 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Background
Estrogen deprivation in the postmenopausal women plays a critical role in progression of type 2 diabetes mellitus (T2DM).
Aim
The present study investigated the overlaid effect of ovariectomy on T2DM and the possible underlying mechanism.
Materials
Forty female Wistar rats were divided into four groups (10 rats each): sham control, ovariectomized control, sham diabetic and diabetic ovariectomized groups. At the end of experiment, estimation of body weight gain percentage, food intake, fasting blood glucose concentration, and insulin tolerance test were done. Then, rats were euthanized and blood samples were taken for measurement of serum concentration of insulin, HOMA-IR, lipid parameters, tumor necrosis factor-α, interleukin 1 beta, interleukin 4, interleukin 10, malondialdehyde and total thiol. Also, histopathological and immunohistochemical examination of the pancreas were done.
Results
The present study revealed that ovariectomy aggravated the diabetic induced glucose metabolic disturbance as implied by impaired insulin tolerance test, increased insulin resistance alongside lipid dyshomeostasis. These metabolic disturbances might claim to exacerbation of oxidative stress and inflammatory response along with apparent histopathological and immunohistochemical changes on the pancreas.
Conclusion
We concluded that metabolic disturbances induced by diabetes might be getting worse after ovariectomy via intensification of oxidative stress and inflammatory state.
1 Introduction
Type 2 diabetes mellitus (T2DM) is a progressive chronic metabolic disease characterized by elevated blood glucose concentration and insulin resistance.Citation1
Estrogen deficiency after menopause correlates with metabolic disorders as T2DM, and cardiovascular disease;Citation2 this designates its role in the pathogenesis of these conditions.Citation3 Beside, the role of estrogens in female physiology and reproduction, now they are considering important regulators of multiple physiological and pathological processes.Citation4 Consequential to menopause, the relation between estrogen concentrations and the risk of developing diabetes is complex. Margolis et al.Citation5 showed lower risk of diabetes in women taking hormone replacement therapy. Meanwhile, Ding et al.Citation6 reported insulin resistance and diabetes in women with elevated endogenous estrogen levels after menopause.
Low-grade inflammation plays a critical role in the pathophysiology of various chronic age related conditions such as T2DM,Citation7 alongside increased level of pro-inflammatory cytokines observed in T2DM.Citation8 Cytokines are small soluble proteins or membrane-bound proteins. Almost all nucleated cells generate these proteins and respond to them. Cytokines include “interleukins, growth factors, chemokines, hematopoietins and colony stimulating factors”.Citation9
Estrogen deficiency after menopause is coupled with oxidative stress,Citation10 a state occurs when the production of reactive oxygen species (ROS) exceed the body's anti-oxidant defenses.Citation11 ROS are oxidative physiological processes end productsCitation12 that bind with protein, lipid, carbohydrates and DNA within the cells, result in cellular damage include cell membranes and genetic materials.Citation11 Given that the amplified levels of ROS contribute to the pathogenesis of T2DM,Citation13 this may suggest an increased risk of development of T2DM in postmenopausal females.
The present study was planned to examine the superimposed impact of surgically induced menopause (ovariectomy) on body weight gain percentage and food intake, glucose and lipid metabolism, insulin tolerance, and insulin resistance in a model of T2DM induced by high fat diet and low dose streptozotocin and to elucidate the potential mechanisms of this effect.
2 Materials and methods
2.1 Animals
Forty Wistar female rats, initially weighing from 150 to 200 grams, aged 3 months obtained from Animal house of Faculty of Medicine, Assiut University. Rats housed in clean stainless steel cages, the cage size was 65 cm × 25 cm × 15 cm, and each cage contained 5 rats. Rats maintained on natural light/dark cycle in an aerated room under controlled hygienic conditions and provided with free access to water. All procedures conducted in accordance to the Guide of the Care and Use of Laboratory AnimalsCitation14 and approved by the Ethical Committee for Scientific Research at the Faculty of Medicine.
Following acclimation to laboratory circumstances, 24 h food intake and body weight measured 3 times per week for 2 weeks between 8.00 a.m. and 10.00 a.m. These values utilized as baseline values. 24 h food intake estimated by offering a standard amount of rodent food pellets (70 g for each rat) every day. Then, food intake measured by subtracting the leftover food from what added at the previous day.
2.2 Experimental design
The rats randomly divided into four groups (10 rats per group):
| 1. | Group 1 (Sham control group): rats subjected to simulated surgery. | ||||
| 2. | Group 2 (Ovariectomized control group): rats subjected to bilateral ovariectomy. | ||||
| 3. | Group 3 (Sham diabetic group): T2DM was induced then; rats underwent sham surgical procedure. | ||||
| 4. | Group 4 (Diabetic ovariectomized group): diabetic rats were subjected to bilateral ovariectomy. | ||||
2.3 Induction of T2DM
T2DM was induced as mentioned by Wang et al.Citation15 by feeding rats with a high-fat diet (HFD), which prepared by adding 20% sucrose (w/w) and 10% ram tail fat (w/w) into basal diet, for 4 weeks, then rats injected with a single intraperitoneal (i.p.) administration of freshly prepared streptozotocin (STZ) dissolved in ice-cold sterile saline 9% (CAT #18883664; Sigma, Germany) in a dose of 30 mg/kg body weight, the combination of HFD and low-dose STZ surrogates as an alternative animal model resembling the human type 2 diabetes. This STZ low dose exhibited a slight trauma to pancreatic beta cells to simulate the condition of chronic hyperinsulinemic insulin resistance.Citation16 To prevent the STZ induced hypoglycemia, rats injected with 10% dextrose solution 6 h after STZ administration and for the next 24 h.Citation17 After that rats continued to feed HFD for another 4 weeks.
2.4 Surgical procedure: ovariectomy
After establishment of diabetes, rats sedated with pentobarbital sodium anesthesia (60 mg/kg, i.p.; Sigma–Aldrich Chemie GmbH, Steinheim, Germany). Bilateral ovariectomy performed according to the method of Khajuria et al.Citation18 Sham-operated rats anaesthetized, the skin and muscle layers opened; ovaries manipulated but not removed.
Rats allowed to recover in individual cages for one week, during which they received a commercial pelleted diet and water ad libitum, anti-inflammatory drug ketoprofen (2 mg/kg) for three days, and antibiotic ampicillin sodium (30 mg/kg) for five days as described by Tavares et al.Citation19
2.5 Blood glucose Determination
Occurrence of diabetes was confirmed by measuring fasting blood glucose concentration (FBG) with strips using glucometer (Accu-Chek Active, Roche Diagnostic Corporation, Mannheim, Germany). Rats with FBG concentrations more than 200 mg/dl considered diabetic.Citation20
2.6 Insulin tolerance test
Rats were fasted for 6 hours (from 7 a.m. to 1 p.m.) and each rat received a single intraperitoneal injection of insulin (1 IU/kg body weight) and tail blood samples used for determining concentration of blood glucose at 0, 30, 60, and 120 min.
2.7 Animal euthanize
Fasting rats (18 h) anesthetized and euthanized by lethal dose of pentobarbital (100 mg/kg body weight).Citation21 4 ml of blood collected from the retro-orbital venous plexus before euthanize. Serum separated by centrifugation at 3000g for 20 min. The separated serum aliquotted and stored frozen at −20 °C until use.
2.8 Determination of homeostasis model of insulin resistance (HOMA-IR)
Serum insulin concentration measured using a rat insulin enzyme-linked immunosorbent assay (ELISA) kit (CAT # RAB0904; Sigma, Germany) according to the manufacturer’s instructions. The insulin resistance index (IRI) assessed by homeostasis model assessment estimate of insulin resistance (HOMA-IR):
2.9 Determination of serum lipids
Total cholesterol (TC), triglycerides (TG) and high density lipoprotein-cholesterol (HDL-C) determined by enzymatic methods using an automated analyzer (Dimension RxL Max, DADE Behring, Marburg, Germany). Low density lipoprotein-cholesterol (LDL-C) concentrations estimated according to the formula specified by Friedwald et al.Citation22 as follows:
2.10 Measurement of cytokines
ELISA performed to measure serum concentrations of TNF-α (CAT # K0331196), IL1β (CAT # K0331212), IL4 (CAT # K0332133) and IL10 (CAT # K0332134) using KOMA Biotech commercial kits and following the instructions supplied with each kit.
2.11 Measurement of serum concentrations of malondialdehyde and total thiol
Serum concentration of malondialdehyde (MDA) estimated by the method of Uchiyama and Mihara.Citation23 Calculation of concentrations = A × 3.84 where A = absorbance.
Total thiol (TT) serum concentration measured according to the method of Ellman.Citation24 TT concentrations in mmoles/L calculated according to the following Co = A/E × D, where Co = Original concentration, A = absorbance at 420 nm, E = extinction coefficient = 13.1000 M/cm and D = diluted factor = 36.8 M/cm. The concentrations of TT in serum = 36.8/5 × A.
2.12 Histopathological examination
Specimens took from tail area of the pancreas, fixed in buffered formalin solution, dehydrated conventionally and embedded in paraffin. 4 µm sections were cut with a Leica sliding microtome (SM 2000R, Nussbach, Germany), then the slides were stained with haematoxylin and eosin as described by the method of Durry and Wallinngton.Citation25
2.13 Immunohistochemistry of pancreas
Pancreatic tissue excised, fixed, and stained for insulin as previously described by Kluth et al.Citation26 The changes in the immunohistochemical reaction of the pancreatic tissues were detected by by dad chromogen and counter-staining was done using Hematoxylin.
2.14 Statistical analysis
Data expressed as mean ± SD (standard deviation) using GraphPad Prism software version 6 (Graph Pad Software, San Diego, CA). Differences among the groups compared by one-way analysis of variance (ANOVA) test followed by Bonferroni’s multiple comparison tests. P value of <0.05 considered to be significant.
3 Results
3.1 Body weight gain percentage and food intake
Ovariectomy following diabetes exhibited no impact on the body weight gain percentage and food intake (A,B).
Fig. 1 Body weight gain percentage (A) and food intake (B) in the studied groups. Data were presented as mean ± standard deviation, n = 10 rats per group. OVX: ovariectomized rats. Significant difference from the sham control group **: P < 0.01 and ***: P < 0.001, using one-way analysis of variance (ANOVA) followed by Bonferroni test.

3.2 FBG, insulin concentrations and insulin resistance
FBG concentration elevated significantly in the sham diabetic and OVX diabetic rats when compared to the sham control rats (P < 0.001 for each) and OVX control rats (P < 0.001 for each) (A).
Fig. 2 Fasting blood glucose concentration (A), serum insulin concentration (B) and HOMA-IR (C) in the studied groups. Data were presented as mean ± standard deviation, n = 10 rats per group. OVX: ovariectomized rats. Significant difference from the sham control group ***: P < 0.001, a: significantly different from the OVX group and b: significantly different from the sham diabetic group, using one-way analysis of variance (ANOVA) followed by Bonferroni test.

OVX diabetic rats demonstrated a significant increase in insulin concentrations when compared to the sham control rats (P < 0.001), OVX control rats (P < 0.001) and sham diabetic rats (P < 0.01) (B).
OVX diabetic rats revealed a significant increase in HOMA-IR value as compared with the sham control (P < 0.001), OVX control (P < 0.001) and sham diabetic group (P < 0.001 for each) (C). Thus, ovariectomy aggravated the diabetes-associated insulin resistance.
3.3 Insulin tolerance test
Insulin tolerance test was displayed in . OVX diabetic and diabetic rats exhibited a lower effect of insulin on fasting blood glucose concentrations than sham control and OVX control rats.
Fig. 3 Insulin tolerance test in the studied groups. Data were presented as mean ± standard deviation, n = 10 rats per group. OVX: ovariectomized rats. Significant difference from the sham control group ***: P < 0.001, a: significantly different from the OVX group and b: significantly different from the sham diabetic group, using one-way analysis of variance (ANOVA) followed by Bonferroni test.

3.4 Lipid profile
The OVX diabetic rats displayed significant elevations in serum concentrations of TC, LDL-C and TG as compared to the sham control rats (P < 0.001 for each parameter), the OVX control rats (P < 0.001, P < 0.01 and P < 0.001, respectively), and the sham diabetic rats (P < 0.01, P < 0.01 and P < 0.001, respectively). On the other hand, serum concentration of HDL-C exhibited a significant decline in OVX control, sham diabetic and OVX diabetic rats when compared with, P < 0.001 and P < 0.001, respectively). Additionally, HDL-C concentration decreased significantly in the OVX diabetic rats when compared with the sham control rats (P < 0.001) and the OVX control rats (P < 0.05) ().
Fig. 4 Serum concentrations of total cholesterol (TC), HDL-C (high density lipoprotein-cholesterol), LDL-C (low density lipoprotein-cholesterol) and triglycerides (TG) in the studied groups. Data were presented as mean ± standard deviation, n = 10 rats per group. OVX: ovariectomized rats. Significant difference from the sham control group *: P < 0.05, **: P < 0.01, ***: P < 0.001, a: significantly different from the OVX group and b: significantly different from the sham diabetic group, using one-way analysis of variance (ANOVA) followed by Bonferroni test.

3.5 Serum concentrations of TNF-α, IL1β, IL4 and IL10
As shown in , the OVX diabetic rats revealed a significant increase in TNF-α and IL1β when compared with the sham control rats (P < 0.001 for each), OVX control (P < 0.01 and P < 0.001, respectively) and sham diabetic groups (P < 0.01 and P < 0.001, respectively).
Fig. 5 Serum concentrations of TNF-α (A), IL-1β (B), IL4 (C) and IL10 (D) in the studied groups. Data were presented as mean ± standard deviation, n = 10 rats per group. OVX: ovariectomized rats; TNF-α: tumor necrosis factor-alpha; IL1β: interleukin 1 beta; IL4: interleukin 4 and IL10: interleukin 10. Significant difference from the sham control group *: P < 0.05 and ***: P < 0.001, a: significantly different from the OVX group and b: significantly different from the sham diabetic group, using one-way analysis of variance (ANOVA) followed by Bonferroni test.

Regarding serum concentration of IL4, it decreased significantly in OVX diabetic group in comparison to sham control, OVX control and sham diabetic groups (P < 0.001 for each). Furthermore, serum concentration of IL10 exhibited a significant decrease in the OVX control, sham diabetic and OVX diabetic group in comparison with (P < 0.05, P < 0.05 and, respectively). the OVX diabetic group showed a significant decrease in serum concentration of IL10 as compared to the sham control group (P < 0.001), the OVX control group (P < 0.01) and the sham diabetic group (P < 0.001).
3.5.1 Serum concentrations of MDA and TT
Serum concentrations of MDA and TT in studied groups displayed in . Concerning serum concentration of MDA, the OVX diabetic group showed a significant increase in serum concentration of MDA in comparison to the sham control group (P < 0.001), the OVX control group (P < 0.001) and the sham diabetic group (P < 0.001).
Fig. 6 Serum concentrations of malondialdehyde (A) and total thiol (B) in the studied groups. Data were presented as mean ± standard deviation, n = 10 rats per group. OVX: ovariectomized rats. Significant difference from the sham control group *: P < 0.05, **: P < 0.01 and ***: P < 0.001, a: significantly different from the OVX group and b: significantly different from the sham diabetic group, using one-way analysis of variance (ANOVA) followed by Bonferroni test.

With regard serum concentration of TT, it decreased significantly in the OVX diabetic group as compared to the sham control group (P < 0.001), the OVX control group (P < 0.001) and the sham diabetic group (P < 0.01).
3.6 Histopathological results
Histopathology changes of pancreatic islets including immunohistochemistry of insulin in all experimental groups were displayed in and . Pancreas of the sham control group showed regularly rounded islets and interlobular ducts (a) that showed strong immune positivity “dark brown, completely positive islets” (a).
Fig. 7 Photomicrographs of paraffin sections of the pancreas in the studied groups. 1a: Pancreas of the sham control group showed regularly round islets (L) and interlobular ducts (d) (H&E, X400). 1b: Pancreas of the OVX control group showed a more or less rounded islet (L) and proliferating cells around a small rounded interlobular duct (d) (H&E, X400). 1c: Pancreas of the sham diabetic group showed a irregular shaped hyperplastic islet (L), proliferating cells (arrow) related to interlobular ducts (d), and an interlobar duct (H&E, X200). 1d & 1e: Pancreas of the OVX diabetic group, 1d: Showed islets (L) surrounded by a zone of dark cells (arrows) and interlobar ducts (d) that are related on both sides by masses of proliferating cells (P). (H&E, X200). 1e: Showed an islets (L) contained many cells with vacuolated cytoplasm and flat dark nucleus and small rounded interlobular ducts (d) surrounded by proliferating cells, some of them are small with dark nucleus (H&E, X400).
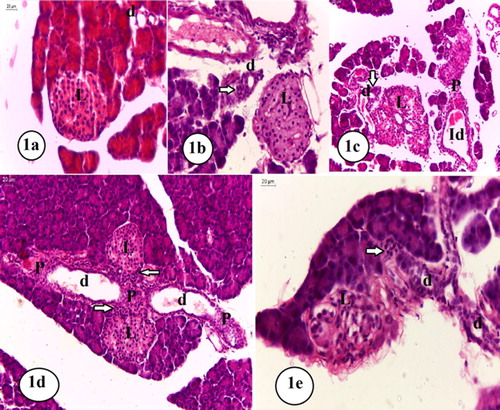
Fig. 8 Photomicrographs of the immune stained pancreas in the studied groups. 2a: Pancreas of the sham control group showed a strong positive dark brown completely immune stained positive islet (anti-insulin antibody, X400). 2b: Pancreas of the OVX control group showed a golden brown immune positive islet with a thin rim of immune negative cells, an interlobular duct (d) surrounded by proliferating cells some of them are immune positive (arrow), and an interlobar duct (Id) with a related group of immune negative proliferating cells (P) (anti-insulin antibody, X400). 2c-2f: Pancreas of the sham diabetic group, 2c: Showed a strong positive completely immune stained islet and a group of immune positive proliferating cells (arrow) related to an interlobular duct (d) (anti-insulin antibody, X400). 2d: Showed a hyperplastic strong immune stained islet that showed some immune negative cells (arrows) (anti-insulin antibody, X400). 2e: Showed an islet that showed few immune positive cells (anti-insulin antibody, X400). 2f: Showed some immune positive proliferating cells (arrow) related to an interlobular duct (d), and other groups of immune positive cells within the acini of the exocrine pancreas (anti-insulin antibody, X400). 2 g and 2 h: Pancreas of the OVX diabetic group, 2 g: Showed a golden brown immune stained islet with peripheral groups of immune negative cells (arrows) (anti-insulin antibody, X400). 2 h: Showed proliferating cells related to the acini of the exocrine pancreas, some of them were immune positive while others were immune negative (arrow) (anti-insulin antibody, X400).

Pancreas of the OVX control group showed regularly rounded islets and proliferating cells around interlobular ducts (b). By immune stain some islet cells were golden brown and some were immune negative. Some of these proliferating cells around some interlobular ducts are immune positive, others related to the interlobar ducts were immune negative (b).
Pancreas of the sham diabetic group revealed that most islets were hyperplastic and irregular shaped islets with groups of endocrine-like proliferating cells of variable sizes observed in relation to both interlobular and interlobar ducts (c). By immune stain, most islets were strong positive completely immune stained (c). Some islets are strongly immune stained with some immune negative cells (d). Other islets contained only few immune positive cells (e). Also we observed groups of endocrine-like proliferating cells of variable sizes in relation to both interlobular and interlobar ducts (c, 8f); some of these cells were immune positive. Also, groups of immune positive cells of variable sizes were frequently observed within the acini of the exocrine pancreas (f).
Examination of the OVX diabetic group showed islets that were mostly surrounded by a zone of cells with dark flat nuclei (d). Small sized islets that contain cells with vacuolated cytoplasm and dark flat nuclei were also observed (e). Cellular proliferation around the interlobar and interlobular ducts was also detected (d). Some of these proliferating cells showed small dark nuclei (e). By immune stain some islets were immune positive with golden brown color and showed some peripheral immune negative cells (g). Occasionally, groups of proliferating immune positive cells within the acini of the exocrine pancreas together with other groups of proliferating immune negative cells were observed, most of the observed proliferating cells around the pancreatic ducts were immune negative (h).
4 Discussion
Menopause is an important period in women's life, characterized by loss of ovarian hormones and altered internal milieu.Citation27
The present study revealed that ovariectomy following HFD/STZ diabetes neither affect body weight gain nor food intake. Consistent with previous studiesCitation28,Citation29 ovariectomy increased body weight gain percentage and food intake. This finding could be explained by increasing anorexigenic and decreasing orexigenic gene expression subsequent to abolish the protective effect of estrogen as proposed by Eckel.Citation30 Additionally, reduction of energy disbursement and physical activity after ovariectomy might be the underlying mechanism as suggested by Ludgero-Correia et al.Citation31 Moreover, increased body weight gain and food intake detected in HFD/STZ diabetic rats confirmed the finding of Liu et al.Citation32 This finding might be attributed to utilization of an energy rich diet and fat accumulation in diverse body fat pads and decreased energy disbursement as explained by Srinivasan et al.Citation33 On the contrary, Wang et al.Citation15 showed no difference in body weight gain between HFD/STZ diabetic and control rats and they claimed this to balanced lipogenesis and lipolysis with augmentation of metabolic rate to a new value.
The present study revealed that when ovariectomy was following HFD/STZ diabetes had no impact on FBG concentration, but it worsened glucose metabolism disturbance (as indicated by impaired insulin tolerance test) and increased insulin resistance (as indicated by increased insulin concentration and elevated HOMA-IR index). This suggested that loss of ovarian hormones might be involved in insulin resistance independent of their effects on body weight. The present study confirmed the finding of previous studies demonstrating that ovariectomy disturbed glucose metabolism and impaired insulin tolerance.Citation10,Citation29
The result of the present study confirmed the finding of Ben-Shmuel et al.Citation29 who showed disturbance of lipid metabolism (as indicated by higher serum concentrations of TC, HDL-C and TG) in OVX diabetic rats more than either ovariectomy or HFD/STZ diabetes. It has been known that diabetes is considering as a disease of lipid metabolism disturbance more than carbohydrate.Citation13 The results of the present study were consistent with the findings of Saad et al.Citation16 and Liu et al.Citation32 that showed elevation of serum concentrations of TG, TC and free fatty acid in rat model of T2DM. This proposed to enhance lipolysis in insulin-resistant adipocytes.Citation34 Furthermore; our results were consistent with the studies whose reported impairment of lipid metabolism in OVX animalsCitation35 and human.Citation36
Total thiols, the most predominant non-protein thiol, are involved in many biological activities including neutralization of reactive oxygen species (ROS), detoxification of xenobiotics and maintenance of -SH level in proteins.Citation37 Depletion of total thiols pool severely compromised antioxidant capacity. Thus, a compromised antioxidant defense system might be the key factor in contributing towards oxidative stress, reflected in higher levels of oxidatively damaged membrane lipids and proteins ultimately leading to tissue injury and dysfunction.Citation38
The present study demonstrated that when OVX was following HFD/STZ diabetes, oxidative stress state exacerbated. Oxidative stress manifested by high MDA (an indicator of oxidative stress) and low TT (a major antioxidant component) concentrations. MDA and TT concentrations revealed the oxidant/antioxidant balance in the body.Citation39 This exacerbation of oxidative stress could be explained by loss of estrogens those were efficient antioxidants and diminished pro-oxidant enzymes expression concurrently with increased antioxidant enzymes expression as proposed by Sankar et al.Citation10 Oxidative stress has a critical role in the development of diabetic complications.Citation40 Matsuda and ShimomuraCitation41 suggested that both oxidative stress and decreased antioxidant defense mechanisms had a role in increasing insulin resistance in diabetic rats.
The present study revealed an aggravation of inflammatory state when OVX was following HFD/STZ diabetes as indicated by high pro-inflammatory cytokines (IL1β and TNF-α) and low anti-inflammatory cytokines (IL4 and IL10). This inflammatory state might has a role in insulin resistance. Minihane et al.Citation7 proposed that pro-inflammatory mediators released from adipose tissue impaired the insulin signaling and induced insulin resistance.
Histological findings of the pancreas revealed that pancreatic islets were clearly influenced by both ovariectomy and diabetes. Pancreas of OVX rats exhibited immune negative cells in some islets that fail to secrete insulin. This might be a result of loss of the direct hypertrophic effect of estrogen on β cellsCitation42 or due to cytotoxic effect of IL-1β on β cells.Citation43 In addition, OVX rats showed proliferating cells around both interlobular and interlobar ducts (nesidioblastosis) and some of them were immune positive. These changes reflected a compensating mechanism for β cell destruction or functional insufficiency.Citation44 HFD/STZ diabetic pancreas showed islets hyperplasia and proliferating cells around interlobular and interlobar ducts, indicating higher degree of β cells compensation. On the other hand, some islets showed signs of decompensation (as reflected by the presence of immune negative cells in some islets). Islet β cell failure in T2DM might be result from enhanced oxidative stress,Citation45 augmented endoplasmic reticulum stress, exacerbated glucolipotoxicity,Citation46 advanced glycation end product stress, inflammatory pathways activation and islet amyloid polypeptide accumulation.Citation47
When ovariectomy was following HFD/STZ diabetes, the pancreatic changes became more apparent than the effect of any of them alone. The pancreatic architecture showed more decompensatory changes in β cells with evidence of degenerative changes (indicated by vacuolated cytoplasm and dark flat nuclei). Our findings were in agreement with those of Mojtaba et al.Citation43
Thus, the present study theorized that estrogen might have a protective role against glucose and lipid disturbance induced by diabetes in rat as ovariectomy reduced this defense. Future studies using estrogen agonists will be needed to establish this protection.
In conclusion, ovariectomy-induced estrogen deficiency exacerbated glucose and lipid metabolic disturbance of diabetic female rats. This might be contributed to amplification of oxidative stress and inflammatory states. These findings suggested that estrogen deficiency in post-menopausal females could aggravate glucose and lipid dyshomeostasis. Consequently, for efficiency of the treatment, the menopausal status of diabetic female should be addressed.
Conflict of interest
The authors declare that there is no duality of interest associated with this manuscript.
Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 12 June 2017
References
- S.H.TawfikB.F.MahmoudM.I.SaadM.ShehataM.A.KamelM.H.HelmySimilar and additive effects of ovariectomy and diabetes on insulin resistance and lipid metabolismBiochem Res Int2015201556794510.1155/2015/567945
- K.VerasF.N.AlmeidaR.T.NachbaraDHEA supplementation in ovariectomized rats reduces impaired glucose-stimulated insulin secretion induced by a high-fat dietFEBS Open Bio18201414114610.1016/j.fob.2014.01.005
- H.ZhangX.ChenM.R.SairamNovel Genes of Visceral Adiposity: Identification of mouse and human mesenteric estrogen-dependent adipose (MEDA)-4 gene and its adipogenic functionEndocrinol15320122665267610.1210/en.2011-2008
- N.HeldringA.PikeS.AnderssonEstrogen receptors: How do they signal and what are their targetsPhysiol Rev872007905931
- K.L.MargolisD.E.BondsR.J.RodaboughEffect of estrogen plus progestin on the incidence of diabetes in postmenopausal women: results from the women’s health initiative hormone trialDiabetologia47200411751187
- E.L.DingY.SongJ.E.MansonN.RifaiJ.E.BuringS.LiuPlasma Sex steroid hormones and risk of developing type 2 diabetes in women: a prospective studyDiabetologia50200720762084
- A.M.MinihaneS.VinoyW.R.RussellLow-grade inflammation, diet composition and health: Current research evidence and its translationBr J Nutr1142015999101210.1017/S0007114515002093
- N.G.1CruzL.P.SousaM.O.SousaN.T.PietraniA.P.FernandesK.B.GomesThe linkage between inflammation and type 2 diabetes mellitusDiabetes Res Clin Pract992013859210.1016/j.diabres.2012.09.003
- A.C.StanleyP.LacyPathways for cytokine secretionPhysiology (Bethesda)201025201021822910.1152/physiol.00017
- P.SankarB.ZachariahV.VickneshwaranS.E.JacobM.G.SridharAmelioration of oxidative stress and insulin resistance by Soy isoflavones (from Glycine Max) in ovariectomized Wistar rats fed with high fat diet: The molecular mechanismsExp Gerontol632015677510.1016/j.exger.2015.02.001
- S.GuptaJ.GhulmiyyahR.SharmaJ.HalabiA.AgarwalPower of proteomics in linking oxidative stress and female infertilityBiomed Res Int2014201491621210.1155/2014/916212
- R.VukovićS.BlažetićI.OršolićImpact of ovariectomy, high fat diet, and lifestyle modifications on oxidative/antioxidative status in the rat liverCroat Med J552014218227
- D.AhmedV.KumarA.VermaAntidiabetic, renal/hepatic/pancreas/cardiac protective and antioxidant potential of methanol/dichloromethane extract of lbizzia Lebbeck Benth. Stem Bark (ALEx) on streptozotocin induced diabetic ratsBMC Complement Altern Med14201424310.1186/1472-6882-14-243
- K.BayneRevised guide for the care and use of laboratory animals available. American Physiology SocietyPhysiologist391996208211
- H.J.WangY.X.JinW.ShenLow dose streptozotocin (STZ) combined with high energy intake can effectively induce type 2 diabetes through altering the related gene expressionAsia Pac J Clin Nutr162007412417
- M.I.SaadM.A.KamelM.Y.HanafiModulation of adipocytokines production and serum NEFA level by metformin, glimepiride, and sitagliptin in HFD/STZ diabetic ratsBiochem Res Int2015201513813410.1155/2015/138134
- V.SabithaS.RamachandranK.R.NaveenK.PanneerselvamAntidiabetic and antihyperlipidemic potential of Abelmoschus esculentus (L.) Moench. in streptozotocin-induced diabetic ratsJ Pharm Bioallied Sci3201139740210.4103/0975-7406.84447
- D.K.KhajuriaR.RazdanD.R.MahapatraDescription of a new method of ovariectomy in female ratsRev Brasileira Reumatologia522012466470
- S.S.TavaresN.B.C.MariaF.C.F.SouzalimaA.N.JoséCalcium and caffeine interaction in increased calcium balance in ovariectomized ratsRev Nutrição26201314155273
- K.GhazanfarB.A.GanaiS.AkbarAntidiabetic activity of Artemisia Amygdalina Decne in streptozotocin induced diabetic ratsBiomed Res Int201418567610.1155/2014/185676
- M.AllahtavakoliN.HonariI.PourabolliVitex Agnus Castus extract improves learning and memory and increases the transcription of estrogen receptor α in hippocampus of ovariectomized ratsBasic Clin Neurosci62015185192
- W.T.FriedewaldR.I.LevyD.S.FredricksonEstimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifugeClin Chem181972499502
- M.UchiyamaM.MiharaDetermination of malondehyde precursor in tissue by thiobarbituric acid testAnal Biochem861978271278
- G.L.EllmanTissue sulphydryl groupsArch Biochem Biophys8419597077
- R.B.DurryE.A.WallinngtonCarleton's histological technique5th ed.1980Oxford University PressToranto
- O.KluthF.MirhashemiS.ScherneckDissociation of lipotoxicity and glucotoxicity in a mouse model of obesity associated diabetes: Role of forkhead box O1 (FOXO1) in glucose-induced beta cell failureDiabetologia54201160561610.1007/s00125-010-1973-8
- Z.HouM.U.ImamM.IsmailD.J.OoiA.IderisR.MahmudNutrigenomic effects of Edible Bird's Nest on insulin signaling in ovariectomized ratsDrug Des Devel Ther920154115412510.2147/DDDT.S80743
- C.LampertD.M.ArcegoD.P.LaureanoEffect of chronic administration of tamoxifen and/or estradiol on feeding behavior, palatable food and metabolic parameters in ovariectomized ratsPhysiol Behav1192013172410.1016/j.physbeh.2013.05.026
- S.Ben-ShmuelE.J.ScheinmanR.RashedOvariectomy is associated with metabolic impairments and enhanced mammary tumor growth in MKR miceEndocrinol227201514315110.1530/JOE-15-0310
- L.A.EckelThe Ovarian hormone estradiol plays a crucial role in the control of food intake in femalesPhysiol Behav104201151752410.1016/j.physbeh.2011.04.014
- A.Ludgero.-CorreiaJrM.B.AguilaC.A.Mandarim-de-LacerdaT.S.FariaEffects of high-fat diet on plasma lipids, adiposity, and inflammatory markers in ovariectomized C57BL/6 miceNutrition28201231632310.1016/j.nut.2011.07.014
- Y.LiuC.LiuM.-L.LuVibration exercise decreases insulin resistance and modulates the insulin signaling pathway in a type 2 diabetic rat modelInt J Clin Exp Med820151313613144
- K.SrinivasanB.ViswanadL.AsratC.L.KaulP.RamaraoCombination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screeningPharm Res522005313320
- A.D.MooradianDyslipidemia in type 2 diabetes mellitusNat Clin Pract Endocrinol Metab5200915015910.1038/ncpendmet1066
- T.M.D’EonS.C.SouzaM.AronovitzM.S.ObinS.K.FriedA.S.GreenbergEstrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathwaysJ Biol Chem28020053598335991
- Whayne TF, Mukherjee D. Women, the menopause, hormone replacement therapy and coronary heart disease. Curr Opin Cardiol 2015; Epub Feb 18. doi: 10.1097/HCO.0000000000000157.
- H.SiesGlutathione and its role in cellular functionsFree Radic Biol Med271999916921
- S.ChattopadhyayS.ChoudhuryA.RoyG.B.ChainyL.SamantaT3 fails to restore mitochondrial thiol redox status altered by experimental hypothyroidism in rat testisGen Comp Endocrinol16920103947
- Z.Yalniz-AkkayaV.FidanciA.KilincA.BurcuG.O.UneyF.OrnekThe effect of systemic amantadine sulfate on malondialdehyde and total thiol levels in rat corneasJ Ophthalmic Vis Res92014339342
- H.RazaA.JohnF.C.HowarthIncreased metabolic stress in Zucker diabetic fatty rat kidney and pancreasCell Physiol Biochem3220131610162010.1159/000356597
- M.MatsudaI.ShimomuraIncreased oxidative stress in obesity: Implications for metabolic syndrome, diabetes, hypertension, dyslipidemia, atherosclerosis, and cancerObes Res Clin Pract72013e330341
- M.ZhuA.MizunoM.KuwajimaT.OginoT.MurakamiOvarian hormone-induced beta-cell hypertrophy contributes to the homeostatic of β-cell mass in OLETF female rat, a model of type II diabetesDiabetologia411998799805
- E.MojtabaK.MahdiJ.R.MehdiS.AmirSerum interleukin-1 beta plays an important role in insulin secretion in type II diabeticInt J Biosci120119399
- J.E.ChoiS.J.NohJ.J.SungW.S.MoonNesidioblastosis and pancreatic non-functioning islet cell tumor in an adult with type 2 diabetes mellitusKorean J Path472013489491
- Y.LiX.CaoL.X.LiP.L.BrubakerH.EdlundD.J.DruckerBeta cell Pdx1 expression is essential for the glucoregulatory, proliferative, and cytoprotective actions of glucagon-like peptide-1Diabetes542005482491
- K.Chang-ChenR.MullurE.Bernal-MizrachiΒ-cell failure as a complication of diabetesRev Endocr Metab Disord9200832934310.1007/s11154-008-9101-5
- I.HameedS.R.MasoodiS.A.MirM.NabiK.GhazanfarB.A.GanaiType 2 diabetes mellitus: from a metabolic disorder to an inflammatory conditionWorld J Diabetes6201559861210.4239/wjd.v6.i4.598
