Box-Whiskar plot in .
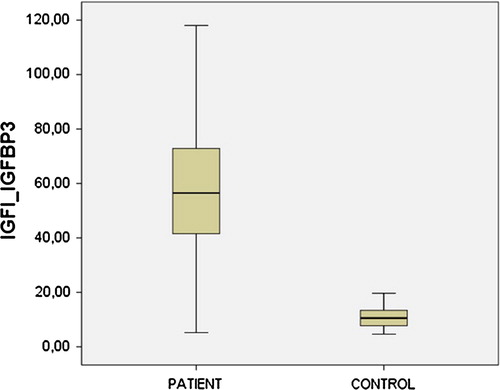
IGF-I/IGFBP-3 ratios of patient and control groups are shown in a Box-Whiskar plot in . There is a statistically significant (p < 0.05) difference between IGF-I/IGFBP-3 ratios of the patient and control groups.
For the correlation analysis, 5 samples with CEA levels above 1000 ng/mL were excluded as those values were exceeding analytical detection range.
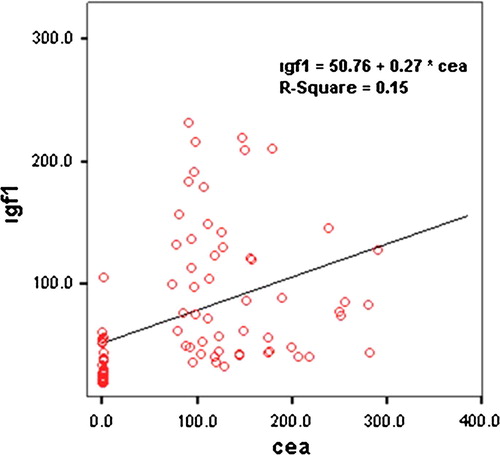
A statistically significant moderately positive correlation is found between CEA and IGF-I levels (r = 0.533, p = 0.001). shows the linear regression graphic between CEA and IGF-I levels.
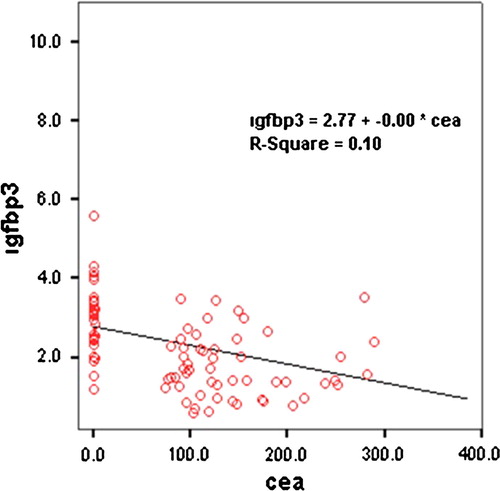
A statistically significant moderately negative correlation is found between CEA and IGFBP-3 levels (r = −0.573, p = 0.001). shows the linear regression graphic between CEA and IGFBP-3 levels.
5 Discussion
Colorectal cancer is one of the most common cancers seen in developed countries and it is one of the most widespreadly diagnosed and mortal type of cancer in both gender among cancer cases. Various tumor markers have been suggested and examined for the diagnosis and monitoring of colorectal cancer in recent years. Among these markers, CEA is the most commonly accepted parameter.Citation20,Citation21
The principle use of CEA in colorectal cancer is the survival follow-up after the curative resection of the primary tumor. Like any other marker; low level of sensitivity and specificity of CEA limits usage in clinical practice in early onset colorectal cancer. But especially in the post-op period, early onset metastasis can be detected with serial CEA measurements.Citation22 Also it is reported that the risk of early relapse is more likely to occur with increasing serum CEA level.Citation23 Besides this, the detection of pre-operative CEA levels after diagnosis will provide a baseline value for the serial CEA measurements during treatment and also give prognostic information.Citation24 CEA levels usually drop back to normal values after 2–4 weeks following curative resection. In our study, there is a statistically significant difference between serum CEA levels of patient and control groups (p < 0.05).
Nowadays, there is increased evidence about the relationship of IGF-I and cancer because it has been shown that there is a strong relationship between growth factors and oncogenes. Several studies have been focused on the increased levels of IGF-I in breast, prostate, esophagus, lung and liver cancer patients to show that this is also a factor triggering carcinogenesis.Citation25–Citation28 Krajcik et al. declared that IGF-I increase induces breast cancer in premenopausal women but not in postmenopausal ones and explained this with a reduction in IGF-I levels with age.Citation26 It is a real fact that IGF-I levels drop with age. Therefore the reference values should be divided into periods of 10 years when it comes to IGF-I. In our study, no statistically significant difference was present between the mean ages of patient and control groups (p = 0.085). That’s why there was no need to classify the IGF-I levels according to reference groups defined by age. The patient and control groups were in the same age interval and there was a statistically significant difference between IGF-I levels of the two groups (p < 0.05).
It is known that only less than 1% of IGF-I is found free in circulation. The majority of IGF-I is bound and carried with IGFBPs. More than 90% of this bound portion is known to be attached to the carrier protein IGFBP-3.Citation27,Citation29 Oh et al. worked with estrogen-receptor-negative breast cancer cells and showed that IGFBP-3 binds to cell surface receptors in a dose dependent manner.Citation30 Similarly, IGFBP-3 associated proteins detected to be membrane-bound in prostate cancer cells.Citation31
IGF levels are known to be increased in carcinogenesis. This increase in IGF-I is caused by an increase in it’s free form. As the level of IGFBPs decrease, free IGF-I levels increase and it stimulates it’s receptor by binding to it. Macdonald et al. transfected Caco-2 human colon cancer cells with IGFBP-3 cDNA and observed that cell density and cleavage decreased after a 10–15 days period.Citation32
IGFBP-3 levels were also analyzed in different types of cancer. In their study Krajcik et al. showed that IGFBP-3 levels dropped in women with breast cancer especially in the postmenopausal period.Citation26 Again in a study with postmenopausal women; it has been shown that lower IGFBP-3 level is a significant risk factor for endometrium CA.Citation33 Prostate cancer and benign prostate hyperplasia are also a group of diseases in which levels of IGF-I and IGFBP-3 are evaluated frequently.Citation17,Citation34 In our study, it was shown that IGFBP-3 levels of the patient group are significantly lower than the IGFBP-3 levels of the control group. These results support the fact that IGFBP-3 level drops by different mechanisms in various carcinomas and leads to increased risk of cancer either dependent or independent of IGF; as mentioned above.
Like all other types, IGFBP-3 levels found to be dropped in colorectal cancer patients in different studies. Ma et al. in their study with male colorectal cancer patients have revealed that high IGF-I and low IGFBP-3 levels are important factors that increase the risk of cancer.Citation27 In many studies parallel to this; both the genetic expression and the biological and physiological aspects of IGFBP-3 was analyzed and lower levels of this peptide have shown to be an independent risk factor for colorectal carcinoma.Citation35,Citation36
In our study, we also found that IGFBP-3 levels differ in a statistically significant manner between patient and control group subjects (p < 0.05).
In recent years, there is an attempt to evaluate IGF-I/IGFBP-3 ratio in studies instead of evaluating IGF-I and IGFBP-3 levels seperately. A decrease in level of IGFBP-3 for any reason causes IGF-I to be more bioavailable to tissues. And this leads to hyperactivation of the IGF-I receptor to promote cell growth and carcinogenesis. In many studies designed principally based on these data, cases of prostate, breast, endometrium and colon cancer have been evaluated and IGF-I/IGFBP-3 ratio found to be increased in all of them.Citation17,Citation26,Citation27,Citation33
Ma et al. declared that high IGF-I and low IGFBP-3 levels are independent risk factors for colorectal cancer development but they also associated the increase in IGF-I/IGFBP-3 ratio in combination with an increased risk of cancer.Citation33 Amenable with these results; our study revealed that not only IGF-I and IGFBP-3 levels are significantly different in control and patient groups, but also IGF-I/IGFBP-3 ratio is significantly higher in patient group when compared to control group. Results are statistically significant (p < 0.05).
In some studies evaluating the relationship of IGF-I and IGFBP-3 with different cancer types; correlation analysis of these peptides with the conventionally used tumor markers in routine cancer follow-up and therapy monitoring have been made. Khosravi et al. carried out correlation analysis of IGF-I and IGFBP-3 levels with free PSA in prostate cancer patients. This study put out positive and negative correlations between IGF-I, IGFBP-3 and free PSA levels respectively. From this point, they hypothesized that IGF-I and IGFBP-3 levels can be used together with free PSA in prostate cancer follow-up.Citation33
Yılmaz et al. carried out correlation analysis between IGF-I and IGFBP-3 levels with the tumor markers CEA and CA 19-9 separately and suggested that serum IGF-I and IGFBP-3 levels may be used as tumor markers in oesophageal carcinoma.Citation25
Especially in recent years, the IGF pathway and it’s relation with carcinogenesis have been evaluated from the genetic perspective. In a meta-analysis, IGF1(CA)19 and IGFBP-3-202A/C gene polymorphisms were shown to be associated with common cancer risk including colorectal, breast, prostate, and lung cancer.Citation37 Liu et al. evaluated the role of mRNA expression of IGF-1 and IGF-1R in patients with colorectal adenocarcinoma and concluded that mRNA levels of IGF-I and IGF-IR were significantly higher in CA patients.Citation38 Besides this, Stanilov et al. focused mainly on genetic polymorphisms in IGF-IR in colorectal cancer development and showed that a relationship between the +3179G > A polymorphism of the IGF-1R and serum IGF-1 with the progression of colorectalcarcinoma.Citation39
Parallel to our work, the study of Jiang et al. investigated the possible roles of insulin, IGF-1 and IGFBPs in initiation and progression of colorectal cancer but they concluded that these factors have an influence on cancer initiation but they are not related to progression and outcome of the disease.Citation40
In this study we made correlation analysis between IGF-I and IGFBP-3 levels and CEA respectively. Paralel to Khosravi et al’s results, we found positive correlation between CEA and IGF-I levels and negative correlation between CEA and IGFBP-3 levels.
As a result, this study revealed that there is an increase in IGF-I levels, a decrease in IGFBP-3 levels and an increase in IGF-I/IGFBP-3 ratio which is accepted as free IGF-I expression in colorectal cancer patients. A moderately significant correlation is detected between IGF-I, IGFBP-3 and CEA which is the most commonly used tumor marker in colorectal cancer treatment and monitoring. When these findings are combined with the results of the previous studies, we suggest that the serum IGF-I and IGFBP-3 levels may be used as tumor markers in colorectal carcinoma among with CEA. But to be used in clinical practice, these data need to be supported with wider and more extensive studies.
Ethical considerations
The study has been approved by the Ethic Committee of the hospital at 10.02.2011 with the approval number 218-2011
Acknowledgements
We are grateful to the many colleagues and patients in our institution who contributed to this study. The study is supported by Ankara Numune Training and Research Hospital.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 7 March 2017
References
- J.DeitrickW.M.PruittWnt/β catenin-mediated signaling commonly altered in colorectal cancerProg Mol Biol Transl Sci1442016 49-6
- P.BoyleJ.S.LangmanABC of colorectal cancer: epidemiologyBMJ32172642000805808
- R.BendardafH.LamlumS.PyrhönenPrognostic and predictive molecular markers in colorectal carcinomaAnticancer Res244200425192530
- Proceedings of the First International Conference on the Clinical Uses of Carcinoembryonic Antigen. Cancer 1973; 42(3 Suppl): 1397–1659.
- D.M.GoldenbergA.M.NevilleA.C.CarterCEA, (carcinoembryonic antigen): its role as a marker in the management of cancerJ Cancer Res Clin Oncol10131981239242
- M.TurrizianiM.FantiniM.BenvenutoCarcinoembryonic antigen (CEA)-based cancer vaccines: recent patents and antitumor effects from experimental models to clinical trialsRecent Pat Anticancer Drug Discov732012265296
- R.E.HumbelInsulin-like growth factors I and IIEur J Biochem1901990445462
- D.LeRoithC.BondyS.YakarJ.L.LiuA.ButlerThe somatomedin hypothesisEndocr Rev2220015374
- G.WrobelP.RoerigF.KokocinskiMicroarray-based gene expression profiling of benign, atypical and anaplastic meningiomas identifies novel genes associated with meningioma progressionInt J Cancer1142005249256
- W.HartmannA.KochH.BruneInsulin-like growth factor II is involved in the proliferation control of medulloblastoma and its cerebellar precursor cellsAm J Pathol166200511531162
- C.M.PerksJ.M.HollyThe insulin-like growth factor (IGF) family and breast cancerBreast Dis1820034560
- C.M.PerksJ.M.HollyIGFBPs and breast cancerBreast Dis17200391104
- S.RajaramD.J.BaylinkS.MohanInsulin-like growth factor-binding proteins in serum and other biological fluids: regulation and functionsEndocr Rev181997801831
- G.R.DeviD.H.YangR.G.RosenfeldY.OhDifferential effects of insulin-like growth factor (IGF)-binding protein-3 and its proteolytic fragments on ligand binding, cell surface association, and IGF-1 receptor signallingEndocrinol141141200041714179
- J.M.RicortInsulin-like growth factor binding protein (IGFBP) signallingGrowth Horm IGF Res142004277286
- S.A.RosenzweigWhat’s new in the IGF-binding proteins?Growth Horm IGF Res142004329336
- Anand P.ChokkalingamMichaelPollakCapri-MaraFillmoreInsulin-like growth factors and prostate cancer: a population-based case-control study in ChinaCancer Epidemiol, Biomarkers Prevention102001421427
- A.GrimbergP.CohenRole of insulin-like growth factors and their binding proteins in growth control and carcinogenesisJ Cellular Physiol183200019
- E.YılmazObez ve obez olmayan kadınlarda kardiyovasküler risk faktörleri ile insülin benzeri büyüme faktörü-1 ve insülin benzeri büyüme faktörü bağlayıcı protein 3 (igfbp3) arasındaki ilişki2009Tıpta Uzmanlık Teziİstanbul
- M.J.DuffyCEA as a marker for colorectal cancer: is it clinically usefulClin Chem472001624630
- R.BendardafH.LamlumS.PyrhonenPrognostic and predictive molecular markers in colorectal carcinomaAnticancer Res24200425192530
- G.M.JefferyB.E.HickeyP.HiderFollow-up strategies for patients treated for nonmetastatic colorectal cancer (Cochrane Review)The Cochrane Library Issue 22004John Wiley & Sons Ltd.Chichester (UK)
- J.J.SzymenrdaM.P.NowackiA.W.SzawlowskiJ.A.KaminskaPredictive value of plasma CEA levels: preoperative prognosis and postoperative monitoring of patients with colorectal carcinomaDis Colon Rectum2519824652
- M.J.DuffyaA.van DalencC.HaglunddTumour markers in colorectal cancer: European Group on Tumour Markers (EGTM) guidelines for clinical useEur J Cancer43200713481360
- O.YılmazA.EroğluE.DağN.KaraoglanogluSerum levels of IGF-I and IGFBP-III and their relation with carcinoembryonic antigen and carbohydrate antigen 19–9 in cases of esophageal cancerInt J Clin Pract6012200616041608
- Rozlyn A.KrajcikNancy D.BorofskyStephenMassardoNormanOrentreichInsulin-like growth factor I (IGF-I), IGF-binding proteins, and breast cancerCancer Epidemiol, Biomarkers Prevention1111200215661573
- JingMaMichael N.PollakEdwardGiovannucciProspective study of colorectal cancer risk in men and plasma levels of insulin-like growth factor (IGF)-I and IGF-binding protein-3J Natl Cancer Inst9171999
- R.J.TroisiA.N.FreedmanS.S.DevesaIncidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975–1994Cancer85199916701676
- R.C.BaxterInsulin-like growth factor binding proteins in the human circulation, a reviewHorm Res421994140144
- Y.OhH.L.MüllerH.PhamR.G.RosenfeldDemonstration of receptors for insulin-like growth factor binding protein-3 on Hs578T human breast cancer cellsJ Biol Chem26819932604526048
- R.RajahB.ValentisP.CohenInsulin-like growth factor (IGF)-binding protein-3 induces apoptosis and mediates the effects of transforming growth factor-b1 on programmed cell death via a p53 – and IGF-independent mechanismJ Biol Chem27219971218112188
- R.G.MacDonaldB.S.SchafferI.J.KangS.M.HongE.J.KimJ.H.ParkGrowth inhibition and differentiation of the human colon carcinoma cell line, Caco-2, by constitutive expression of insulin like growth factor binding protein-3J Gastroenterol Hepatol1419997278
- James V.LaceyJr.NancyPotischmanM. PatriciaMadiganInsulin-like growth factors, insulin-like growth factor-binding proteins, and endometrial cancer in postmenopausal women: results from a U.S. Case-control studyCancer Epidemiol Biomarkers Prev1342004
- J.KhosraviA.DiamandiJ.MistryA.ScorilasInsulin-like growth factor I (IGF-I) and IGF-binding protein-3 in benign prostatic hyperplasia and prostate cancerJ Clin Endocrinol Metab28622001694699
- J.M.CulouscouM.Remacle-BonnetF.GarrousteJ.FantiniJ.MarvaldiG.PommierProduction of insulin-like-growth factor II (IGF-II) and different forms of IGF-binding proteins by HT-29 human colon cancer carcinoma cell lineJ Cell Physiol1431990405415
- G.PommierF.GarrousteF.el AtiqJ.MarvaldiM.Remacle-BonnetPotential role of IGFBPS in the regulation of the differentiation state of human colonic carcinoma cellsGrowth Regul3119938082
- H.QuanH.TangL.FangJ.BiY.LiuH.LiIGF1(CA)19 and IGFBP-3-202A/C gene polymorphism and cancer risk: a meta-analysisCell Biochem Biophys6912014169178
- R.LiuL.L.HuA.SunMRNA expression of IGF-1 and IGF-1R in patients with colorectal adenocarcinoma and type 2 diabetesArch Med Res4542014318324
- N.S.StanilovI.A.KarakolevT.S.DeliyskyJ.P.JovchevS.A.StanilovaAssociation of insulin-like growth factor-I receptor polymorphism with colorectal cancer developmentMol Biol Rep412201480998106
- B.JiangX.ZhangL.L.DuPossible roles of insulin, IGF-1 and IGFBPs in initiation and progression of colorectal cancerWorld J Gastroenterol206201416081613