 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Aim
Apelin, an adipocyte-derived factor, exhibited a number of cardioprotective properties; however, its effect in diabetes which is a major risk factor for cardiovascular disease (CVD) needs to be further studied. So this work was designed to evaluate the effect of apelin on diabetes and its associated cardiac hypertrophy with its possible underlying protective mechanisms.
Experimental protocol
Thirty male adult Wistar rats were categorized into three groups, 10 rats each, normal control group: received standard food and water regime. Diabetic control group: received streptozotocin (STZ) at a dose of (55 mg/kg, i.p., once) dissolved in citrate buffer (pH 4.5). Apelin-13 treated diabetic group: STZ diabetic rats received an intra peritoneal injection of apelin-13 at a dose of (100 nmol/kg/day), and given daily for 8 weeks. at the end of the experiment, oral glucose tolerance test (OGTT) was assayed, then rats were sacrificed and serum glucose, insulin, triglyceride (TG), total cholesterol, high density lipoprotein (HDL) cholesterol, serum lactate dehydrogenase (LDH) and serum creatine kinase – MB (CK-MB) were measured, together with cardiac hypertrophy index (CHI), left ventricular hypertrophy index (LVHI) and left ventricular protein and collagen content levels. Myocardial superoxide dismutase (SOD), catalase, reduced glutathione (GSH) and malondialdehyde (MDA) were determined in the myocardial tissue of experimental rats.
Results
Treatment with apelin-13 improved hyperglycemia and hyperlipidemia, and significantly protected against STZ-induced structural alterations in cardiac tissue, it also produced a significant reduction in MDA while it elevated the level of antioxidant enzymes in hearts of diabetic rats.
Conclusion
This study suggested that apelin can ameliorate diabetes and its associated myocardial hypertrophy through mainly its anti diabetic, anti hyperlipidemic and anti oxidative stress properities.
1 Introduction
Diabetes mellitus (DM) is a group of metabolic diseases caused by a complex interaction of genetic, immunological and environmental factors. DM is very common and it is a prime risk factor for CVD, including diabetic cardiomyopathy.Citation1
Table 1 Effect of apelin-13 (100 nmol/kg/day) on biochemical parameters in experimental animals after 8 weeks treatment.
Table 2 Effect of apelin-13 (100 nmol/kg/day) on serum level of cardiac enzymes of experimental animals after 8 weeks treatment:
Table 3 Effect of apelin-13 (100 nmol/kg/day) on oxidative stress related markers in hearts of experimental animals after 8 weeks treatment.
Diabetic cardiomyopathy has been defined as a ventricular dysfunction that occurs in diabetic patients independent of a recognized cause, such as coronary artery disease or hypertension.Citation2 The hallmark characteristic of diabetic cardiomyopathy is a subclinical phase associated with cellular structural abnormalities including cardiomyocyte hypertrophy, cardiac inflammation, fibrosis and increased apoptosis which lead initially to diastolic dysfunction, later to systolic dysfunction and eventually to heart failure.Citation3
The pathophysiology of the link between diabetes and CVD is complex and multifactorial, though unclear.Citation4 However, the occurrence of hyperglycemia, hyperlipidemia, and oxidative stress in diabetes has been extensively documented, and is implicated in the pathogenesis of various cardiovascular complications including cardiomyopathy.Citation5 Thus, among the various therapeutic strategies, antihyperglycemic, antihyperlipidemic, and antioxidant agents can be useful in the prevention of cardiomyopathy in STZ induced diabetes.Citation6
Apelin, a small regulatory peptide, had been identified as the endogenous ligand of the human orphan G protein coupled receptor (GPCR) which is a receptor structurally related to the angiotensin II (ANG II) receptor AT1 but does not bind to angiotensin II, thus it was previously designated as an “orphan” GPCR, having no known ligand, and was named APJ.Citation7 Later, it was identified that apelin is the endogenous ligand for this receptor, thus The approved Human Genome Organization (HUGO) gene symbol for APJ is now apelin receptor (APLNR).Citation8
Apelin is synthesized as a 77 amino acid pre-pro-peptide that can be cleaved into fragments of different sizes that activate APLNR, with apelin-13 is the final active product, being the most potent isoform and more resistant to enzymatic cleavage.Citation9 Apelin is secreted mainly from white adipose tissue, however it can also be expressed in different tissues as kidney, heart, lung and many areas in the brain.Citation10
Apelin peptides have been shown to affect many biological functions in mammals including neuroendocrine, cardiovascular, and immune systems and it can act via autocrine, paracrine, endocrine, and exocrine signaling.Citation11
In recent years, apelin have been studied for its anti-obesity and anti diabetic properties and it was recorded that apelin-13 may have beneficial effects in metabolic disorders and its associated complications.Citation7
Acute intravenous injection of apelin had been shown to produce a powerful glucose-lowering effect associated with improved glucose tolerance in high fat fed mice which were glucose intolerant or frankly diabetic,Citation12 also chronic treatment with apelin in obesity-induced type II diabetic model improved hyperglycemia, and insulin sensitivity but it was associated with elevated levels of TG.Citation10 Furthermore, it was recorded that there was a negative correlation between apelin blood levels and glycosylated hemoglobin (HbA1c) levels in diabetic patients, this indicated that higher apelin levels were associated with lower HbA1c levels and better glucose control, but this was only significant for type II diabetic patient.Citation13
In type I diabetes, the effect of apelin is markedly questionable. Early studies recorded that the increased circulating level of apelin in children with type I DM had no significant relation with glucose, lipids, adiponectin levels, and insulin sensitivity,Citation14 however, it had been shown recently that apelin had a hypoglycemic effect and could improve pancreatic beta cell function and lower the serum triglycerides in type I diabetic model.Citation10 Thus apelin’s role in glucose and lipid homeostasis in type I diabetes needed further investigations.
Furthermore, It had been shown that dysregulation of the apelin/APLNR system may be involved in the predisposition of cardiovascular diseases, and enhancing apelin action may have important therapeutic effects, especially that some studies recorded decreased serum level of apelin in cardiovascular diseases.Citation15 also, apelin proved its cardioprotective effect in different animal models, as in cardiac ischemia reperfusion injury,Citation16,Citation17 isoproterenol-induced cardiotoxicityCitation18 and heart failure models,Citation19 however its effect in diabetic cardiomyopathy is still needed to be further highlighted.
Thus, I extended this current study to investigate the anti diabetic and cardioprotective effects of apelin in STZ induced diabetic rat model, delineating its different mechanisms of action in ameliorating the course of this disease progression.
2 Material and methods
2.1 Animals and experimental design
This study was carried out on thirty male adult Wistar rats weighing about 200–250 g. The rats were housed, four per cage, under standard laboratory conditions at room temperature (24 ± 2 °C), and had free access to water and food. The rats were fasted during the night before the experiment. All animal experiments were undertaken with the approval of Ethical Animal Research Committee of Tanta University.
The rats were randomly divided into three groups (10 rats each).
2.2 Normal control group
Rats were maintained on standard food and water regime.
2.3 Diabetic control group
An experimental model of diabetes was induced by intra peritoneal injection of STZ (55 mg/kg body weight) (Sigma–Aldrich Chemical, Steinheim, Germany) dissolved in Na citrate solution adjusted at pH 4.5, The control animals were injected with equal volume of vehicle.Citation20
STZ is anti microbial agent with a selective cytotoxicity to pancreatic β-cells that has the ability to induce a specific necrosis and destruction of the pancreatic β-cells and thus it is the first choice for diabetes induction in animals.Citation21 This cytotoxicity to β-cells is mediated through DNA break, nitric oxide production and Free radical generation.Citation22
Because of streptozotocin’s ability to induce fatal hypoglycemia as a result of massive pancreatic insulin release, the rats were provided with 10% glucose solution after 6 h of STZ administration for the next 48 h to prevent hypoglycemia. Rats with diabetes (blood glucose >200 mg/dl) were selected for this experiment.Citation23
2.4 Apelin-13 treated diabetic group
Intraperitoneal injection of apelin-13 (Apelin, Phoenix Pharmaceutical, Belmont, CA, USA), was given at a dose of (100 nmol/kg/day) dissolved in saline and treatment was started after 3 days of STZ injection, furthermore control diabetic group was injected daily with saline via the intraperitoneal (IP) route.Citation24
Weekly body weight gain was measured and at the end of the experiment (8 weeks) from induction of diabetes, the following parameters were determined.
2.5 Oral glucose tolerance test
The animals were subjected to oral glucose tolerance test (OGTT).Citation25 To perform OGTT, the animals were fasted at least 5 h after the last meal, then they were orally administered with 1.5 g/kg glucose and blood samples were collected from the tail vein under light ether anesthesia before, and at intervals of 0 min and 30, 60 and 120 min after oral glucose administration. Samples were analyzed for glucose and insulin as mentioned above. Plotting the glucose concentration versus time gives a curve showing rise and fall in glucose and insulin levels with time and results are expressed as integrated area under the curve (AUC) for glucose and insulin.
AUC glucose and insulin levels were calculated using the trapezoidal method which depends on an interpolation between data points of glucose and insulin levels measured during OGTT. For a given time interval (t1–t2), the AUC can be calculated as follows:
In essence, the first two terms (C1 + C2) calculate the average concentration over the time interval, while the last piece (t1–t2) is the duration of time. So this method takes the average concentration of glucose and insulin and applies it to the entire time interval, sum of all the intervals together, gives the total AUC.Citation27
2.6 Blood sampling and biochemical analysis
The rats were sacrificed by cervical decapitation and overnight fasting blood samples (8 ml/rat) were taken, and allowed to clot for 2 h at room temperature before centrifugation for 20 min at approximately 5000 rpmCitation28 was done. The separated serum was stored at −20 °C till the time of measurement. Repeated freezing and thawing were avoided.
The separated sera were used for measurement of the following parameters:
| (1) | Serum glucose level: | ||||
Spectrophotometric determination of serum glucose level was assayed according to Randox kit manufacturer’s guidelines depending on glucose oxidase method.Citation29
| (2) | Serum insulin level: | ||||
Serum insulin levels were measured using insulin ELISA kits which include an enzyme immunoassay for the quantitative determination of insulin in sera of rats according to the manufacturer instructions (American Diagnostica Inc., South San Francisco, CA, USA).
| (3) | Serum TG level: | ||||
A peroxidase-coupled method based onCitation30 was used for colorimetric determination of serum triglycerides using TG assay kit (Cayman Chemical, USA).
| (4) | Serum total cholesterol and HDL cholesterol level: | ||||
According to the method of,Citation29 serum total cholesterol and HDL cholesterol levels were determined spectrophotometrically, using enzymatic colorimetric BioMed assay kits.
2.7 Serum cardiac enzymes assay
Serum LDH and CK-MB were measured by a quantitative sandwich enzyme immunoassay technique using their respective kits (Biosource, International, Camarillo, California, USA) and all instructions of the manufacturer were followed.
2.8 Cardiac and left ventricular hypertrophy indicies
The hearts of the experimental animals were isolated and weight of the hearts were noted down to calculate the index of cardiac hypertrophy as indicated by heart weight to body weight (HW/BW) and wet left ventricular weight to body weight (LV/BW) to calculate the index of left ventricular hypertrophy.Citation1
2.9 Tissue homogenate preparation
Snap-frozen hearts were thawed and were dissected, left ventricles (LV) from freshly excised hearts were weighed, minced and homogenized in 80 mM Tris–HCl (pH 6.8) The homogenate was used to determine LV protein and collagen contents together with oxidative stress related markers.
2.10 Assesment of LV protein content
The tissue homogenate was centrifuged at 800g for 10 minCitation31 and the clear supernatant was used to estimate total protein content using a detergent compatible kit (DC protein assay, BioRad) according to the method ofCitation32 which is based on the reaction of protein with an alkaline copper tartrate solution and folin reagent given a characteristic blue colour with absorbance at 750 nm.
2.11 Left ventricular collagen content assay
The left ventricular soluble collagen concentration was measured spectrophotometrically by a technique based on Sirius Red binding to collagenCitation33 using a commercially available kit (Sircol collagen assay, Biocolor, Westbury, NY, USA) according to manufacturers’ guidelines.
Sircol collagen assay depend on binding of sircol dye with collagen by incubation for 30 min on a shaker at room temperature, then the collagen-bound dye is recovered by centrifugation, collagen is eluted with alkali, and measured using a spectrophotometer at 540 nm.
2.12 Oxidative stress-related markers assay
The clear supernatant of LV homogenate was used for assessment of antioxidant enzyme levels as SOD, catalase and GSH together with amount of lipid peroxides that is indicated by MDA level. SOD was determined according to the method of Misra and Fridovich.Citation34 Catalase was assessed by the method of Aebi.Citation35 While GSH was measured as described by Moron et al.Citation36 Malondialdehyde (MDA) formation was measured according to the study of Slater and Sawyer.Citation37
3 Statistical analysis
Values are expressed as mean ± standard deviation (SD). The results were analyzed using one-way factorial analysis of variance (ANOVA) followed by Tukey’s multiple comparison test using Graph Pad Instat, 32 bit for win 95/NT (Version 3.05). The value of P < 0.05 was considered as statistically significant.
4 Results
4.1 Effect of apelin treatment on biochemical parameters
The results of this study revealed that STZ- induced diabetes was associated with a significant hyperglycemia, hypo insulinemia and impaired OGTT () indicated by a significant increase in AUCglucose and a significant decrease in AUC insulin in diabetic control group when compared with that of the normal control one. Treatment with apelin-13 at a dose of (100 nmol/kg/day) for 8 weeks significantly decreased the elevated levels of serum glucose and AUCglucose, on the other hand there was insignificant difference in serum insulin and AUCinsulin levels in treated diabetic group compared to diabetic control one.
Fig. 1 Effect of apelin-13 (100 nmol/kg/day) on serum glucose and insulin levels during OGTT in experimental animals after 8 weeks treatment. NC = normal control group; DC = Diabetic control group; AD = Apelin-13 treated diabetic group.
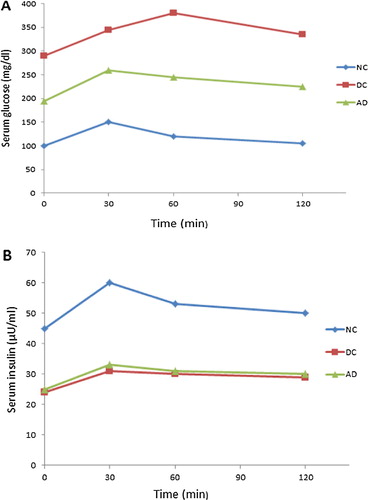
Regarding the serum lipids, STZ-diabetic rats were found to exhibit a significant increase in TG and cholesterol levels with a significant decrease in HDL cholesterol levels comparable to normal control rats. Apelin treatment significantly lowered the elevated level of TG and cholesterol and significantly increased HDL cholesterol levels in treated diabetic group compared to diabetic control one.
4.2 Effect of apelin treatment on serum LDH and CK–MB
It is obvious from the results of this work that STZ injection produced a significant increase in serum level of LDH and CK-MB in diabetic control group compared to normal control one. Apelin treatment significantly attenuated the increased serum level of these enzymes in treated diabetic group compared to diabetic control one.
4.3 Effect of apelin treatment on cardiac and left ventricular hypertrophy indicies
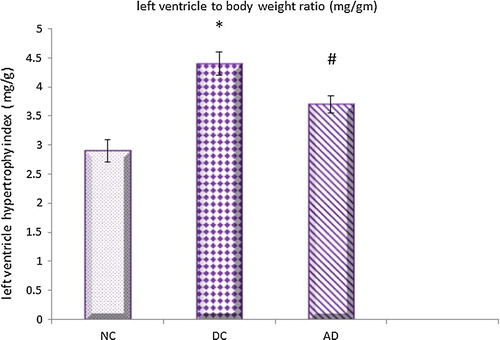
The results of this work showed that both cardiac and left ventricular hypertrophy indicies were significantly higher in diabetic control group compared to normal control one which were significantly reduced under apelin treatment ( and ).
Fig. 2 Effect of apelin-13 (100 nmol/kg/day) on cardiac hypertrophy index in experimental animals after 8 weeks treatment NC = normal control group; DC = Diabetic control group; AD = Apelin-13 treated diabetic group. Data are expressed as mean ± (SD). *At P < 0.05 versus normal control group, #at P < 0.05 versus control diabetic group.
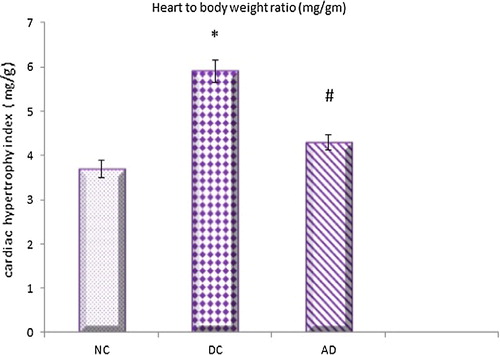
Fig. 3 Effect of apelin-13 (100 nmol/kg/day) on left ventricular hypertrophy index in experimental animals after 8 weeks treatment. NC = normal control group; DC = Diabetic control group; AD = Apelin-13 treated diabetic group. Data are expressed as mean ± (SD). *At P < 0.05 versus normal control group, #at P < 0.05 versus control diabetic group.
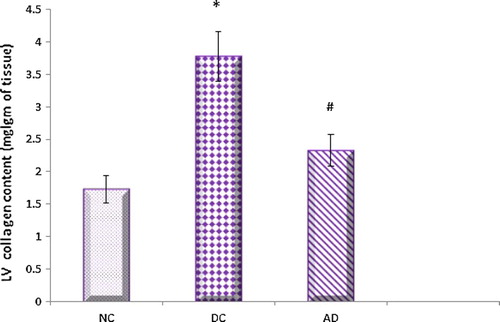
4.4 Effect of apelin on left ventricular protein and collagen contents
The present investigations revealed that the left ventricular protein and collagen content levels were found to be significantly higher in diabetic control rats compared to normal control ones. Apelin significantly reduced these elevated levels in treated diabetic rats when compared with that of diabetic untreated ones ( and ).
Fig. 4 Effect of apelin-13 (100 nmol/kg/day) on left ventriclular protein content in experimental animals after 8 weeks treatment. NC = normal control group; DC = Diabetic control group; AD = Apelin-13 treated diabetic group. Data are expressed as mean ± (SD). *At P < 0.05 versus normal control group, #at P < 0.05 versus control diabetic group.
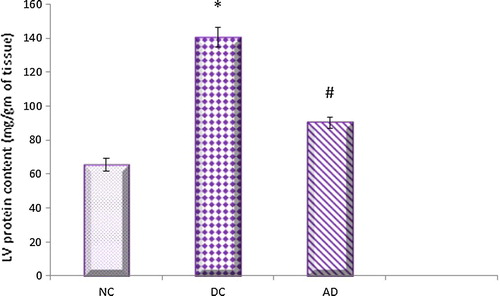
Fig. 5 Effect of apelin-13 (100 nmol/kg/day) on left ventricular collagen content in experimental animals after 8 weeks treatment. NC = normal control group; DC = Diabetic control group; AD = Apelin-13 treated diabetic group. Data are expressed as mean ± (SD). *At P < 0.05 versus normal control group, #at P < 0.05 versus control diabetic group.
4.5 Effect of apelin on oxidative stress parameters in heart
Compared with normal control group, STZ induced diabetes was associated with marked oxidative stress indicated by a significant increase in myocardial MDA and decrease in SOD, CAT and GSH levels. Apelin treatment exhibited a marked antioxidant effect with a significant reduction in myocardial MDA and elevation in SOD, CAT and GSH levels in treated diabetic group compared to diabetic control one.
5 Discussion
Diabetes mellitus is an important and prevalent risk factor for congestive heart failure, there is a large subset of diabetic patients with underlying cardiovascular morbidity.Citation38 Thus a multifactorial approach for the management of DM addressing its microvascular and macrovascular complications is the need of the hour. Therefore, an anti diabetic drug with additional therapeutic benefits against cardiovascular complications may be beneficial in this group of diabetic patients.
Many studies recorded a beneficial effect of apelin in different heart models.Citation39,Citation40 However its cardio protective effect in diabetes and its different mechanisms are further wanted to be elucidated which was the aim of this current study.
The results of the present study showed that STZ injection produced a significant weight loss, hyperglycemia, hypo insulinemia with impaired OGTT indicated by a significant increase in AUCglucose associated with a significant decrease in AUCinsulin values in diabetic control animals as compared to normal control ones.
Increasing evidence suggested that hyperglycemia is a major etiological factor in the development of diabetic cardiomyopathy. It leads to abnormalities in substrate supply and utilization with impaired lipid metabolism. Furthermore, it promotes excessive production and release of reactive oxygen species leading to abnormal gene expression, faulty signal transduction, and cardiomyocytes apoptosis.Citation41
Furthermore, it was suggested that altered glucose supply and utilization by cardiac myocytes could be the primary injury in the pathogenesis of this specific heart muscle disease. Therefore, it is necessary to increase glucose utilization or increase the rate of glucose transport in the diabetic heart to improve its function.Citation1
It was observed in this current study that apelin treatment significantly decreased serum glucose level without concomittent change in insulin output, Furthermore, it significantly attenuated STZ-induced increase in AUC glucose but did not alter the AUC insulin value.
These data collectively indicated that apelin has the ability to produce a glucose lowering effect through a most likely insulin-independent manner which may be due to its ability to increase glucose utilization. As many studies showed that apelin increased glucose utilization by different tissues as skeletal muscle, adipose tissue and myocardial muscle and this was suggested to be mediated through a pathway involving adenosine mono phosphate kinase (AMPK).Citation12,Citation42,Citation43
In addition, hemodynamic factors may be associated with increased glucose utilization under apelin treatment, as it was suggested that vasodilatation is associated with increased glucose utilization, whereas vasoconstriction results in decreased glucose utilization and it was recorded that apelin cause endothelium-dependent vasodilatation by triggering the release of nitric oxide (NO) which increased glucose utilization by tissues.Citation12
Despite the insignificant effect of apelin treatment on insulin secretion in this study, apelin recorded some beneficial effects on beta cells of the pancreas of Akita mice (a well-established type I diabetic model) by reducing endoplasmic reticulum stress of pancreatic cells which leads to modification of pancreatic islet mass reduction and preservation of insulin content.Citation44
As regarding apelin’s effect on lipid profile that represent an important issue in management of diabetes and its associated cardiac changes, this current study recorded an increased level of triglycerides and total cholesterol in STZ diabetic rats while HDL-cholesterol levels were significantly lower. These results were improved by apelin treatment proving its anti hyperlipidemic effect.
Earlier studies have shown that in STZ-diabetic rats, hyperglycemia is associated with hypercholesterolemia and hypertriglyceridemia which play a key role in the pathogenesis of diabetic cardiomyopathy and that the rate of free fatty acid (FFA) uptake by myocardium is inversely proportional to the severity of the myocardial dysfunction.Citation1 Thus reducing the serum lipid levels through dietary or drug therapy decreases the risk of cardiovascular disease and its related cardiovascular complications.Citation45
The elevated circulating FFA produces inhibition of glucose oxidation, abnormal high oxygen requirements during FFA metabolism and the intracellular accumulation of potentially toxic intermediates of FFA, all of which lead to impaired myocardial performance and severe morphological changes. Thus, strategy to be employed to produce improvement in cardiac function is to improve upon these metabolic disarrangements.Citation6
Apelin’s ability to improve lipid metabolism and to decrease FFA release is attributed to its dual inhibition on both adipogenesis and lipolysis; as apelin suppresses adipogenesis through adenosine monophosphate protein kinase (MAPK) dependent pathways, while it inhibits lipolysis by preventing lipid droplet fragmentation by increasing the amount of perilipin surrounding the lipid vacuoles, giving them a greater stability and a resistance to lipases.Citation46
According to the results of this study, STZ - induced diabetes evoked a deleterious effect on heart indicated by high serum LDH and CK-MB activity level in STZ diabetic rats compared to normal control ones and this was attenuated by apelin treatment.
It was claimed that abnormally high activity of serum LDH and CK-MB is a specific and extremely sensitive index of myocardial damage, necrosis or ischemia.Citation47
Increased cardiac enzymes level in diabetes was also recorded in the study of Fuleshwor et al.Citation48 indicating myocardial damage and atherosclerosis was referred to be one of the contributing factors of this damage, as it leads to deposition of cholesterol and cholesterol esters, that cause narrowing of the coronary artery with subsequent decrease in the necessary nutrients to the heart affecting its function. Apelin may limit atherosclerosis progression by promoting intracellular cholesterol efflux and reducing macrophage foam cell formation thus improving the cardiac function in diabetic patients.Citation49
Also the decrease in serum levels of LDH and CK-MB under apelin treatment was recorded in myocardial infarction (MI) modelCitation50 which was mediated by its ability to decrease lipid peroxidation and enhancement of No production. Hyperglycemia also increased oxidative stress and reduced NO bioavailability which resulted in endothelial cell dysfunction in the vasculature of diabetic heart with subsequent cardiac tissue injury and elevated serum cardiac enzymes level,Citation51 this was attenuated by apelin explaining its cardioprotective effect in diabetic cardiomyopathy.
The results of this current study revealed that, the cardiac and left hypertrophy indicies together with LV protein and collagen contents were increased in diabetic hearts and decreased significantly under apelin treatment.
These findings are consistent with the published data by Wang et al.Citation52 who reported that STZ-induced diabetic rats exhibited cardiac hypertrophy after 8 weeks of diabetes induction. Furthermore echocardiographic evidence revealed that LVH is a common structural and functional alternation in diabetic patients even in the absence of coronary artery disease or hypertension and may contribute to reduced myocardial compliance.Citation3
Also, Poornima et al.Citation53 recorded that myocardial fibrosis and myocyte hypertrophy are the most frequently proposed mechanisms to explain cardiac changes in diabetic cardiomyopathy, as increased collagen formation and accumulation of protein within the interstitium lead to cardiac wall stiffness and fibrosis which result in cardiac dysfunction.Citation38
The diabetic heart is metabolically characterized by increased fatty acid oxidation, leading to lipid accumulation in the myocardium with subsequent cardiac hypertrophy.Citation54 Thus the beneficial effect of apelin in ameliorating this diabetes-induced cardiac hypertrophy could be directly attributable to its anti hyperlipidemic effect as mentioned above.
In addition, previous studies showed that the increased fibrous tissue formation and accumulation of collagen in STZ diabetes cause LVH, and this is may be due to impaired collagen degradation resulting from glycosylation of the lysine residues on collagen, suggesting that good diabetic control is associated with the improvement of LV hypertrophyCitation6 which was provided by apelin through its hypoglycemic effect.
Furthermore, apelin’s ability to decrease collagen and fibrous tissue formation may be attributed to its inhibition to transforming growth factor-Beta (TGF-β) and this is mediated through a sphingosine kinase 1 (SphK1) dependant mechanism.Citation55 TGF-β is a cytokine that has a key role in mediating expression and differentiation of fibroblasts into myofibroblasts and this process is a key event in the progression of cardiac fibrosis, and it was recorded that TGF-β gene expression is enhanced by diabetes and thus, its inhibition has a beneficial role in decreasing cardiac fibrosis and stiffness.Citation56
Also, its ability to decrease myocardial fibrosis may be explained by its anti-inflammatory properities, as it has the ability to suppress effectively the expression of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) and interleukin (IL)-1b protein.Citation57 These cytokines trigger a chronic inflammatory process and cell injury in the heart ultimately inducing cardiac fibrosis as a consequence, thus suppression of these cytokines have been shown to reduce cardiac fibrosis, and enhance cardiac function.Citation58
It was obvious from the present investigation that, the level of myocardial SOD, GSH, and catalase enzymes were decreased, while MDA was increased on STZ induction, all of which was improved by apelin treatment indicating its antioxidant properties.
Yosuke et al.Citation3 concluded that hyperglycemia and metabolic abnormalities of diabetes lead to production of reactive oxygen species (ROS) which plays a pivotal role in the development of diabetes-induced cardiovascular disease. Increased ROS generation causes activation of maladaptive signaling pathways with subsequent cell death and abnormal cardiac remodeling which lead to the characteristic morphological and functional abnormalities associated with diabetic cardiomyopathy. Thus, strategies that either reduce ROS or augment myocardial antioxidant defense mechanisms might have therapeutic efficacy in improving myocardial function in diabetes mellitus.Citation59
Improvement of myocardial antioxidants enzymes and decreased MDA under apelin treatment had been recorded previously in myocardial I/R injury.Citation60
The antioxidant effect of apelin is mediated through its upregulation to the cardiac antioxidant enzymes by increasing their mRNA expression, also apelin has the ability to decrease excessive mitochondrial ROS production with subsequent preservation of myocardial metabolic status in cardiomyocytes. In addition, Zhang et al.Citation61 suggested that apelin could suppress ROS generation by down-regulation of inducible nitric oxide synthase expression which improved myocardial injury.
6 In conclusion
The results of this study had clearly demonstrated that apelin protected against STZ induced metabolic abnormalities and its associated cardiac changes. Thus diabetics, who have an increased risk of cardiac dysfunction, may benefit both from an improvement in glucose and lipid homeostasis as well as from the antioxidant and cardioprotective effects of apelin.
Assuming that these animal studies can be extended to humans, apelin may be useful as an adjunct therapy with oral hypoglycemic agents in the management of diabetes mellitus and its cardiac complications.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 9 June 2017
References
- K.ParmarA.PrajapatiS.PatelM.PatelB.PatelCardioprotective effect of lisinopril in streptozotocin induced type – II diabetic ratsActa Endocrinol (Buc)82012177188
- V.PalmieriB.CapaldoC.RussoUncomplicated type 1 diabetes and preclinical left ventricular myocardial dysfunction: insights from echocardiography and exercise cardiac performance evaluationDiabetes Res Clin Pract792008262268
- K.YosukeJ.AnnA.MattiDiabetic cardiovascular disease induced by oxidative stressInt J Mol Sci1620152523425263
- B.D.BetsyPathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipidDiabetes Spectrum212008160165
- M.RajeshP.MukhopadhyayS.BatkaiCannabidiol attenuates cardiac dysfunction, oxidative stress, fibrosis, and inflammatory and cell death signaling pathways in diabetic cardiomyopathyJ Am Coll Cardiol56201021152125
- S.P.SnehalK.G.RameshCardioprotective effects of gallic acid in diabetes-induced myocardial dysfunction in ratsPharmacogn Res32011239245
- L.A.MonasteroloStrategies in diabetic nephropathy: apelin is making its wayJ Physiol5922014423424
- S.L.PitkinJ.MaguireT.I.BonnerA.P.DavenportInternational union of basic and clinical pharmacology. LXXIV. Apelin receptor nomenclature, distribution, pharmacology, and functionPharmacol Rev622010331342
- J.J.MaguireM.J.KleinzS.L.PitkinA.P.DavenportPyr1 Apelin-13 identified as the predominant apelin isoform in the human heart vasoactive mechanisms and inotropic action in diseaseHypertension542009598604
- M.M.A.AbulmeatyS.A.AbdullahA.M.AlmajwalApelin is promising in management of diabetes complicating high fat diet induced obesity in ratsProg Nutr152013245254
- L.V.DeguanL.I.HeningC.LinxiApelin and APJ, a novel critical factor and therapeutic target for atherosclerosisActa Biochim Biophys Sin452013527533
- C.DrayC.KnaufD.DaviaudApelin stimulates glucose utilization in normal and obese insulin-resistantMice Cell Metab82008437445
- M.HabchiL.DuvillardV.CottetCirculating apelin is increased in patients with type 1 or type 2 diabetes and is associated with better glycaemic controlClin Endocrinol (Oxf)812014696701
- C.MeralE.TascilarF.KarademirElevated plasma levels of apelin in children with type 1 diabetes mellitusJ Pediatr Endocrinol Metab232010497502
- M.R.SeyyedK.BajestaniV.B.PatelW.WangG.Y.OuditTargeting the ACE2 and apelin pathways are novel therapies for heart failure: opportunities and challengesCardiol Res Practice2012 Article ID 823193) 11
- M.J.KleinzG.F.BaxterApelin reduces myocardial reperfusion injury independently of PI3K/Akt and P70S6 kinaseRegul Pept1462008271277
- A.T.Abd ElmouttalebE.E.EbrahemK.A.MarghanyE.M.BayomyA.A.HassaboPlasma apelin concentrations in non-obese acute myocardial infarction patients with type 2 diabetes mellitusAm J Med Med Sci620165765
- V.L.LakomkinA.A.AbramovE.V.LukoshkovaThe action of apelin-12 and its analog on hemodynamics and cardiac contractile function of rats with isoproterenol-induced myocardial lesionKardiologiia5520155462
- S.TerukiS.TakashiW.HiroyukiApelin is a positive regulator of ACE2 in failing heartsJ Clin Invest123201352035211
- A.A.HamedH.A.MalekEffect of telmisartan in experimentally induced diabetetes mellitus in ratsInt J Health Sci120072
- B.J.GoudV.DwarakanathB.K.Chikka swamyStreptozotocin – a diabetogenic agent in animal modelsIjppr Human32015253269
- O.ChinedumK.EleazuE.ChinedumC.SoniaN.UdemeReview of the mechanism of cell death resulting from streptozotocin challenge in experimental animals, its practical use and potential risk to humansJ Diabetes Metab Disord12201360
- P.PalsamyS.SubramanianResveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocinnicotinamide induced experimental diabetic ratsBiomed Pharmacother47200818
- K.HiguchiT.MasakiK.GotohApelin, an APJ receptor ligand, regulates body adiposity and favors the messenger ribonucleic acid expression of uncoupling proteins in miceEndocrinology148200726902697
- J.M.OlefskyInsulin resistance and insulin action. An in vitro and in vivo respectiveDiabetes301981118162
- D.W.A.BourneFirst Course in Pharmacokinetics & Biopharmaceutics1995University of Oklahoma College of Pharmacy
- R.D.PurvesOptimum numerical integration methods for estimation of area-under-the-curve (AUC) and area-under-the-moment-curve (AUMC)J Pharmacokin Biopharm201992211227
- S.I.LulatY.C.YadavR.BalaramanR.MaheshwariAntiurolithiatic effect of lithocare against ethylene glycol-induced urolithiasis in Wistar ratsIndian J Pharmacol4820167882
- Tietz NW, Cook T, McNiven MA. Clinical Guide to Laboratory Tests. WB Saunders Co; 1995:509–512.
- M.W.McgowanJ.D.ArtissD.R.StrandberghB.ZakA peroxidase-coupled method for the colorimetric determination of serum triglyceridesClin Chem291983538542
- Saini AS, Taliyan R, Sharma PL. Protective effect and mechanism of Ginkgo biloba extract-EGb 761 on STZ-induced diabetic cardiomyopathy in rats. Pharmacogn Magasine. 2014;10:172–178.
- O.H.LowryN.J.RosenbroughA.L.FarrR.J.RandallProtein measurement with the Folin phenol reagentJ Biol Chem931951265275
- A.KhaidarM.MarxB.LubecG.L.LubecL-arginine reduces heart collagen accumulation in the diabetic db/db mouseCirculation901994479483
- Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 1972;247:3170.
- Aebi H. Oxidoreductases acting on groups other than CHOH: catalase. Methods in Enzymology. London 7: Academic Press 1984;105:121–25.
- M.S.MoronJ.W.DepierreB.MannervikLevels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liverBiochim Biophys Acta58219796778
- T.F.SlaterB.C.SawyerThe stimulatory effects of carbon tetrachloride and other halogenoalkanes or peroxidative reactions in rat liver fractions in vitro. General features of the systems usedJ Biochem1231971805814
- B.SihemD.A.EvanDiabetic cardiomyopathy, causes and effectsRev Endocr Metab Disord1120103139
- B.H.ZhangC.X.GuoH.X.WangCardioprotective effects of adipokine apelin on myocardial infarctionHeart Vessels292014679689
- W.KoguchiN.KobayashiH.TakeshimaM.IshikawaF.SugiyamaT.IshmitsuCardioprotective effect of apelin-13 on cardiac performance and remodeling in end-stage heart failureCirculation762012137144
- I.Falcão-PiresA.F.Leite-MoreiraDiabetic cardiomyopathy: understanding the molecular and cellular basis to progress in diagnosis and treatmentHeart Fail Rev172012325344
- S.XuP.HanM.HuangIn vivo, ex vivo, and in vitro studies on apelin’s effect on myocardial glucose UptakePeptides372012320326
- C.AttanéD.DaviaudC.DrayApelin stimulates glucose uptake but not lipolysis in human adipose tissue ex vivoJ Mol Endocrinol4620112128
- H.ChenC.ZhengX.ZhangJ.LiL.ZhengK.HuangApelin alleviates diabetes-associated endoplasmic reticulum stress in the pancreas of Akita micePeptides32201116341639
- H.NakayamaT.MorozumiS.NantoAbnormal myocardial free fatty acid utilization deteriorates with morphological changes in the hypertensive heartJpn Circ652001783787
- A.ThanY.ChengL.C.FohApelin inhibits adipogenesis and lipolysis through distinct molecular pathwaysMol Cell Endocrinol3622012227241
- G.M.Howard-AlpeJ.W.SearP.FoexMethods of detecting atherosclerosis in non cardiac surgical patients; the role of biochemical markersBrit J Anaesth972006758769
- M.FuleshworA.PratikK.DeepakStudy of lipid profile and the effect of cardiac enzymes in type 2 diabetes mellitus patient developing coronary heart diseaseInt Res J Pharm App Sci320135154
- X.Y.LiuQ.LuX.P.OuyangApelin-13 increases expression of ATP-binding cassette transporter A1 via activating protein kinase C a signaling in THP-1 macrophage-derived foam cellsAtherosclerosis2262013398407
- Y.AziziM.FaghihiA.ImaniM.RoghaniA.NazariPost-infarct treatment with [Pyr1]-apelin-13 reduces myocardial damage through reduction of oxidative injury and nitric oxide enhancement in the ratmodel of myocardial infarctionPeptides4620137682
- B.B.DokkenThe pathophysiology of cardiovascular disease and diabetes: beyond blood pressure and lipidsDiabetes Spectrum212008160165
- G.G.WangW.LiX.H.LuX.ZhaoL.XuTaurine attenuates oxidative stress and alleviates cardiac failure in type I diabetic ratsCroat Med J542013171179
- I.G.PoornimaP.ParikhR.P.ShannonDiabetic cardiomyopathy: the search for a unifying hypothesisCir Res982006596
- J.L.EvansI.D.GoldfineB.A.MadduxG.M.GrodskyAre oxidative stress-activated signaling pathways mediators of insulin resistance and beta-cell dysfunction?Diabetes52200318
- D.PchejetskiC.FoussalC.AlfaranoApelin prevents cardiac fibroblast activation and collagen production through inhibition of sphingosine kinase 1Eur Heart J33201223602369
- C.R.BanS.M.TwiggFibrosis in diabetes complications: pathogenic mechanisms and circulating and urinary markersVasc Health Risk Manag42008575596
- M.KhullarA.A.Al-ShudiefatA.LudkeG.BinepalP.K.SingalOxidative stress: a key contributor to diabetic cardiomyopathyCan J Physiol Pharmacol882010233240
- C.DuerrschmidJ.R.CrawfordE.ReinekeTNF receptor 1 signaling is critically involved in mediating angiotensin-II-induced cardiac fibrosisJ Mol Cell Cardiol5720135967
- X.ShenS.ZhengN.S.MetreveliP.N.EpsteinProtection of cardiac mitochondria by overexpression of Mn SOD reduces diabetic cardiomyopathyDiabetes552006798805
- O.PisarenkoV.LankinG.KonovalovaApelin-12 and its structural analog enhance antioxidant defense in experimental myocardial ischemia and reperfusionMol Cell Biochem3912014241
- H.X.ZhangC.JiaojiaoS.TaoGW26-e0194 Apelin ameliorates myocardial insulin resistance and improves myocardial injury in diabetesJ Am Coll Cardiol66201520
