Abstract
Introduction: Obesity and type 2 diabetes mellitus have reached epidemic proportions worldwide. Abnormal sleep has been linked to both incident and prevalent obesity and type 2 diabetes. We aimed to characterize abnormal sleep patterns [ASP's] in patients with obesity, type 2 diabetes, or both. Subjects: The study included 92 subjects divided into four groups: Group 1, 23 obese patients (BMI > 30) with type 2 diabetes mellitus; Group 2, 23 non-obese diabetic patients; group 3, 23 obese subjects without diabetes; group 4, 23 matched healthy control subjects. Methods: Waist circumference and BMI [body mass index] estimation, fasting and post challenge plasma glucose “groups 2 & 4”, HOMA-IR [Homeostatic model assessment- Insulin resistance] estimation, and finally evaluation for ASP's using a CDC [Centers for Disease Control and prevention] validated questionnaire. Results: Post-prandial glucose and BMI significantly predicted Sleep latency and sleep hours at night respectively. Both group 1 and 3 compared to group 4 showed higher prevalence of: Insomnia [p < .01], snoring [p < .01], fragmented sleep [p < .05], excessive day time sleepiness [p < .001], and daytime dysfunction [p < .001]. Group 2 compared to group 4 showed higher prevalence of: Insomnia, snoring, fragmented sleep, and finally, daytime dysfunction [All p < .01]. Group 1 compared to groups 3 and 4 had significantly less hours of sleep at night [p < .01]. Group 1 compared to group 2 showed higher prevalence of: Insomnia, fragmented sleep, excessive day time sleepiness, and daytime dysfunction [All p < .05]. Finally, group 3 compared to group 2 showed higher prevalence of: Excessive day time sleepiness, and daytime dysfunction [p < .01]. Conclusion: The combination of obesity and diabetes mellitus is associated with poor quality and quantity of sleep with resultant significant daytime dysfunction. Glycemic, and adiposity measures predicted sleep latency and hours.
1 Introduction
Diabetes Mellitus (DM) is a global emergency and epidemic. Current estimates of world population with diabetes ranges between 415 and 422 million according to IDF (International Diabetes Federation) and WHO (World Health Organization) respectively, and expected to reach 642 million by the year 2040.Citation1,Citation2
Obesity is a major global health challenge, the prevalence has been increasing in the past 30 years, so that in 2013, it was estimated that worldwide 37%, and 38% of adult men and women respectively, and 24%, and 23% of boys and girls respectively are either overweight or obese.Citation3
Sleep disorders and short sleep duration are an emerging public health issue. According to the National Heart, Lung, and Blood Institute of the National Institutes of Health, around 50 to 70 million US (United States) adults suffer from a sleep disorder or report short sleep duration. The prevalence of insomnia symptoms at any given time ranges from 30% to 45%, however, only 6% of people with insomnia receive a diagnosis. Sleep apnea has an estimated prevalence of 27% to 34% among men and 9% to 28% among women, 30 to 70 years of age. Around 27.5% to 29.1% of American adults reported short sleep duration.Citation4
Meta-analyses of cross-sectional and prospective studies have shown that short sleep duration is linked to both prevalent and incident obesity, as well as prevalent and incident type 2 diabetes, respectively.Citation5–Citation8
Possible mechanisms to explain the association between sleep deprivation and sleep disordered breathing and the increased likelihood of obesity and diabetes include.
Increased ghrelin, NPY (Neuropeptide Y), orexin-A, and endocannabinoids, decreased leptin, GLP-1 (Glucagon like peptide-1), and melatonin combined lead to increased appetite, Increased desire to eat high caloric food, increased both hedonic and homeostatic eating, increased adipose tissue accumulation and decreased gastric fullness scores, ultimately leading to Increased caloric intake by a range from 297 to 559 kcal per day. This is aggravated by decreased physical activity, doubled sedentary activity and TV watching leading to obesity. Genetic correlation between increased levels of EDS and increased measures for adiposity traits; BMI, and WC was recently reported.Citation9–Citation16
Short sleep, sleep fragmentation, and OSA were shown to induce insulin resistance through: prolonged nocturnal GH (Growth Hormone) secretion, increased afternoon concentrations of cortisol, increased early morning NE (Nor-Epinephrine) concentrations, Increased release of free fatty acids, obesity, Low grade inflammation, Decreased adiponectin, decreased nocturnal melatonin affecting both insulin secretion and action. Insulin resistance along with a decrease in cerebral glucose utilization eventually lead to diabetes.Citation17–Citation24
The aim of the present study was to characterize abnormal sleep patterns [ASP's] across the continuum of type 2 diabetes and obesity and their relation to insulin resistance.
2 Subjects
The study included 92 subjects divided into four groups: Group 1, 23 patients who are obese (BMI > 30) and suffering from type 2 diabetes mellitus; Group 2, 23 patients who are Diabetics but non-obese; group 3, 23 subjects who are obese with normal glucose tolerance; group 4, 23 healthy control subjects who are non-obese with normal glucose tolerance. We excluded from the study subjects who are: under 18 years of age, or with known liver, cardiac, pulmonary, and renal disorders. Alexandria Faculty of Medicine Ethical committee approved the protocol of the study, and all study participants provided a written informed consent after being explained the nature and aim of the study.
3 Methods
All participants were subjected to: WC in centimeters (waist circumference) and BMI (Body mass index) estimation [Body weight in Kg/Height in m2], fasting and post challenge plasma glucose [with 75 grams of oral glucose load, FBG < 100 mg/dl and post challenge glucose < 140 mg/dl were considered normal glucose tolerance excluding diabetes] “groups 2 & 4”, HOMA-IR (Homeostatic model assessment-Insulin resistance) estimation [Fasting plasma glucose mg/dl × fasting insulin mIU/L/ 405], and finally evaluated for ASP's using NHANES (National Health and Nutrition Examination Survey) sleep questionnaire that included items from 2 validated tools: Sleep Heart Health Study Sleep Habits Questionnaire, Functional Outcomes of Sleep Questionnaire.Citation25–Citation30
The questionnaire is divided into 3 parts, the first is about sleeping habits in 7 questions, the second is about sleeping habits in the past month in 9 questions, and finally the third is about difficulty carrying out certain activities because of being too sleepy in 8 questions. In our presentation of the results, we referred to the 3 different parts of the NHANES questionnaire as questionnaires I, II, and III.Citation11
The following sleep related parameters and ASP’s in the studied groups were assessed using the following corresponding questions highlighted in quotation marks:
Total sleep time: “Number of hours of sleep at night on weekdays”.
Snoring as a surrogate marker of SDB (Sleep disordered breathing) or OSA (Obstructive sleep apnea): “How often do you snore while sleeping”.
Insomnia defined as difficulty initiating or maintaining sleep: “Time taken to fall asleep at bedtime”, i.e. sleep latency period, “Having trouble falling asleep”, “Having trouble getting back to sleep after waking up during night”, “Waking up too early in the morning”.
Sleep abnormalities that may lead to sleep fragmentation: “Having nocturia”, “Having leg jerks while trying to sleep”, “Having leg cramps while trying to sleep”.
Excessive day time sleepiness “EDS: “Feeling excessively or overly sleepy during the day”, “Not getting enough sleep”.
Daytime dysfunction or impairment: “Having difficulty concentrating because of feeling sleepy”, “Having difficulty remembering because of feeling sleepy”, “Having difficulty working on a hobby”, “Having difficulty getting things done”, “Having difficulty performing employed work because of feeling sleepy”, “Having difficulty maintaining a telephone conversation because of feeling sleepy”.
3.1 Statistical methods
Data were analyzed using IBM SPSS software package version 20.0, Armonk, NY: IBM Corp. Comparison between different groups regarding categorical variables was tested using Chi-square test. When more than 20% of the cells have expected count less than 5, correction for chi-square was conducted using Monte Carlo correction. The distributions of quantitative variables were tested for normality. For normally distributed data, comparison between the different studied groups were analyzed using F-test (ANOVA), while for abnormally distributed data Kruskal Wallis test was used. Significance of the obtained results was judged at the 5% level.
4 Results
The four studied groups were matched for age, however, BMI, WC, FBG, 2HPPG, and HOMA-IR showed significant intergroup differences as expected based on selection criteria for each group [].
Table 1 Clinical, anthropometric, and biochemical characteristics of the 4 studied groups.
In our study, diabetic non-obese individuals “group 2” compared to non-diabetics non-obese “group 4” showed significantly higher prevalence of:
Insomnia in terms of more time to fall asleep, and waking up too early in the morning. Snoring; a surrogate marker of sleep disordered breathing. More fragmented sleep in terms of having leg cramps while trying to sleep. Finally, more daytime dysfunction in terms of having difficulty concentrating, remembering, working on a hoppy, and maintaining a telephone conversation because of feeling sleepy [ and ] [ and ].
Fig. 1 Sleep latency period (Time to fall asleep) and sleep hours at night across the four studied groups.
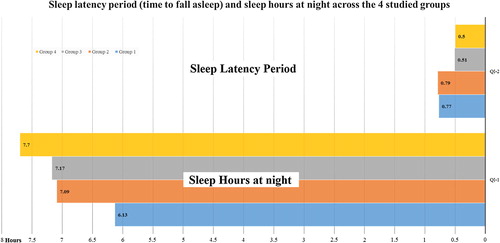
Fig. 2 Responses of the four studied groups to questionnaire III about difficulty carrying out daily activities because of being too sleepy.
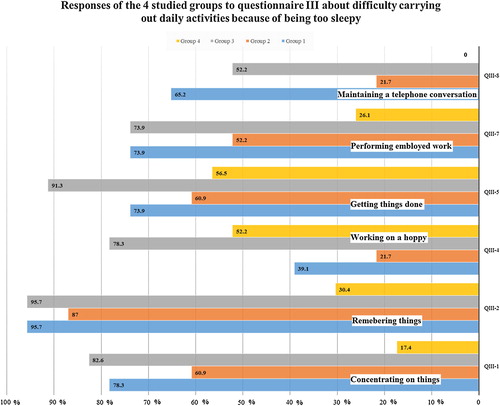
Table 2 Comparison between the studied groups according to Questionnaire I.
Table 3 Comparison between the studied groups according to Questionnaires II, III.
Also, both diabetic obese individuals “group 1” and obese non-diabetic individuals “group 3” compared to non-diabetics non-obese “group 4” showed significantly higher prevalence of:
Insomnia in terms of more time to fall asleep, having trouble falling asleep, having trouble getting back to sleep after waking up during night, and waking up too early in the morning. Snoring; a surrogate marker of sleep disordered breathing. More fragmented sleep in terms of having nocturia, leg jerks, and leg cramps while trying to sleep. Excessive day time sleepiness in terms of not getting enough sleep, and feeling excessively or overly sleepy during the day. Finally, more daytime dysfunction in terms of having difficulty concentrating, remembering, performing employed work, and maintaining a telephone conversation because of feeling sleepy [ and ] [ and ].
Diabetic obese individuals “group 1” compared to obese non-diabetic individuals “group 3” and non-diabetics non-obese “group 4” showed significantly shorter sleep duration in terms of having significantly less hours of sleep at night on weekdays [] [].
Diabetic obese individuals “group 1” compared to diabetic non-obese individuals “group 2” showed significantly higher prevalence of:
Insomnia in terms of having trouble falling asleep. More fragmented sleep in terms of having nocturia. Excessive day time sleepiness in terms of not getting enough sleep, and feeling excessively or overly sleepy during the day. Lastly, more daytime dysfunction in terms of having difficulty maintaining a telephone conversation because of feeling sleepy [ and ] [].
Finally, obese non-diabetic individuals “group 3” compared to diabetic non-obese individuals “group 2” showed significantly higher prevalence of:
Excessive day time sleepiness in terms of not getting enough sleep, and feeling excessively or overly sleepy during the day. More daytime dysfunction in terms of having difficulty getting things done, working on a hoppy, and maintaining a telephone conversation because of feeling sleepy [] [].
In the whole study group, time to fall asleep or sleep latency period (LP) was significantly positively associated with FBG, 2HPPG, HOMA, and WC, while number of sleep hours at night were significantly negatively associated with WC and BMI. Using a stepwise regression model, only 2HPPG and BMI significantly predicted sleep latency period (LP) and number of sleep hours respectively [ and ].
Table 4 Correlation between sleeping hours and time to fall asleep with different parameters in total sample.
Table 5 Stepwise regression models for predictors of time to fall asleep and sleeping hours.
5 Discussion
The aim of the present study was to evaluate the prevalence of abnormal sleep patterns [ASPs] across the continuum of type 2 diabetes and obesity and their relation to insulin resistance. Our results showed a high prevalence of ASP's in our cohort of diabetics, obese, or both combined in terms of Insomnia, snoring as surrogate marker of sleep disordered breathing, sleep abnormalities that may lead to sleep fragmentation [nocturia, leg jerks and leg cramps], excessive day time sleepiness, and daytime dysfunction. Sleep latency period correlated significantly with insulin resistance and glycemic parameters while number of sleep hours correlated significantly with anthropometric measures of adiposity.
In a recent study by Plantinga et al. of self-reported sleep problems in 9848 adults (aged ≥20 y) participating in the NHANES 2005 through 2008 where Sleep problem information was elicited via the same questionnaire used in our study, Diabetes was associated with increased odds of inadequate sleep, frequent daytime sleepiness, restless legs symptoms, sleep apnea, and nocturia. BMI partly explained this association. Waist circumference was associated with sleep apnea with OR (Odds ratio), 1.03 per 1-cm increase. Apnea, leg symptoms, daytime sleepiness, and nocturia showed greater odds with increasing severity of diabetes in a significant, graded fashion. Diabetes duration was significantly associated with the same problems; risk increased 20% to 30% per 10 years since diagnosis.Citation31
Similarly, a high prevalence of sleep abnormalities among T2DM patients have been reported in the literature. Poor sleep quality as assessed by Pittsburgh Sleep Quality Index (PSQI) questionnaire in 49%, Excessive daytime sleepiness assessed by Epworth sleepiness score in 28.9 to 46%, and obstructive sleep apnea assessed by polysomnography in 48.7% of patients with T2DM.Citation32–Citation34
A correlation between sleep related parameters and glycemic and adiposity measures has also been reported. Among diabetics, higher sleep fragmentation or the presence of insomnia assessed using questionnaire and actigraphy was associated with 12.6 to 28.8 mg/dl higher fasting plasma glucose and 43 to 82% higher HOMA respectively.Citation35 Among subjects with metabolic syndrome, Epworth sleepiness score showed significant associations with both BMI, and FBG.Citation34
ASPs are not only related to the presence of diabetes but can also influence the micro- and macrovascular complications arising due to diabetes. T2DM Subjects with short sleep had a higher risk of developing diabetic neuropathy; DKD (Diabetic kidney disease) [OR 1.3]; CVD (Cardiovascular disease) [OR 1.6); and PAD (Peripheral arterial disease) [OR 1.34). T2DM Subjects with insomnia had a higher risk of developing CVD [OR 1.35], while those with excessive daytime sleepiness had a higher risk of diabetic neuropathy.Citation36,Citation37
The observed higher prevalence of abnormal sleep patterns in obese, diabetics, or obese diabetics in our study may be interpreted in two ways: poor sleep causing diabetes and obesity or diabetes and obesity causing poor sleep.
6 Evidence in favor of poor sleep causing diabetes and obesity
Two meta-analysis of prospective studies that assess the relationship between sleep quality and quantity and the incidence of type 2 diabetes, including 10 studies each, with follow up duration ranging from 2.5 to 32 years; showed that short sleep duration, long sleep duration, difficulty in initiating sleep, and difficulty in maintaining sleep were associated with a significantly increased risk of developing type 2 diabetes.Citation8,Citation38
Li et al. recently reported, in a prospective trial with up to 10 years of follow-up, that women who reported having one, two, three, and all four sleep conditions namely; sleeping difficulty, frequent snoring, sleep duration ≤6 h and sleep apnea, had one and a half fold, twofold, threefold, and more than a fourfold increased likelihood of type 2 diabetes, respectively.Citation39
Two prospective studies aimed to assess regular snoring as a risk factor for incident T2DM during 10 years of follow up. In males, the combination of obesity and habitual snoring were independently associated with the development of diabetes, however, Habitual snoring without obesity was not a risk factor for the development of diabetes.Citation40 In females, both occasional snoring and regular snoring were associated with risk of diabetes compared to no snoring.Citation41
In a recent meta-analysis of prospective studies examining the association between sleep duration and risk of obesity, short sleep duration increased OR for obesity, both in males and in females, and both in short and long follow-up duration (>5 years and ≤5 years).Citation6 This was confirmed in the most recent prospective study examining the association between Night-time sleep duration and the incidence of obesity, the OR of becoming obese was significantly higher in subjects with short sleep duration, at both 6-years and 11-years follow-up.Citation42
7 Evidence in favor of diabetes causing poor sleep
Diabetes was associated with both obstructive and central sleep apnea, obesity present in up to 86% and diabetic neuropathy present in up to half of diabetic patients mediate this association respectively. In the Sleep Heart Health Study (SHHS), the association between diabetes and OSA was largely dependent on obesity, in the same study, autonomic neuropathy directly affects central chemoreceptors and through impaired cardiovascular function affects cardiac chemoreceptors, combined they may lead to a periodic (Cheyne Stokes) breathing pattern.Citation43,Citation44
In a recent study, both macrovascular and microvascular diabetic complications, namely nephropathy and retinopathy, independently predicted early awakening, short sleep, and long sleep.Citation17 In another study, diabetic peripheral neuropathy predicted the presence of RLS, and poor sleep quality as assessed by PSQI was related to the presence of RLS, and peripheral neuropathy.Citation45
Nocturia, reported in Up to 80% of patients with type 2 diabetes mellitus, is highly prevalent in patients with intrinsic sleep disorders like insomnia, poor sleep quality, EDS, snoring, and OSA. individuals awakened by nocturia are more likely to report poor sleep quality, and EDS.Citation46,Citation47
Nocturnal hypoglycemia characterized by a rapid decline in glucose levels, rather than the absolute degree of hypoglycemia, is associated with increased arousal from sleep which may lead to sleep fragmentation.Citation48
Diabetes confers an increased risk of heart failure that is more in women compared to men and more in middle aged compared to elderly. Heart failure is associated with early and late insomnia, as well as, obstructive and central sleep apnea.Citation49,Citation50
Diabetes impaired pineal melatonin synthesis machinery, and relief of pineal hyperglycemia restores melatonin production to normal. Melatonin disruption may have detrimental effects on sleep maintenance and lead to abnormal sleep.Citation51
Limitations of the current study include: (a) the small number of subjects included, (b) the CDC formulated questionnaire used in the current study is not validated against ESS, PSQI, or polysomnography which are considered gold standard methods for assessment of daytime sleepiness, overall sleep quality, and sleep disordered breathing respectively, however, the purpose of this study is to globally characterize sleep abnormalities in diabetic patients rather than assessing specific domains of abnormal sleep and this questionnaire better serves this purpose and secondly it has proven to be effective in characterizing sleep abnormalities on a much larger sample in the study by Plantinga et al. done on almost 10 thousand American subjects.Citation15
Other limitations include: (c) not using glycated hemoglobin which assesses long term glycemic control rather than fasting and prandial glucose which assess short term glycemic state because laboratory at our institution is not NSGP (National Glycohemoglobin Standardization Program) certified as recommended by the American diabetes association, in addition, other authors similarly reported correlations between abnormal sleep and fasting glycemia.Citation19,Citation20,Citation52 Finally, (d) inclusion of overweight subjects in groups 2 and 4 may have undermined the differences in sleep related measures between different groups attributable to obesity despite the fact that BMI differed significantly between obese groups [1 & 3] and non-obese groups [2 & 4].
In conclusion, obesity, type 2 diabetes mellitus, or their combination are associated with poor quality and quantity of sleep with resultant significant daytime dysfunction. Post-prandial glycemic status and adiposity significantly predicted Sleep latency, and number of sleep hours at night.
Conflict of interest
We have no conflict of interest to declare.
supplementary-material 1
Download PDF (72.6 KB)Notes
Peer review under responsibility of Alexandria University Faculty of Medicine.
Available online 15 November 2017
References
- International Diabetes FederationIDF Diabetes Atlas7th ed.2015International Diabetes FederationBrussels, Belgium
- World Health OrganizationGlobal Report on Diabetes2016World Health OrganizationGeneva
- N.G.MarieT.FlemingM.RobinsonGlobal, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013: a systematic analysis for the Global Burden of Disease Study 2013Lancet3842014766781
- M.P.St-OngeM.A.GrandnerD.BrownAmerican Heart Association Obesity, Behavior Change, Diabetes, and Nutrition Committees of the Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular Disease in the Young; Council on Clinical Cardiology; Stroke Council. Sleep duration and quality: impact on lifestyle behaviors and cardiometabolic health: a Scientific Statement from the American Heart AssociationCirculation1342016e367e386
- F.P.CappuccioF.M.TaggartN.B.KandalaMeta-analysis of short sleep duration and obesity in children and adultsSleep312008619626
- Y.WuL.ZhaiD.ZhangSleep duration and obesity among adults: a meta-analysis of prospective studiesSleep Med15201414561462
- A.TeresaT.ShahradSleep optimization and diabetes control: a review of the literatureDiabetes Ther62015425468
- Z.ShanH.MaM.XieSleep duration and risk of type 2 diabetes: a meta-analysis of prospective studiesDiabetes Care382015529537
- M.P.St-OngeThe role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditureJ Clin Sleep Med920137380
- K.L.KnutsonE.V.CauterAssociations between sleep loss and increased risk of obesity and diabetesAnn N Y Acad Sci11292008287304
- H.K.J.GonnissenR.HurselF.RuttersEffects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy menBr J Nutr1092013748756
- E.C.HanlonE.TasaliR.LeproultSleep restriction enhances the daily rhythm of circulating levels of endocannabinoid 2-arachidonoylglycerolSleep392016653664
- F.G.AmaralA.M.CastrucciJ.Cipolla-NetoEnvironmental control of biological rhythms: effects on development, fertility and metabolismJ Neuroendocrinol262014603612
- L.KlingenbergA.SjödinU.HolmbäckShort sleep duration and its association with energy metabolismObes Rev132012565577
- F.NduhirabandiE.F.du ToitA.LochnerMelatonin and the metabolic syndrome: a tool for effective therapy in obesity-associated abnormalities?Acta Physiol (Oxf)2052012209223
- J.M.LaneJ.LiangI.VlasacGenome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traitsNat Genet192016https://doi.org/10.1038/ng.3749 [Epub ahead of print]
- J.L.BroussardF.ChapototV.AbrahamSleep restriction increases free fatty acids in healthy menDiabetologia582015791798
- J.L.BroussardD.A.EhrmannE.V.CauterImpaired insulin signaling in human adipocytes after experimental sleep restriction: a randomized, crossover studyAnn Intern Med1572012549557
- R.LeproultG.CopinschiO.BuxtonSleep loss results in an elevation of cortisol levels the next eveningSleep201997865870
- J.M.MullingtonN.S.SimpsonH.K.Meier-EwertSleep loss and inflammationBest Pract Res Clin Endocrinol Metab242010775784
- N.S.SimpsonS.BanksS.ArroyoEffects of sleep restriction on adiponectin levels in healthy men and womenPhysiol Behav1012010693698
- CJ1McMullanG.C.CurhanE.S.SchernhammerAssociation of nocturnal melatonin secretion with insulin resistance in nondiabetic young womenAm J Epidemiol1782013231238
- M.A.CornierD.DabeleaT.L.HernandezThe metabolic syndromeEndocr Rev292008777822
- S.ReutrakulE.Van CauterInteractions between sleep, circadian function, and glucose metabolism: implications for risk and severity of diabetesAnn N Y Acad Sci13112014151173
- American Diabetes AssociationClassification and diagnosis of diabetesDiabetes Care40Suppl. 12017S11S24
- D.R.MatthewsJ.P.HoskerA.S.RudenskiHomeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in manDiabetologia281985412419
- Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS). National Health and Nutrition Examination Survey Questionnaire. Hyattsville, MD: US Department of Health and Human Services, CDC, 2005–2006. http://www.cdc.gov/nchs/data/nhanes/nhanes_05_06/sp_slq_d.pdf. [accessed September 1, 2017].
- G.T.O’ConnorB.K.LindE.T.LeeVariation in symptoms of sleep-disordered breathing with race and ethnicity: The Sleep Heart Health StudySleep2620037479
- T.E.WeaverA.M.LaiznerL.K.EvansAn instrument to measure functional status outcomes for disorders of excessive sleepinessSleep201997835843
- E.R.ChasensS.J.RatcliffeT.E.WeaverDevelopment of the FOSQ-10: a short version of the Functional Outcomes of Sleep QuestionnaireSleep322009915919
- L.PlantingaM.N.RaoD.SchillingerPrevalence of self-reported sleep problems among people with diabetes in the United States, 2005–2008Prev Chronic Dis92012110244
- Y.SongX.YeL.YeDisturbed subjective sleep in Chinese females with type 2 diabetes on insulin therapyPLoS ONE82013e54951
- T.Nakanishi-MinamiK.KishidaT.FunahashiSleep-wake cycle irregularities in type 2 diabeticsDiabetol Metabolic Syndrome4201218
- A.S.BediwyY.M.MansourE.A.Abo AliExcessive daytime sleepiness among patients with metabolic syndromeEgypt J Chest Dis Tuberc652016259263
- K.L.KnutsonCross-sectional associations between measures of sleep and markers of glucose metabolism among subjects with and without diabetes the coronary artery risk development in young adults (CARDIA) sleep studyDiabetes Care34201111711176
- R.RamanA.GuptaK.VenkateshAbnormal sleep patterns in subjects with type II diabetes mellitus and its effect on diabetic microangiopathies: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetic StudyActa Diabetol492012255261
- L.MengY.LiuR.GengAssociation of diabetic vascular complications with poor sleep complaintsDiabetol Metab Syndr8201680
- F.P.CappuccioQuantity and quality of sleep and incidence of type 2 diabetes a systematic review and meta-analysisDiabetes Care332010414420
- Y.LiX.GaoJ.W.WinkelmanAssociation between sleeping difficulty and type 2 diabetes in womenDiabetologia592016719727
- A.ElmasryC.JansonE.LindbergThe role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male populationJ Intern Med24820001320
- W.K.Al-DelaimyJ.MansonW.C.WillettSnoring as a risk factor for type II diabetes mellitus: a prospective studyAm J Epidemiol1552002387393
- C.Gutiérrez-RepisoF.SoriguerE.Rubio-MartínNight-time sleep duration and the incidence of obesity and type 2 diabetes. Findings from the prospective Pizarra studySleep Med15201413981404
- ResnickDiabetes and sleep disturbances findings from the sleep heart health studyDiabetes Care262003702709
- C.DaousiI.F.CassonG.V.GillPrevalence of obesity in type 2 diabetes in secondary care: association with cardiovascular risk factorsPostgrad Med J822006280284
- LopesRestless legs syndrome and quality of sleep in type 2 diabetesDiabetes Care28200526332636
- S.FurukawaNocturia and prevalence of erectile dysfunction in Japanese patients with type 2 diabetes mellitus: The Dogo StudyJ Diabetes Investig72016786790
- D.FurtadoNocturia × disturbed sleep: a reviewInt Urogynecol J232012255267
- G.PillarInteractions between hypoglycemia and sleep architecture in children with type 1 diabetes mellitusJ Pediatr1422003163168
- R.E.GilbertH.KrumHeart failure in diabetes: effects of anti-hyperglycaemic drug therapyLancet385201521072117
- N.S.RedekerSleep disturbance in people with heart failure implications for self-careJ Cardiovasc Nurs232008231238
- F.G.AmaralMelatonin synthesis impairment as a new deleterious outcome of diabetes-derived hyperglycemiaJ Pineal Res5720146779
- American Diabetes AssociationClassification and diagnosis of diabetes. Sec. 2. In Standards of Medical Care in Diabetes 2017Diabetes Care40Suppl. 12017S11S24
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://doi:10.1016/j.ajme.2017.10.004.
